Abstract
A greenhouse microcosm study investigated the impacts of recovered iron oxyhydroxide mine drainage residuals (MDRs) on phosphorus (P) and trace metal distributions at the sediment layer/water column interface in Grand Lake o’ the Cherokees, a large reservoir receiving waters impacted by both historic mining and current agricultural land uses. Each mesocosm included 5 kg of lake sediment and 20 L of on-site groundwater. Three treatments were examined in triplicate: control (C) with no additions, low MDR (LM) with 0.3 kg added MDR, and high MDR (HM) with 0.9 kg added MDR. In the first 10 days, aqueous soluble reactive phosphorous (SRP) concentrations decreased likely due to colonizing biomass uptake with no significant differences among the three treatments. LM and HM treatments showed delayed peaks in dissolved oxygen (DO) and lesser peaks in chlorophyll-a (Chl-a) concentrations compared to the C treatment, indicating MDR addition may suppress biomass growth. During days 11 to 138, the C treatment demonstrated increasing pH, decreasing ORP, and biomass decay resulting in significantly increased SRP concentrations. In LM and HM treatments, sufficient P sorption by the MDR maintained low SRP concentrations. Although the MDRs are derived from metal-rich mine waters, all aqueous concentrations were below both hardness-adjusted acute and chronic criteria, except for Pb with regard to the chronic criterion. Metal concentrations in sediments were below the Tri-State Mining District (TSMD)–specific Sediment Quality Guidelines (SQGs). MDR additions may serve as stable long-term P sinks to prevent P release from dead biomass, decrease internal P cycling rates, and mitigate eutrophication, with limited concern for trace metal release.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Excess nutrient inputs into aquatic ecosystems have raised concerns about eutrophication and resulting harmful algal blooms (Sibrell et al. 2009; Ho et al. 2019). Phosphorus (P), a common limiting nutrient in freshwater systems, often enters lakes and reservoirs via both external and internal loading sources (Perkins and Underwood 2001; Chen et al. 2018). External loading includes contributions from rivers and streams, surface run-off, and precipitation. Internal loading refers to release from legacy P sinks in lake or reservoir sediments (Perkins and Underwood 2001; Jin et al. 2006; Nilolai and Dzialowski 2014). Therefore, eutrophication concerns are often managed by decreasing external P loading through watershed best management practices (e.g., Yang et al. 2014) or water treatment (e.g., Sibrell and Tucker 2012), and internal sources are often addressed by dredging and removal of P-rich sediments (e.g., Yu et al. 2017).
Phosphorus-sorbing materials (PSMs) have been investigated as a means to decrease external P loading. Generally, PSMs can be divided into two categories: calcium (Ca) and/or magnesium (Mg)-based materials for P precipitation and iron (Fe) and/or aluminum (Al)-based materials for P adsorption (Penn et al. 2007; Christianson et al. 2017). The P-removal mechanism for Ca/Mg-based PSMs is the precipitation of Ca and Mg phosphate minerals, which is usually a slow process but provides a stable and permanent burial of P. The P-removal mechanism for Fe/Al PSMs is ligand exchange reactions on sorption sites provided by amorphous Fe/Al oxide flocs. This mechanism is rapid, but P may be released upon changes in the oxidation-reduction profile (Penn et al. 2007; Sibrell et al. 2009; Chen et al. 2015; Penn et al. 2017; Qin et al. 2018). Fe/Al salts such as alum (aluminum sulfate) and ferric sulfate have been applied in municipal and industrial wastewater treatment systems for decades, but the cost of using commercial products for P removal in lakes and reservoirs is often prohibitive (Penn et al. 2007; Sibrell et al. 2009; Sibrell and Tucker 2012). Therefore, a need exists to develop cost-efficient PSMs, perhaps by recycling waste products, and corresponding methods to utilize them to address internal and external loadings.
Several studies have been conducted to evaluate P removal efficiencies of different industrial waste products, including steelmaking slags, fly ash, wood ash, drinking water treatment residuals, paper mill sludge, sander dust, mag dust (i.e., waste products from building practices, composed mainly of calcium oxide and silicon dioxide) and waste gypsum, all of which demonstrated P removal potential, but restricted widespread application due to lack of local availability and transportation costs (e.g., O’Reilly and Sims 1995; Drizo et al. 2006; Penn et al. 2007; Sibrell et al. 2009; Spears et al. 2013). Iron and aluminum oxides generated from water treatment at mining sites, known as mine drainage residuals (MDRs), are another option due to their wide availability (Spears et al. 2013).
Acid mine drainage (AMD) occurs often at abandoned coal and metal mines. Exposure of sulfide minerals (e.g., pyrite, FeS2) to water and air results in oxidation reactions producing sulfuric acid and dissolved metal species. Other metals in host rock and adjacent strata (e.g., aluminum (Al), manganese (Mn), zinc (Zn), and lead (Pb)) can dissolve and be released into the solution (Stumm and Morgan 1996; Sibrell et al. 2009). Therefore, AMD can cause low pH and elevated ecotoxic trace metal concentrations in receiving water bodies. To sustainably treat AMD without constant chemical and energy inputs, an economical approach including a sequence of ecologically engineered process units has been developed, known as passive treatment (e.g., Hedin et al. 1994; Watzlaf et al. 2004; Hedin 2008; Skousen et al. 2017; Nairn et al. 2020). Passive treatment systems improve water quality by acid neutralization, metal retention, and alkalinity generation through a suite of designed yet naturally occurring biogeochemical processes. Oxidation ponds and aerobic wetlands, common in passive treatment systems, produce MDRs rich in iron oxyhydroxides (Hedin 2008; Sibrell et al. 2009). Over time, MDRs accumulate in these process units, and identification and evaluation of reuse opportunities are critical to system sustainability.
Sibrell et al. (2009) processed MDRs from six different coal mine drainage sites in PA, composed primarily of Fe and Al oxyhydroxides, with minor amounts of gypsum and unreacted calcite. After drying and pelletization, phosphorus adsorption capacity (PAC) tests were performed resulting in values as high as 20,000 mg P per kg MDR. Their column tests showed rapid sorption with 60–90% P removal within the first 5 min of contact time. Stripping recycled MDRs with a 0.1 M NaOH solution showed up to 76% P loss and demonstrated that MDR materials can be regenerated and reused for P removal. Wei et al. (2008) performed MDR batch studies to examine both treatment potential for secondary wastewater effluent and metal leachability. They built Freundlich isotherm sorption models to estimate PAC ranges using different wastewater effluent concentrations. In the PAC tests, they found over 98% P removal efficiency and, in later leaching tests, no appreciable trace metal release over a typical pH range of 6–8. The study carried out by Han et al. (2005), instead of directly using MDRs, examined modified lignocellulosic fibers with surface-precipitated AMD materials to remove at least 40% of P from water with 59 mg P/L influent concentrations. Their study showed that the sorption capacity of such modified fibers was higher than other conventional adsorbents and a pseudo second-order kinetic model fit well. Heal et al. (2005) tested the P removal performance of MDR from wastewater in laboratory experiments, achieving a 90% P removal rate, and in field investigations where MDR added into constructed horizontal flow wetlands achieved a mean P removal efficiency of 27 ± 28% during a 15-month monitoring period. They found that a hydraulic design was key to P removal as they documented short-circuiting which limited the full P removal capacity of the MDR. Dobbie et al. (2009) report on a follow-up study which involved two long-term field tests (a 3-year experiment at Leitholm in Scotland and a 9-month experiment at Windlestone in England) and concluded that MDR-based treatment systems had considerable potential to remove P with a lifetime estimated to be ten times longer than other tested substrates, with no observed toxic metal releases.
Although these results demonstrate potential utility, most MDR P-removal studies are based on treating wastewater with much higher levels (often >10 mg P/L) than eutrophic lake waters. Therefore, the sorption performance achieved at such elevated P levels may not apply to natural water conditions (typically less than 1 mg P/L). Most of the cited MDR-based studies were performed in flow-through columns, batch reactors, or experimental wetlands to address external P loading. However, no research was found on direct application of MDR in lakes and reservoirs to address internal P loading. In previous studies, examinations of trace metal leachability focused on water column concentrations without investigation into sediment levels. Therefore, this study aimed to fill these data gaps by attempting to (i) estimate MDR-based P removal performance at natural water concentrations and for reservoir sediments to address internal loading and (ii) evaluate trace metal changes in both the water column and sediments.
2 Material and Methods
2.1 Study Site Description
Grand Lake o’ the Cherokees (GLOC) is a large eutrophic multipurpose reservoir (surface area approximately 19,000 ha) which experiences seasonal algal blooms (Morrison et al. 2017). Historically, P has been an important excess nutrient in this 27,000-km2 predominantly agricultural watershed, and internal P loading contributes to seasonal variations in TN:TP ratios such that limiting nutrients change (Nilolai and Dzialowski 2014).
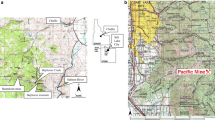
Background water quality and sediment samples were collected from GLOC in the Sycamore Flats area (Fig. 1; 36° 44′ 44.2″ N, 94° 44′ 9.6″ W). Water samples were collected using a depth-discrete horizontal water sampler just above the sediment layer/water column interface. Water depths were approximately 1.1 m. Lake surface sediments were collected using a Ponar dredge from 0 to 10 cm sediment depth. After collection, all samples were stored and transported at 4 °C in coolers filled with ice before delivery to the University of Oklahoma Center for Restoration of Ecosystems and Watersheds (CREW) laboratories.
a Location of Grand Lake o’ the Cherokees, Tar Creek watershed, and Tri-State Mining District showing Sycamore Flats (water and sediment sampling location), and Mayer Ranch passive treatment system (MDR sampling location). Insert map b shows the location of study site to Grand Lake o’ the Cherokees watershed. Insert map c shows the location of the watershed in Oklahoma, Kansas, Missouri, and Arkansas
2.2 Phosphorus-Sorbing Material: Mine Drainage Residuals
The headwaters of GLOC are located approximately 33 km downstream (via Tar Creek and the Neosho River) from the center of the Tar Creek Superfund Site, the northeast Oklahoma portion of the now derelict Tri-State Lead-Zinc Mining District (TSMD) (Fig. 1). Currently, four National Priority List (NPL) Superfund Sites are located in TSMD: Tar Creek in Oklahoma, Cherokee County in southeast Kansas, and two additional sites in Jasper and Newton Counties in southwest Missouri (Garvin et al. 2017). The entire TSMD drains to GLOC through the Neosho and Spring Rivers.
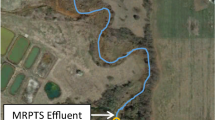
At the Tar Creek Superfund Site, the Mayer Ranch passive treatment system (MRPTS) has received artesian flows of trace metal–contaminated mine water since late 2008 (Fig. 1). Iron is successfully retained in the oxidative process units (including an oxidation pond and two surface flow wetland units), and 99% overall iron removal is achieved, decreasing concentrations from 175 ± 25 to 0.65 ± 0.98 mg Fe/L (n = 184). Approximately 57,000 kg iron is retained annually (Oxenford 2016). MDR used in this study was collected from the MRPTS oxidation pond (Table 1, Oxenford 2016). MDRs were air-dried and ground to <0.85 mm before use in this mesocosm study.
2.3 Microcosm Setup
Greenhouse microcosms were established at the University of Oklahoma Aquatic Research Facility using 25-L borosilicate glass vessels, each with 5 kg wet GLOC sediment and 20 L on-site ground water. To avoid chemical and biological variability due to long-distance transport of lake water, on-site ground water was used and periodic sampling demonstrated similar quality to the lake water. Three treatments were established in this study: control (C) with only sediment and ground water; low MDR addition (LM) with ground water and 0.3 kg dry MDR addition (to 5 kg lake sediments); and high MDR addition (HM) with ground water and 0.9 kg dry MDR addition. The ratios of 1:10 and 3:10 MDR to lake sediment (dry weight:dry weight) were selected based on preliminary bench-scale studies. MDR was added on the surface of the lake sediments without mixing to mimic potential future applications of dosing MDR onto the surface of eutrophic lake sediments.
Each treatment included triplicates, resulting in nine experimental vessels. After initial setup, all vessels were allowed to equilibrate for 2 days before initial sample collections.
2.4 Sampling Process and Chemical Analysis
Water samples were collected at days 0, 1, 2, 4, 6, 8, and 10 (short-term) and days 40, 75, 102, and 138 (long-term). Water samples were obtained at 10 cm above the sediment layer/water column interface using a siphoning technique. For each sampling event, approximately 750 mL of water was siphoned from each vessel and replaced by supplemental ground water to replenish water losses due to sampling and evaporation. All samples were analyzed for soluble reactive phosphorus (SRP) using the ascorbic acid method (EPA Method 365.3).
Although no biomass was intentionally added to any vessel, algal biomass blooms and subsequent decay were observed during the study. Therefore, chlorophyll-a (Chl-a) concentrations were analyzed using a hot-ethanol extraction method (Chen et al. 2006), in which the water samples were filtered with Whatman glass microfiber filters (GF/F) and extracted with 90% ethanol in an 85 °C water bath for 2 min and stored at room temperature for 5 h, then analyzed by using a Cole-Parmer SQ2800 UV/visible spectrometer at 665 and 750 nm.
Samples for aqueous total metal (TM) and dissolved metal (DM) analyses underwent microwave hot HNO3 digestion (EPA Method 3015 using a CEM MARS Xpress Digestion System) and were analyzed via inductively coupled plasma-optical emission spectrometry (ICP-OES) (EPA Method 6010 using a Varian Vista-Pro simultaneous axial ICP-OES) for a suite of trace metals: silver (Ag), aluminum (Al), arsenic (As), barium (Ba), calcium (Ca), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), lithium (Li), magnesium (Mg), manganese (Mn), sodium (Na), nickel (Ni), lead (Pb), selenium (Se), silicon (Si), and zinc (Zn). Only selected metals are reported.
A YSI 600QS multiparameter datasonde coupled with a YSI 650 display was used to collect and record physicochemical water quality data approximately 10 cm above the sediment layer/water column interface. These data include pH, water temperature (Temp), salinity, specific conductance, total dissolved solids (TDS), dissolved oxygen (DO) (in mg/L and % saturation), and oxidation-reduction potential (ORP). Samples of any supplemental ground water were collected and analyzed as described above.
Sediment and MDR samples were collected at the initial stage (before setup) and final stage (after the last set of water sample collections) of the study and measured gravimetrically for moisture content (MC) and organic content (OC) as loss-on-ignition through the American Society for Testing and Materials methods (ASTM D2974). Solid-phase P was extracted with ammonium bicarbonate-diethylenetriaminepentaacetic acid (AB-DTPA) (Kuo 1996), representing the P fraction bound to Fe, Al, and Mn oxides and hydroxides, and was analyzed following EPA Method 365.3 (SSSA 1996). Sediment samples for total recoverable metal analyses underwent microwave hot HNO3 digestion (EPA Method 3051) and were analyzed via ICP-OES (EPA Method 6010), similar to the water samples.
2.5 Statistical Analysis
Statistical significance of differences analyses were performed with one-way analysis of variance (ANOVA) using the SPSS 24.0 software. A p value <0.05 was considered significant. When differences between treatments were significant, least significant difference (LSD) tests were performed as a post hoc study. Variations in results were reported in terms of standard deviations (± SD).
3 Results and Discussion
3.1 Physicochemical Water Quality Parameters and Chl-a
Over the summer-fall study period, water temperatures ranged from 21.3 to 27.2 °C with no significant differences among the three treatments (p = 0.934) but significant initial decreasing and later increasing trends over time (p < 0.0005), due to prevailing weather conditions. pH ranged between 6.9 and 7.7 during the first 8 days and 8.2 and 10.0 from day 10 to day 138 with no significant differences among treatments (p = 0.974) and significant increasing trends over time (p < 0.0005) (Fig. 2a). For these figures, smaller inset figures are inserted to the upper right corner, showing the entire 138-day study period, while the larger figures show details for the first 10 days of the study. The insert figures share the same x- and y-axis units as the original figures. Temporal increases in pH were likely due to photosynthetic activity of colonizing biomass (Jin et al. 2006; Chen et al. 2018). ORP ranged between 189 and 242 mV during the first 8 days and 32 and 234 mV from day 10 to day 138 with no significant differences among treatments (p = 0.923) but significant decreasing trends over time (p < 0.0005) (Fig. 2b). Decreasing ORP values over extended time periods have been documented by other studies (Drizo et al. 2002; Dobbie et al. 2009), probably due to existing Fe (II) in the MDR.
DO concentration, ranged between 7.0 and 27.6 mg/L (Fig. S1), showed no significant differences among treatments (p = 0.660), and demonstrated a significant increasing trend over the first 4–6 days followed by a decreasing trend until day 138 (p < 0.0005). During the first 6 days, the time to peak DO concentration may have been delayed by MDR addition (C < LM < HM) indicating the potential effects of mixed valence iron precipitates on water chemistry.
No biomass growth was observed at the initial setup stage of the study, and no algal biomass was added into treatments during the entire study period. However, colonizing algal biomass blooms and decay were observed during the study. Chl-a concentrations ranged between 1.4 and 121.8 μg Chl-a /L (Fig. S2), showed no significant differences among treatments (p = 0.976), and showed no significant trends over time (p = 0.080). MDR addition treatments had lesser peak Chl-a concentration (LM and HM) and delayed peak over time (HM), which may indicate that MDR additions inhibited initial algal biomass growth.
3.2 Nutrient Concentrations in Water and Sediment
SRP concentrations ranged from 0.001 to 0.678 mg P/L (Fig. 3). From day 0 to day 75, SRP showed no significant differences among the three treatments (p = 0.876) and significant decreases over time (p < 0.0005). During the initial stages, the added MDR did not result in significant water column P concentration changes compared to C treatments. However, from day 75 to day 138, SRP showed significant differences among three treatments (p = 0.003), with C treatments showing significant increase in SRP (p = 0.010) while LM and HM treatments showing no significant changes in SRP (p = 0.127 for LM, p = 0.208 for HM, respectively). Three major mechanisms may contribute to increasing SRP concentrations. First, increasing pH over time (pH 9.3–10.0 between day 40 and day 138) exceeded the point of zero charge (PZC) of natural iron oxyhydroxides, typically between 5 and 8 (Schwertmann and Fechter 1982). Neely (2010) found a PZC of 7.24 ± 0.42 for MRPTS MDRs. Available binding sites for phosphate were decreased due to the negatively charged solid surface and competition with hydroxyl ions (Jin et al. 2006; Peng et al. 2007; Wei et al. 2008). Second, decreasing redox potentials may have impacted Fe(III)-bound phosphorus leading to a release of P from the sediment (Jin et al. 2006; Chen et al. 2014; Wang et al. 2019). Third, biomass growth and subsequent death and decay in the latter stages of the study also drove changes in pH and ORP that caused sediment P release as well as the P release from degraded biomass cells (Chen et al. 2014; Chen et al. 2018). The C treatment showed a significant SRP release, while LM and HM treatments did not, because the added MDR had substantial P sorption capacity and provided a greater buffer regarding increased P release due to changes in pH, ORP, and biomass decay.
Solid-phase P concentrations for lake sediments and MDRs were compared between the initial and final stages (Table 2). P concentrations in lake sediments showed no significant difference among the four sediment groups analyzed (initial sediment, control sediment, sediments with low MDR addition, and sediment with high MDR addition) (p = 0.356). The LSD post hoc tests showed no differences between groups (p > 0.05). P concentrations in MDRs showed significant differences among the three groups analyzed (initial MDR, MDR from low MDR addition, MDR from high MDR addition) (p = 0.001). The LSD post hoc tests also showed treatments were different, except between MDR from the low and high additions (p < 0.0005 for all, except p = 0.919 for low addition MDR vs high addition MDR).
The initial lake sediment had almost 13 times greater P concentration than the MDR at setup. By the end of the 138-day study, P concentrations in the lake sediments were ranged between 32 and 37 mg P/kg. However, P concentrations in the final MDRs increased almost tenfold from 2.52 to around 25 mg P/kg. These increased final P concentrations in MDRs show that the added MDR sorbed substantial P from this system.
The change of P mass in the three major components of the study system (water column, sediment and MDR, if applicable) was compared for all nine vessels (Table 3). Water P masses were calculated based on the SRP concentrations, while sediment and MDR P masses were calculated based on the AB-DTPA extracted P concentrations. Compared to the C treatment, both LM and HM treatments showed decreased water P mass and increased MDR P mass, demonstrating that added MDR arrested P release into the water column. HM treatments showed greater increases in solid-phase P mass, which showed that the added MDR served as a P sink in this system.
3.3 Metal Concentrations in Water and Sediment
For all dissolved metal (DM) samples, Fe, As, and Cd concentrations were below the practical quantitation limits (PQLs) of 0.003 mg Fe/L, 0.02 mg As/L, and 0.0006 mg Cd/L, respectively. Compared to the hardness-adjusted National Recommended Water Quality Criteria (NRWQC) (U.S.EPA 2014), other dissolved metal concentrations were below both the acute criterion maximum concentration (CMC) and the chronic criterion continuous concentration (CCC), except for Pb with regard to the CCC. Also, compared to the National Secondary Drinking Water Regulations (NSDWRs) (U.S.EPA 2008), all dissolved metal concentrations were below secondary maximum contaminant levels (SMCLs) except for Mn and Zn. These results show that the majority of trace metal concentrations were below relevant criteria, and therefore, concerns about trace metal contamination to the water column from added MDRs were minimal, a result supported by Sibrell et al. (2009).
For aqueous total metal (TM) concentrations, As and Cd concentrations for all samples were below the practical quantitation limit (PQL). Measurable concentrations of Fe, Ni, Pb, and Zn concentrations were plotted versus time (Fig. 4). For Ni and Zn, there were no significant differences among the three treatments (p = 0.893 and p = 0.865, respectively), but significant decreasing trends over time (p < 0.0005 and p < 0.0005, respectively). For Fe and Pb, there were no significant trends over time (p = 0.609 and p = 0.838, respectively), but significant differences among the three treatments (p = 0.020 and p < 0.0005, respectively). LSD post hoc tests showed that C treatments were significantly different from HM (p = 0.006) for Fe and different from LM and HM (p = 0.001 and p < 0.0005, respectively) for Pb. It should be noted that metal samples were not collected on day 102 and day 138, due to the observed relatively constant concentrations.
During the first 10 days of the study, HM treatments showed peak total Fe concentration at day 6, and all total Fe concentrations for LM and HM treatments were greater than C treatments, except for LM at day 8, indicating that the MDR addition treatments, not surprisingly, resulted in increased Fe concentrations. Increasing SRP concentrations after day 40 in the C treatments (due to decay of colonizing biomass) indicate that settled dead biomass promoted P release from both the sediment and through biomass decomposition. However, SRP concentrations were unchanged in LM and HM treatments with abundant Fe-binding sites, which captured any released P. Perkins and Underwood (2001) found Chl-a to be significantly correlated to Fe-bound P in the water column, which indicates the link between algal biomass and available P from internal loading. Chen et al. (2014) also observed similar results that settled cyanobacteria/organic matter promoted the mobilization of sediment P and enhanced the sediment P release. These authors concluded that an effective future bloom control method was to remove the current bloom biomass and dredge P-rich sediments, a costly endeavor.
LM and HM treatments demonstrated greater total Pb concentrations at the end of study, indicating that MDR addition may have introduced some level of trace metals. For Ni and Zn, all treatments showed decreasing trends over time, which may be attributed to absorption during biomass growth and adsorption on the dead biomass materials (Fielding 2017). Of these three metals, only Pb is not a critical micronutrient, thus supporting the supposition regarding potential importance of biological uptake.
Solid-phase metal concentrations, for both lake sediments and MDR, were compared between the initial and final stages (Table 4). For lake sediment, there were significant differences among the four groups (initial sediment, control sediment, sediments with low MDR addition, and sediment with high MDR addition) for As, Mn, Ni, Cd, Fe, Pb, and Zn (p < 0.0005, p = 0.001, p = 0.002, p = 0.028, p = 0.010, p = 0.001, p = 0.002, respectively), and LSD post hoc tests showed initial and control treatments were significantly different from LM and HM treatments (p < 0.05) for all metals. For MDR, there was no significant difference among the three treatment groups (initial MDR, MDR from low MDR addition, MDR from high MDR addition) for all studied metals, except for Mn (p = 0.004), and LSD post hoc tests showed that each treatment was different from the others for Mn (p = 0.001 for initial MDR vs MDR from low MDR addition, p = 0.049 for initial MDR vs MDR from high MDR addition, p = 0.017 for MDR from low MDR addition vs MDR from high MDR addition).
At the initial setup, As, Ni, Cd, Fe, Pb and Zn concentrations were greater, but Mn concentration was lesser in MDRs than in the lake sediments. Elevated trace metal concentrations in the LM and HM-associated sediments were likely due to contact with the MDRs as the corresponding concentrations in MDR from both low MDR addition and high MDR addition slightly decreased in the final samples. Mn appeared to be exported from lake sediment to MDRs, as increases were seen in the final MDR from both low MDR additions and high MDR additions, with decreases in all sediments. Metal concentration changes between lake sediments and MDRs were likely due to both reactive exchanges facilitated with pore water (López et al. 2010; Torres et al. 2015) and inevitable incidental mixing at the final solid sample collection.
Sediment metal concentrations were compared to TSMD-specific Sediment Quality Guidelines (SQGs) (Ingersoll et al. 2009). Table 5 lists metal concentrations compared to probable effects concentrations (PECs), above which harmful effects on sediment-dwelling organisms were expected to occur frequently.
For both initial and final lake sediments, results showed that none of the treatments exceeded the TSMD PECs. For MDRs, all treatments exceeded the TSMD PECs, which was not unexpected since MDRs were precipitated from the mine drainage that contains elevated ecotoxic trace metal concentrations. The fact that the sediments after addition of MDRs showed metal concentrations that were still less than SQGs supports future applications of MDR dosing to achieve P removal in eutrophic reservoirs with subsequent withdrawal of MDR after saturation with P. During the entire period when MDRs were in contact with the water column and sediment layer, no significant trace metal release was observed, indicating MDR as an effective and safe P-sorbing material, although Pb was found to be greater than the NRWQC CCC for on-site ground water.
4 Conclusions
In this study, MDR additions to the surface of lake sediments in greenhouse microcosms did not show significant decreases in aqueous P levels during the first 10 days when colonizing biomass was growing compared to the control treatment. However, in the long-term (days 11–138), after growth, death, and decay of colonizing algae, both MDR treatments showed relative constant levels of P and Fe, while C treatments demonstrated significant P increases in the water column. MDR additions appeared to serve as a long-term internal P loading control method to prevent the release of labile P back into the water column after algal bloom growth and decay, a known source which could enhance future blooms. The comparison of aqueous metal concentrations to NRWQCs and NSDWRs showed limited concerns for trace metal release from MDR additions, with only Pb above one criterion, meriting further study. Sediment metal concentrations for all samples were below the TSMD site-specific SQGs, indicating that there was no significant toxicity introduced to the sediment layer after MDR addition. Metal concentrations in MDRs, however, exceeded TSMD site-specific SQGs. MDR additions to lake sediments may provide an effective internal P loading control option. In targeted areas of elevated nutrient concentrations and known initiation of algal blooms, like sheltered coves in reservoirs, limited MDR addition in shallow waters may provide substantial benefit to control eutrophication.
Data Availability
The authors declare that all the data collected in this paper are original and transparent.
Abbreviations
- AB-DTPA:
-
ammonium bicarbonate-diethylenetriaminepentaacetic acid
- AMD:
-
acid mine drainage
- ANOVA:
-
analysis of variance
- ASTM:
-
American Society for Testing and Materials
- C:
-
control treatment without MDR addition
- CCC:
-
criterion continuous concentration
- Chl-a:
-
chlorophyll-a
- CMC:
-
criterion maximum concentration
- CREW:
-
Center for Restoration of Ecosystems and Watersheds
- DO:
-
dissolved oxygen
- DM:
-
dissolved metal
- GLOC:
-
Grand Lake o’ the Cherokees
- HM:
-
high MDR addition treatment
- ICP-OES:
-
inductively coupled plasma-optical emission spectrometry
- LM:
-
low MDR addition treatment
- LSD:
-
least significant difference
- MC:
-
moisture content
- MDR:
-
mine drainage residual
- MRPTS:
-
Mayer Ranch passive treatment system
- NPL:
-
National Priority List
- NRWQC:
-
National Recommended Water Quality Criteria
- NSDWR:
-
National Secondary Drinking Water Regulation
- OC:
-
organic content
- ORP:
-
oxidation-reduction potential
- PAC:
-
phosphorus adsorption capacity
- PEC:
-
probable effect concentration
- PQL:
-
practical quantitation limit
- PSM:
-
phosphorous-sorbing material
- SD :
-
standard deviation
- SMCL:
-
secondary maximum contaminant level
- SRP:
-
soluble reactive phosphorus
- SQG:
-
Sediment Quality Guideline
- Temp:
-
temperature
- TDS:
-
total dissolved solid
- TM :
-
total metal
- TSMD:
-
Tri-State Mining District
References
Chen, Y. W., Chen, K. N., & Hu, Y. H. (2006). Discussion on possible error for phytoplankton chlorophyll-a concentration analysis using hot-ethanol extraction method. Journal of Lake Sciences., 18(5), 550–552.
Chen, M., Ye, T. R., Krumholz, L. R., & Jiang, H. L. (2014). Temperature and cyanobacterial bloom biomass influence phosphorus cycling in eutrophic lake sediments. PLoS One, 9(3), e93130.
Chen, C., Deng, W., Xu, X., He, J., Wang, S., Jiao, L., & Zhang, Y. (2015). Phosphorus adsorption and release characteristics of surface sediments in Dianchi Lake. China. Environmental Earth Sciences., 74, 3689–3700.
Chen, M. S., Ding, S. M., Chen, X., Sun, Q., Fan, X. F., Lin, J., Ren, M. Y., Yang, L. Y., & Zhang, C. S. (2018). Mechanisms driving phosphorus release during algal blooms based on hourly changes in iron and phosphorus concentrations in sediments. Water Research, 133, 153–164.
Christianson, L. E., Lepine, C., Sibrell, P. L., Penn, C., & Summerfelt, S. T. (2017). Denitrifying woodchip bioreactor and phosphorus filter pairing to minimize pollution swapping. Water Research, 121, 129–139.
Dobbie, K. E., Heal, K. V., Aumonier, J., Smith, K. A., Johnston, A., & Younger, P. L. (2009). Evaluation of iron ochre from mine drainage treatment for removal of phosphorus from wastewater. Chemosphere., 75(6), 795–800.
Drizo, A., Comeau, Y., Forget, C., & Chapuis, R. P. (2002). Phosphorus saturation potential: A parameter for estimating the longevity of constructed wetland systems. Environmental Science and Technology., 36(21), 4642–4648.
Drizo, A., Forget, C., Chapuis, R. P., & Comeau, Y. (2006). Phosphorus removal by electric arc furnace steel slag and serpentine. Water Research, 40, 1547–1554.
Fielding, E. 2017. Sorption and release of nickel and zinc using a mixed algae community collected from a mine drainage passive treatment system. (Master Thesis). Center for Restoration of Ecosystems and Watersheds, University of Oklahoma, Norman, U.S.
Garvin, E. M., Bridge, C. F., & Garvin, M. S. (2017). Screening level assessment of metal concentrations in streambed sediments and floodplain soils within the grand Lake watershed in northeastern Oklahoma, USA. Archives of Environmental Contamination and Toxicology., 72(3), 349–363.
Han, J. S., Min, S. H., & Kim, Y. K. (2005). Removal of phosphorus using AMD-treated lignocellulosic material. Forest Products Journal., 55(11), 48–53.
Heal, K. V., Dobbie, K. E., Bozika, E., McHaffie, H., Simpson, A. E., & Smith, K. A. (2005). Enhancing phosphorus removal in constructed wetlands with ochre from mine drainage treatment. Water Science and Technology., 51(9), 275–282.
Hedin, R. S. (2008). Iron removal by a passive system treating alkaline coal mine drainage. Mine Water and the Environment., 27(4), 200–209.
Hedin, R. S., Watzlaf, G. R., & Nairn, R. W. (1994). Passive treatment of acid mine drainage with limestone. Journal of Environmental Quality, 23(6), 1338–1345.
Ho, J. C., Michalak, A. M., & Pahlevan, N. (2019). Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature., 574, 667–670.
Ingersoll, C.G., Ivey, C.D., Brumbaugh, W.G., Besser, J.M., Kemble, N.E., 2009. Toxicity assessment of sediments from the Grand Lake O’ the Cherokees with the amphipod Hyalella azteca. US Geological Survey Administrative Report CERC-8335-FY09–20-01. https://www.fws.gov/souThwesT/es/Oklahoma/documents/contaminants/ingersoll%20et%20al.%20grand%20lake%20sediment%20toxicity%20report%20text%20final%20august%2027%202009.pdf (Accessed 16 May 2014).
Jin, X., Jiang, X., Yao, Y., Li, L., & Wu, F. C. (2006). Effects of light and oxygen on the uptake and distribution of phosphorus at the sediment–water interface. Science of the Total Environment., 357(1–3), 231–236.
Kuo, S. (1996). Phosphorus. In D. L. Sparks. (ed.) methods of soil analysis: Part 3- chemical methods (pp. 869–919). Madison, WI: SSSA.
López, D. L., Gierlowski-kordesch, E., & Hollenkamp, C. (2010). Geochemical mobility and bioavailability of heavy metals in a lake affected by acid mine drainage: Lake Hope, Vinton County, Ohio. Water, Air and Soil Pollution, 213(1–4), 27–45.
Morrison, J.M., Baker, K.D., Zamor, R.M., Nikolai, S., Elshahed, S.M., Youssef, N.H. 2017. Spatiotemporal analysis of microbial community dynamics during seasonal stratification events in a freshwater lake (Grand Lake, OK, USA). PLoS One. (https://doi.org/10.1371/journal.pone.0177488).
Nairn, R W., LaBar, J.A., Oxenford, L.R., Shepherd, N.L., Holzbauer-Schweitzer, B.K., Arango, J.G., Tang, Z., Dorman, D.M., Folz, C.A., McCann, J.I., Ingendorf, J.D., Stanfield, H.T., Knox, R.C. (2020): Toward sustainability of passive treatment in legacy mining watersheds: operational performance and system maintenance. Proc. IMWA 2020 “Mine Water Solutions”. Christchurch, New Zealand, pp.123–129. International Mine Water Association. https://www.imwa.info/docs/imwa_2020/IMWA2020_Nairn_123.pdf. (Accessed 09 November 2020).
Neely, C.A. 2010. Characterization and possible sustainable sorbent use of iron oxides from abandoned mine drainage discharges. (Master Thesis). Center for Restoration of Ecosystems and Watersheds, University of Oklahoma, Norman, U.S.
Nilolai, S. J., & Dzialowski, A. R. (2014). Effects of internal phosphorus loading on nutrient limitation in a eutrophic reservoir. Limnologica-Ecology and Management of Inland Waters., 49, 33–41.
O’Reilly, S. E., & Sims, J. T. (1995). Phosphorus adsorption and desorption in a sandy soil amended with high rates of coal fly ash. Communications in Soil Science and Plant Analysis., 26(17 & 18), 2983–2993.
Oxenford, L. 2016. Iron transport and removal dynamics in the oxidative units of a passive treatment system. (PhD dissertation). Center for Restoration of Ecosystems and Watersheds, University of Oklahoma, Norman, U.S.
Peng, J. F., Wang, B. Z., Song, Y. H., Yuan, P., & Liu, Z. H. (2007). Adsorption and release of phosphorus in the surface sediment of a wastewater stabilization pond. Ecological Engineering., 31(2), 92–97.
Penn, C. J., Bryant, R. B., Kleinman, P. J. A., & Allen, A. L. (2007). Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. Journal of Soil and Water Conservation., 62(4), 269–276.
Penn, C. J., Chagas, I., Klimeski, A., & Lyngsie, G. (2017). A review of phosphorus removal structures: How to assess and compare their performance. Water., 9(8), 583.
Perkins, R. G., & Underwood, G. J. C. (2001). The potential for phosphorus release across the sediment-water interface in an eutrophic reservoir dosed with ferric sulphate. Water Research, 35(6), 1399–1406.
Qin, Z., Shober, A. L., Scheckel, K. G., Penn, C. J., & Turner, K. C. (2018). Mechanisms of phosphorus removal by phosphorus sorbing materials. Journal of Environmental Quality., 47(5), 1232–1241.
Schwertmann, U., & Fechter, H. (1982). The point of zero charge of natural and synthetic ferrihydrites and its relation to adsorbed silicate. Clay Minerals, 17, 471–476.
Sibrell, P. L., & Tucker, T. W. (2012). Fixed bed sorption of phosphorus from wastewater using iron oxide-based media derived from acid mine drainage. Water, Air and Soil Pollution., 223, 5105–5117.
Sibrell, P. L., Montgomery, G. A., Ritenour, K. L., & Tucker, T. W. (2009). Removal of phosphorus from agricultural wastewaters using adsorption media prepared from acid mine drainage sludge. Water Research, 43(8), 2240–2250.
Skousen, J., Zipper, C. E., Rose, A., Ziemkiewicz, P. F., Nairn, R. W., Mcdonald, L. M., & Kleinmann, R. L. (2017). Review of passive systems for acid mine drainage treatment. Mine Water and the Environment., 36(1), 133–153.
Spears, B. M., Meis, S., Anderson, A., & Kellou, M. (2013). Comparison of phosphorus (P) removal properties of materials proposed for the control of sediment P release in UK lakes. Science of the Total Environment., 442, 103–110.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry (third ed.pp. 690–691). New York, NY: Wiley-Interscience.
Torres, E., Couture, R. M., Shafei, B., Nardi, A., Ayora, C., & Cappellen, P. V. (2015). Reactive transport modeling of early diagenesis in a reservoir lake affected by acid mine drainage: Trace metals, lake overturn, benthic fluxes and remediation. Chemical Geology., 419, 75–91.
U.S.EPA. 2008. Secondary drinking water standards. Available from: U.S. EPA https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals
U.S.EPA. 2014. National recommended water quality criteria. Available from: U.S. EPA https://www.epa.gov/sites/production/files/2015-06/documents/nrwqc-2004.pdf
Wang, Z. C., Huang, S., & Li, D. H. (2019). Decomposition of cyanobacterial bloom contributes to the formation and distribution of iron-bound phosphorus (Fe-P): Insight for cycling mechanism of internal phosphorus loading. Science of the Total Environment, 652, 696–708.
Watzlaf, G.R., K.T. Schroeder, R.P. Kleinmann, C.L. Kairies, and R.W. Nairn. 2004. The passive treatment of coal mine drainage. U.S. Department of Energy National Energy Technology Laboratory, DOE/NETL Report 2004/1202. 72 pp.
Wei, X., Viadero, R. C., & Bhojappa, S. (2008). Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants. Water Research, 42(13), 3275–3284.
Yang, C. P., Hsieh, C. Y., & Hseu, Z. Y. (2014). Fate of heavy metals and evaluation of eutrophication in a wetland-reservoir system. Water Environ Research., 86(4), 331–339.
Yu, J., Ding, S., Zhong, J., Fan, C., Chen, Q., Yin, Zhang, L., & Zhang, Y. (2017). Evaluation of simulated dredging to control internal phosphorus release from sediments: Focused on phosphorus transfer and resupply across the sediment-water interface. Science of the Total Environment., 592, 662–673.
Acknowledgements
The authors would like to thank the Grand River Dam Authority (GRDA) for providing help with lake sampling and water quality analyses and to the University of Oklahoma Aquatic Research Facility and Department of Biology for providing greenhouse space. The authors also extend their sincere appreciation to all the colleagues working at the Center for Restoration of Ecosystems and Watersheds (CREW) at University of Oklahoma for their help in field sample collection and laboratory analysis, especially to Brandon Holzbauer-Schweitzer for helping to develop the map used in site description.
Funding
This research supported by Agreements 100052 and A15–0240 with the Grand River Dam Authority.
Author information
Authors and Affiliations
Contributions
Zepei has conducted the literature research, experimental design, and field and laboratory work with the supervision from Dr. Nairn. Zepei has performed the data analysis and drafted the manuscript, and Dr. Nairn has provided comments and revisions based on that.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 34 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, Z., Nairn, R.W. Mine Drainage Residual Additions to Lake Sediments Alter Phosphorus and Trace Metal Distributions. Water Air Soil Pollut 232, 52 (2021). https://doi.org/10.1007/s11270-021-05016-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05016-3