Abstract
Elevated salinity creates degrading conditions for the development of aquatic biota in different regions of the world. There is a need for research on freshwater salinisation in order to understand how this stressor alters ecosystem function and to predict changes in biodiversity globally. Such data are missing from Central Europe, and therefore, the presented study was performed in inland anthropogenic ponds with different salinity levels located in the second largest European hard coal basin. The researcher indicated a positive correlation between water salinity and the biomass and density of macrozoobenthos as well as the percentage of shredders and the abundance of alien species, whereas there was a decrease in taxa diversity and richness and the abundance of filtering and gathering collectors and predators along with increasing salinity. The survey showed that a high level of nutrients and organic matter were also significantly correlated with the distribution of the macroinvertebrate taxa and functional feeding groups. The conducted research confirmed that mining salinisation acts as a strong filter that shapes the biodiversity because it affects the composition, abundance, biomass and functional traits of benthic macroinvertebrates and significantly contributes to the invasion of alien species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic salinisation of inland waters is caused by many human activities on every inhabited continent and will expand globally as a consequence of global climate change (Williams 2001; Kefford et al. 2003; Vineis et al. 2011; Cañedo-Argüelles et al. 2013; Kefford et al. 2016; Olson 2019). This phenomenon is especially common in industrial and urban regions and areas that are connected with mining activity (Rzętała 2008; Machowski 2010; Molenda 2011; Cañedo-Argüelles et al. 2013). The hard coal mining process is inseparably connected with the production of huge amounts of saline underground water that contains high loads of TDS, sulphates and hardness and inflow into the surface and ground waters and contaminate them. The types of foundation and the extraction depth as well as radioactive contamination by radium and uranium also have an impact on water mineralisation (Tiwary 2001; Smoliński 2006). Thus, the problem of salinisation in such areas is particularly significant in the case of the water bodies that have been involved in the process of coal mining or that are located in regions where there was and/or is mining activity (Jankowski and Rzętała 1999; Harat and Grmela 2008).
Previous studies have indicated that secondary salinisation is a major factor that is responsible for biological changes in aquatic ecosystems—the large amounts of ions in the water create adverse conditions for the development of freshwater biota (e.g. Williams et al. 1990; Smoliński 2006; Bäthe and Coring 2011; Braukmann and Böhme 2011; Scheibler and Ciocco 2011; Kang and King 2012; Arle and Wagner 2013; Bąk et al. 2020). The disappearance of aquatic plants in areas that are undergoing salinisation is one of the first visible effects (Williams 2001; Halabowski and Lewin 2020). Exposure to saline conditions may reduce the germination of macrophytes, the diapausing eggs of zooplankton, respiration rates, growth of algae, growth and food intake of amphibians and fishes, while it may promote the growth of bacteria and invasions of alien species (e.g. Nielsen et al. 2003; Bœuf and Payan 2001; Bailey et al. 2004; Piscart et al. 2011; Braukmann and Böhme 2011; Chambers 2011; Cañedo-Argüelles et al. 2013).
Macroinvertebrates are the main components of freshwater habitats and their abundance, biomass, life cycles, growth, development, feeding structure and diversity depends primarily on their salinity tolerance (Williams and Williams 1998; Kennedy et al. 2004; Piscart et al. 2006; Carver et al. 2009). To date, the impact of salinisation on benthic fauna has chiefly been studied only in running waters (e.g. Piscart et al. 2005a, b; Velasco et al. 2006; Kefford et al. 2011; Piscart et al. 2011; Schäfer et al. 2011; Cañedo-Argüelles et al. 2014, 2015; Golovatyuk and Shitikov 2016; Gutiérrez-Cánovas et al. 2019a; Laceby et al. 2019; Halabowski et al. 2020). Data on salinisation in anthropogenic water bodies is relatively rare (Williams 2001; Blasius and Merritt 2002). Moreover, such studies in saline inland settling ponds have never been undertaken. Previous research that focused on the impact of mining activity as a source of secondary salinisation was conducted in Spain (e.g. Ladrera et al. 2016), Australia (e.g. Pinder et al. 2005; Sauer et al. 2016), Germany (e.g. Bäthe and Coring 2011; Braukmann and Böhme 2011; Petruck and Stöffler 2011; Schröder et al. 2015), Finland (e.g. Leppänen et al. 2019), China (e.g. Zhao et al. 2018), Canada (e.g. Luek and Rasmussen 2017) and the USA (e.g. Kennedy et al. 2004; Pond et al. 2008; Echols et al. 2009; Pond 2010; Palmer et al. 2010); however, they mainly concerned on rivers. Similar data are missing for Central Europe; thus, further research is needed in other regions in order to confirm the results that have obtained from previous studies on running waters as well as on small gradient of salinity and to produce robust conclusions that integrate the data about the problem of anthropogenic salinisation from all of the regions of the world, which would lead to a better understanding of how salinity alters ecosystem function and to predict changes in biodiversity globally.
Therefore, the main goals of presented research were to answer the following questions:
-
1.
How water salinity affects the distribution and trophic structure of the macroinvertebrate communities in the ponds located in the hard coal mining region of Poland (Central Europe)?
-
2.
Whether the zoobenthos abundance, biomass and diversity will decline along with a large salinity gradient?
-
3.
Does mining salinity promote the invasion of alien species in anthropogenic water bodies (including settling ponds) as it does in running waters?
-
4.
Do other environmental factors (such as the physico-chemical variables of water, pond size and the content of organic matter in bottom sediments) have a significant impact on the structure and functioning of bottom fauna in ponds with different degrees of salinity?
2 Materials and Methods
2.1 Study Area and Sampling Procedure
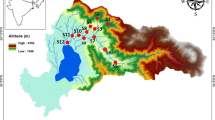
In Poland, a major cause of the secondary salinisation of surface waters results from coal mining activity, and therefore, the presented research was performed in Southern Poland in an area of the Upper Silesian Coal Basin (Fig. 1), which is the second largest European hard coal basin (Smoliński 2006). The entire area is situated in a highly industrialised and urbanised area—68% of the coal mines and 58% of the iron plants in Poland are located here (Strzelec et al. 2006; Jaruchiewicz 2014). Moreover, there are no natural reservoirs such as lakes in this region, whereas it has more than 4770 anthropogenic water bodies (such as subsidence ponds, settling ponds, fish ponds, sand pits and gravel pits) and almost all of them are strongly affected by underground hard coal mining (Rzętała and Jaguś 2012).
We chose nine human-made ponds for the study, and we divided them into three groups with different salinity levels: freshwater (EC up to 1000 μS cm−1), brackish (EC 1000–5500 μS cm−1) and saline (EC of 5500 μS cm−1); we used electrical conductivity to measure the water salinity in the investigated water bodies. All of the ponds were created in the 1970s, and their sizes ranged from 0.2 and 26 ha surface area. The freshwater and brackish water bodies are mining subsidence ponds, while the saline water bodies are used to constantly retain the underground water discharges from the “Knurów-Szczygłowice” coal mine.
Our research was carried out in 2016–2017. Quantitative samples of the benthic macroinvertebrates were gathered from all nine ponds. Within each pond, we developed two study sites along the shoreline (18 study sites in total). The samples were collected by randomly placing a quadrat frame (25 × 25 cm) on the bottom sediments in three locations at each study site. The sites were selected after a brief shoreline survey in order to represent the dominant habitat types that were present in each water body. Material was gathered from each study site eight times during the study period; a total of 143 macroinvertebrate samples were collected (the lack of one sample results from the drying out of the water at one of the sampling sites). All of the material was transported to the laboratory in plastic containers. In the laboratory, the samples were sieved through a 0.23-mm sieve. All of the macroinvertebrates were sorted out of the sediment under a stereoscopic microscope, fixed in 80% ethyl alcohol, identified to the lowest possible taxonomic level and then counted. The wet weight of the collected specimens was weighed on electronic scales with a resolution of 0.001 g. Macroinvertebrate density, family richness, Shannon-Wiener index (H′), frequency and domination were calculated. The functional feeding groups (FFGs) of the macroinvertebrates in relation to their trophic preferences are given after Dodélec and Statzner (2008). Recorded macrophytes at the each sampling site were also identified.
Prior to the biological sampling, the abiotic variables of the studied ponds were measured on the same day as the macroinvertebrate samples were collected. The temperature, pH, conductivity and total dissolved solids were measured in situ with a Hanna Instruments and WTW meters. The content of chlorides, sulphates, potassium, calcium, magnesium, nitrate nitrogen, nitrite nitrogen, ammonium nitrogen and phosphates were estimated in the laboratory using Hanna Instruments and Merck meters. A total of 72 water samples were sampled and analysed. The total content of organic matter in the bottom sediments was determined using the loss-on-ignition (LOI) method according to PN-88/B04481 (Myślińska 2001).
2.2 Statistical Analysis
The response of the benthic macroinvertebrate communities to salinity was assessed using an indirect gradient analysis with non-metric multidimensional scaling NMDS on the log (x + 1)-transformed abundance data and the Bray-Curtis distance measure. The analysis was based on a dataset that comprised 43 taxa from 143 samples. Rare taxa (those that were found in fewer than 5% of the samples) were removed from the analysis. The analysis was performed using the Canoco ver. 5.0 package. The stress value reflects how the ordination summarized the distance between the samples. A stress value of less than 0.05 indicates an excellent representation, a stress value of less than 0.1 indicates a good representation, a stress value of less than 0.2 indicates an acceptable representation and a stress value of more than 0.3 indicates an unsatisfactory representation (Clarke and Warwick 2001). The spatial representation of the samples along the ordination axes was assessed after overlaying the conductivity values of each sample. To examine the statistical significance between the groups of samples with the different salinity levels (freshwater, brackish and saline), an analysis of similarity (ANOSIM) was performed using PAST ver. 3.25 software (Hammer et al. 2001). The Global R statistic from the ANOSIM ranges from 0 to + 1. A value of 0 indicates no differences between the groups, while a value of 1 indicates no similarities between the samples.
The differences in the macroinvertebrate descriptors (the total biomass, the biomass without molluscs, the density of the macroinvertebrate taxa, the diversity of the communities based on the Shannon-Wiener index, the number of taxa, the percentage of the benthic macroinvertebrate functional feeding groups and the share of alien species in the fauna) for the ponds with different salinity levels were calculated by ranks using the Kruskal-Wallis test because the data did not have a normal distribution (using the Kolmogorov-Smirnov test for normality). The Spearman rank correlations were calculated in order to analyse any associations of the macroinvertebrate indicators with the electrical conductivity. All the analyses were performed using Statistica (version 13.1).
A canonical correspondence analysis (CCA) using the Canoco, ver. 5.0, software package (ter Braak and Šmilauer 2012) was performed to explain any variance in the distribution of the functional feeding groups and the abundance of the macroinvertebrate taxa and to indicate their relationships with the environmental variables. The forward selection method was applied to the environmental variables using the Monte Carlo permutation test (499 runs) and Pearson product-moment correlations were calculated among the selected environmental variables in order to check for redundancy. TDS, chlorides, sulphates, potassium, calcium and magnesium were excluded from the analysis because they correlated with both conductivity and rare macroinvertebrate taxa (those that occurred in less than 5% sample) and were removed to reduce the noise in a dataset (Gauch 1982). The analyses were performed on log (x + 1)-transformed biological and environmental data.
3 Results
3.1 Environmental Characteristics of the Ponds with Different Levels of Salinity
The values of the analysed water variables of each type of pond are presented in Table 1. The Kruskal-Wallis ANOVA test revealed statistically significant differences in the median value of conductivity (H = 63.13141, p < 0.0001) and the concentrations of TDS (H = 63.13141, p < 0.0001), chlorides (H = 60.95674, p < 0.0001), potassium (H = 37.00591, p < 0.0001), sulphates (H = 45.23897, p < 0.0001) as well as the content of organic matter in the bottom sediments (H = 68.30246, p < 0.0001) between all of the types of water bodies. The freshwater and brackish ponds differed significantly in the median value of pH (the Kruskal-Wallis ANOVA test H = 11.98829, p = 0.0025). The Kruskal-Wallis ANOVA test showed significant differences in the median concentration of ammonium nitrogen (H = 22.62095, p < 0.0001) between the brackish water bodies and other types of ponds and in median concentrations of nitrite nitrogen (H = 24.35032, p < 0.0001) and calcium (H = 52.13484, p < 0.0001) between the saline water bodies and the other types of ponds.
The highest mean content of organic matter in the sediments was recorded in the saline ponds (16.8%), while its concentration was the lowest in the brackish ponds (7.1%).
A total of 35 taxa of macrophytes, including 5 taxa of macroalgae, were collected across the study sites. Species richness was the highest at the sites from the freshwaters, while the lowest from the saline ponds (Table 2).
3.2 Composition of the Macroinvertebrate Communities Along the Environmental Variables
The results of NMDS showed a split between the samples with different salinity levels. The samples with the highest salinity are located on the right side of the ordination plot and the samples from freshwater ponds are located on the left site of the plot (Fig. 2). The stress value was 0.16, which means that there was an acceptable representation. The results of the ANOSIM indicated statistically significant differences (R = 0.44, p < 0.001) for the macroinvertebrates between the samples with different salinity levels.
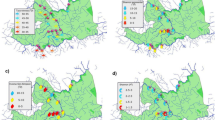
A total of 102,128 benthic macroinvertebrates belonging to 67 families were collected during the entire research period. The structure of the macroinvertebrate communities in the ponds with different salinity levels varied. The macrozoobenthos richness was the highest in the brackish ponds, which had 59 taxa, and the lowest in the most saline ponds, which had 30 macroinvertebrate taxa (Table 3). The dominant groups of macroinvertebrates (> 5.1% of taxa share) that were recorded were oligochaetes, crustaceans, mayflies, dipterans (chironomids larvae) and gastropods (Fig. 3).
The ponds with different salinity levels also varied in the density and biomass of macroinvertebrates as well as in their diversity based on the Shannon-Wiener index and the number of taxa (Table 4). During the entire study period, the lowest abundance of benthic macroinvertebrates and their biomass without molluscs were noted in the freshwaters, 15,445 individuals and 28.826 g, and in the brackish ponds, 35,517 individuals and 51.333 g; in the saline ponds, the highest abundance and biomass were 51,166 individuals and 111.765 g, respectively. The highest total biomass of the macrozoobenthos in the brackish waters (Table 4) was associated with the greatest share of molluscs in these ponds (Table 3). There was a significant positive correlation between the electrical conductivity and the total biomass of macroinvertebrates, biomass without molluscs and the density of the macroinvertebrate communities. However, the diversity of the macroinvertebrate taxa had a significant negative correlation with the electrical conductivity (Fig. 4).
A total of three alien gastropod species including one invasive species (Potamopyrgus antipodarum Gray, 1853), two alien invasive crustacean species (Gammarus tigrinus Sexton, 1935, Orconectes limosus (Rafinesque, 1817)) and five alien oligochaete species were recorded in the studied ponds; however, their percentage share in the fauna varied (Table 5). The highest share of alien species was recorded in the saline ponds and the lowest in the freshwater ponds, but individual taxa exhibited different distribution patterns. Gastropods predominated in the brackish ponds and crustaceans in the saline ponds. Although the share of alien oligochaete species was low in the all of the ponds, it was the highest in the freshwater ponds (Table 4, Fig. 5). There was a significant positive correlation between the electrical conductivity and the percentage of alien species (Fig. 4).
Of the 17 environmental variables that were initially analysed in the CCA, five variables, i.e. electrical conductivity, nitrite nitrogen, ammonium nitrogen, phosphates and the content of organic matter in the sediments were significantly correlated with the distribution of the macroinvertebrate taxa in the studied ponds. In the final CCA analysis, the first axis explained 20.09% of the variance and the second axis explained 5.90% of the variance in the abundance of the macroinvertebrate taxa. The relationship between the composition of the macroinvertebrate taxa and the environmental variables was significant (Monte Carlo test of the significance of the first canonical axis F ratio = 15.3, p = 0.002; test of the significance of all of the canonical axes F ratio = 7.1, p = 0.002).
The electrical conductivity (EC) and the content of organic matter (% OM) in the sediments positively correlated with the first axis, whereas the second axis positively correlated with the nitrite nitrogen, ammonium nitrogen and phosphates. Gammaridae and Hydrobiidae were influenced by the EC, while Ephydridae, Stratiomyidae, Naucoridae, Limoniidae and Noteridae were associated with high nutrient levels (phosphates, nitrite nitrogen and ammonium nitrogen) (Fig. 6).
Seven major functional feeding groups (FFGs) were distinguished in this study: shredders (SH), predators (PR), piercers (PI), filtering collectors (FC), gathering collectors (GC), scrapers (S) and parasites (P). Although all of the types of ponds were dominated by the gathering collectors (Table 4), the shredders had the highest densities in the saline ponds (Fig. 7). The percentage contribution of the functional feeding groups differed significantly in the all of the ponds except for the percentage of parasites (Table 4). There was a significant positive correlation between the electrical conductivity and the percentage of shredders. While the contributions of the filtering collectors, gathering collectors and predators were negatively correlated with the electrical conductivity (Fig. 4).
The CCA analysis showed that electrical conductivity, nitrite nitrogen, ammonium nitrogen and the content of organic matter in the sediments significantly correlated with the distribution of the macroinvertebrate functional feeding groups in the studied anthropogenic ponds. The first axis explained 20.11% of the variance and the second axis explained 6.95% of the variance in biological data. The relationship between the distribution of the functional feeding groups and the environmental variables was also significant (Monte Carlo test of the significance of the first canonical axis F ratio = 34.5, p = 0.002; test of the significance of all of the canonical axes F ratio = 11.2, p = 0.002). Shredders were affected by the EC, while gathering collectors were associated with high nitrite nitrogen and ammonium nitrogen levels (Fig. 8).
4 Discussion
4.1 Shaping of the Macroinvertebrate Communities in Water Bodies with Different Salinity Levels
The presented results showed that changes in water salinity affect the functioning of macrozoobenthos. The preferences of shredders to waters with a high salinity level as we found have not confirmed the previous studies of Munoz and Prat (1994), Piscart et al. (2006) and Zhao et al. (2018), who observed a decrease in their abundance with increasing salinity, while in the study of Piscart et al. (2006), only the predators and parasites were not affected by salinisation. Herbst (2006) also found that a high salinity level favoured the predators who feed on herbivore invertebrates, which in turn controls the macrophytes and the algae biomass. On the other hand, Cañedo-Argüelles et al. (2015) indicated that increase in salinity causes a decline in presence of predators such as Dina lineata. In turn, Castillo et al. (2018) reviewed the global patterns of the responses of freshwater biota to water salinity based on published data and reported that filter feeders and scrapers as well as predators from cold climates have the lowest tolerance to salinisation, while shredders were the most tolerant, which is consistent with our findings and also Szöcs et al. (2014).
The obtained survey results confirm that anthropogenic salt pollution changes the community structure of benthic fauna and causes a degradation of an aquatic habitat (e.g. Nielsen et al. 2003; Piscart et al. 2006; Braukmann and Böhme 2011; Petruck and Stöffler 2011; Arle and Wagner 2013; Schröder et al. 2015; Ladrera et al. 2016; Leppänen et al. 2019). The outcomes show a decrease in taxa diversity and richness along with increasing salinity, which is similar to the disclosures that have been presented in numerous previous works (e.g. Williams et al. 1990; Wollheim and Lovvorn 1995; Brock et al. 2005; Pinder et al. 2005; Piscart et al. 2005a; Carver et al. 2009; Braukmann and Böhme 2011; Kefford et al. 2011; Arle and Wagner 2013; Ladrera et al. 2016; Gutiérrez-Cánovas et al. 2019a, b). Ephemeroptera is an evolutionarily ancient group and thus the group that is most sensitive to environmental changes including elevated conductivity. Although that they are not usually present in waters with a mineralisation higher than 2 g L−1 (Short et al. 1991), the research of Kay et al. (2001) and Rutherford and Kefford (2005) showed that the larvae of some species of the Baetidae and Caenidae families occurred in water that had a salinity as high as 3.8–9.2 g L−1. In our study, the specimens of Baetidae lived in salinity up to 5.5 g L−1. Johnson et al. (2014) claimed that a high content of salt in the water causes a decrease in the body sizes and fecundity of mayflies which, in turn, may have adverse effects on population growth. Kay et al. (2001) and Rutherford and Kefford (2005) observed that caddisflies can live in water with a salinity of 30 g L−1. However, most of the Trichoptera mainly inhabit inland waters and die when the salinity level increases to 5.7 g L−1 (Hart et al. 1991; Short et al. 1991; Zinchenko and Golovatyuk 2013). In the investigated saline ponds, only Limnephilus decipiens occurred among the Typha latifolia. According to Arle and Wagner (2013), increasing salinisation especially adversely affects insects because it prevents them to completing their life cycles.
On the other hand, our outcomes are also consistent with other studies, which show that macroinvertebrates had the highest diversity at slightly elevated salinity levels (e.g. Hammer et al. 1990; Williams et al. 1990; Kefford et al. 2011; Cañedo-Argüelles et al. 2014). Williams et al. (1990) and Piscart et al. (2005b, 2006) suggested that this trend is associated with the broad range of salinity tolerances of the species that occur in such conditions. It is also possible that in an intermediate level of salinisation there are other additional environmental factors that are masked by the salinity or that interact with them (Kefford et al. 2011; Cañedo-Argüelles et al. 2013; Bray et al. 2018). In turn, Kefford et al. (2011) stated that the explanation for the maximal taxa richness could be the fact that many freshwater species have their physiological optimum in moderately saline waters or because of the “mid-domain effect” phenomenon, which was proposed by Colwell and Lees (2000). Moreover, the presented results clearly indicated that especially molluscs reached the highest densities in brackish waters, which had also been registered by Piscart et al. (2005a, 2006). A large share of gastropods and bivalves may also be the cause of the increase of the oligochaete assemblages in these waters because large amounts of nutrients are concentrated to the bottom sediments by molluscs.
In spite of the recorded decline in diversity, the abundance and biomass of recorded macrozoobenthos increased along with increasing salinity. The elevated electrical conductivity led to the elimination of the freshwater taxa and their compensatory increases and resulted in a domination of halophilous species, which are adapted to high salinity levels and create populations with very large densities (Williams et al. 1990; Wollheim and Lovvorn 1995; Piscart et al. 2005a; Boets et al. 2012; Arle and Wagner 2013; Szöcs et al. 2014). Halophilous taxa usually become greatly abundant due to predation and low interspecies competition as a result of a decrease in taxa richness (James et al. 2003; Piscart et al. 2005a; Carver et al. 2009; Bäthe and Coring 2011; Kefford et al. 2016; Bray et al. 2018). Moreover, death or the loss of functions (such as fecundity, growth and osmoregulation) in freshwater species may free up resources for more tolerant species (Kefford et al. 2016). The highest abundance and biomass of benthic fauna in the studied saline settling ponds was due to the significant domination of the alien species Gammarus tigrinus Sexton, 1939. Crustaceans are considered to be the most tolerant group of aquatic macroinvertebrates (Williams et al. 1990; Kefford et al. 2003; Piscart et al. 2005a; Horrigan et al. 2007; Zinchenko and Golovatyuk 2013) because many of them are closely related to marine or estuarine species (Kefford et al. 2012). Besides G. tigrinus, Diptera (Stratiomyidae, Ephydridae), Coleoptera (Chrysomelidae), Odonata (Coenagrionidae) and Heteroptera (Corixidae) also had the highest salinity tolerance, which is in accordance with previous findings (e.g. Piscart et al. 2005a; Rutherford and Kefford 2005; Velasco et al. 2006; Dunlop et al. 2007; Kefford et al. 2012; Schröder et al. 2015; Ladrera et al. 2016; Castillo et al. 2018; Obolewski et al. 2018; Golovatyuk et al. 2019; Gutiérrez-Cánovas et al. 2019b; Mouhoubi et al. 2019). The genera Sigara sp. had the highest resistance to the salinity among the Corixidae, especially species such as Sigara assimilis and Sigara selecta (Velasco et al. 2006; Golovatyuk and Shitikov 2016). We recorded the halophilous S. assimilis in one of the most saline settling ponds, which was the first record of this species in Poland (Sowa et al. 2018).
4.2 Secondary Salinisation—an Important Factor for the Dispersion of Alien Species
The presented results revealed that mining salinisation significantly determined the distribution and spreading of non-native species in inland water bodies. Species that are introduced into rivers and estuary ports can easily get into inland waters; thus, it is assumed that a high tolerance to salinity is one of the factors that enable them to efficiently colonise new areas. Alien species such as the New Zealand mud snail Potamopyrgus antipodarum (Gray, 1843) and the North American amphipod G. tigrinus were brought into Europe through commercial shipping because their high salinity tolerance permits these species to survive in the ballast or drinking water (Piscart et al. 2011).
Previous studies on the anthropogenic small salinity gradients also showed that non-indigenous species colonised the areas with the highest salinity level (Piscart et al. 2005a, 2006; Braukmann and Böhme 2011; Petruck and Stöffler 2011; Piscart et al. 2011; Arle and Wagner 2013; Kašovská et al. 2014; Szöcs et al. 2014; Schröder et al. 2015). Moreover, in some cases, they were the only invertebrate species that were found at some sites, which is in accordance with our findings—alien species that we recorded in most of the saline ponds were represented only by G. tigrinus and P. antipodarum, and they accounted for 70% of the fauna. According to some scientists (Grabowski et al. 2007; Alonso and Castro-Díez 2008; Ba et al. 2010; Bäthe and Coring 2011; Braukmann and Böhme 2011; Piscart et al. 2011), besides their very high salinity tolerance and high reproductive potential, the success of invaders may be promoted by the lack of native species along a salinity gradient, which enables the alien species to become the dominant groups in non-native regions. Other alien species that we have also recorded have a wide range of salinity tolerance, i.e. the spiny-cheek crayfish Orconectes limosus (Rafinesque, 1817), the oligochaete Potamothrix bavaricus (Oschmann, 1913) and the gastropod Physa acuta Draparnaud, 1805.
Our surveys found that in addition to water salinity, the distribution of alien P. antipodarum and G. tigrinus was also influenced by the high content of organic matter in the bottom sediments. A positive correlation between organic matter and the occurrence of eurytopic species has also been demonstrated by Allan and Castillo (2007) and Halabowski et al. (2020). The highest organic matter content in the bottom sediments of the most saline ponds may be explained by flocculation of sediments settlements that are caused by divalent cations due to the aggregation of suspended matter (Grace et al. 1997) as well as by the increased sedimentation in waters with elevated mineralisation (Meiggs and Taillefert 2011; de Nijs and Pietrzak 2012) due to a decrease in the microorganism-mediated leaf litter breakdown (Schäfer et al. 2012; Sauer et al. 2016). The content of organic matter may also have increased due to the high share and activity of the shredders in the saline water bodies (Cummins et al. 2005).
5 Conclusions
The obtained research results completed the knowledge of the salinity effect conducted in the area of underground hard coal mining operations. They showed that the impact of mining salt pollution in anthropogenic water bodies, including settling ponds, is similar to those that occur in running waters. The presented study enables researchers to gain a better understanding of the environmental variables that explain the distribution, diversity, and functioning of macroinvertebrate communities along the water salinity gradient in areas that have underground hard coal mining activity. Our study showed that the communities lost taxa and changed profoundly in terms of the density, biomass and functional traits due to a secondary salinisation process and suggests that this phenomenon plays an essential role in the dispersion and establishment of alien species. Research on the ecosystem function in saline inland stagnant waters is scarce despite the fact that they are of significant ecological importance in areas such as the region of the Upper Silesian Coal Basin where there are no natural water bodies. Therefore, it is necessary to undertake further surveys that should be extended in order to determine the current state of salinisation, not only of the water bodies connected with underground mining but also with the rivers and streams that are connected with these reservoirs. Monitoring of running waters and developing projects that will reduce their pollution is very important due to the applicable provisions of the Water Framework Directive in European Union. This is especially significant because due to the future global climate change and anthropopressure including increasing energy demands, the areas that are threatened by secondary salinisation will most likely expand, which will affect both quality of aquatic ecosystems and of human life and health (Vineis et al. 2011; Cañedo-Argüelles et al. 2013; Olson 2019).
In order to effectively protect the biodiversity of freshwater ecosystems, it is necessary to introduce laws that will regulate salinisation by the type and proportion of ions. This would allow animals that are adapted to different ion concentrations in the water to be protected. Therefore, the content of the ions that are dissolved in water as well as the total hardness and pH of water should be monitored. This is especially important because nowadays there are no maximum permissible limits for these parameters in most of the member states of the EU (Arle and Wagner 2013). Appropriate salinisation monitoring will also be possible by promoting practices that are designed to reduce it. These activities should include implementing agricultural practices that use less water, eliminating the use of salt for road de-icing and using alternative de-icers as well as by reducing the production and discharge of salts into freshwaters and desalinising the water that are rich in salty discharges in urban areas (Cañedo-Argüelles et al. 2016). In addition, future monitoring and the creation of detailed databases can be used to predict the direction of the dispersion of alien species and would contribute to the more effective and precise prevention of biological invasions and their deleterious effects (Petruck and Stöffler 2011; Piscart et al. 2011; Kefford et al. 2012). Moreover, the conservation of invertebrates would contribute to protecting the aquatic biota on the higher trophic levels such as fishes, birds, mammals and reptiles that feed on macroinvertebrates (Cañedo-Argüelles et al. 2013).
References
Allan, J. D., & Castillo, M. M. (2007). Stream ecology: Structure and function of running waters. Dordrecht: Springer. https://doi.org/10.1007/978-1-4020-5583-6.
Alonso, A., & Castro-Díez, P. (2008). What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia, 614, 107–116. https://doi.org/10.1007/s10750-008-9529-3.
Arle, J., & Wagner, F. (2013). Effects of anthropogenic salinisation on the ecological status of macroinvertebrate assemblages in the Werra River (Thuringia, Germany). Hydrobiologia, 701, 129–148. https://doi.org/10.1007/s10750-012-1265-z.
Ba, J., Hou, Z., Platvoet, D., Zhu, L., & Shuqiang, L. (2010). Is Gammarus tigrinus (Crustacea, Amphipoda) becoming cosmopolitan through shipping? Predicting its potential invasive range using ecological niche modelling. Hydrobiologia, 649, 183–194. https://doi.org/10.1007/s10750-010-0244-5.
Bailey, S. A., Duggan, I. C., van Overdijk, C. D. A., Johengen, T. H., Reid, D. F., & Maclsaac, H. J. (2004). Salinity tolerance of diapausing eggs of freshwater zooplankton. Freshwater Biology, 49(3), 286–295. https://doi.org/10.1111/j.1365-2427.2004.01185.x.
Bąk, M., Halabowski, D., Kryk, A., Lewin, I., & Sowa, A. (2020). Mining salinisation of rivers: Its impact on diatom (Bacillariophyta) assemblages. Fottea, 20(1), 1–16. https://doi.org/10.5507/fot.2019.010.
Bäthe, J., & Coring, E. (2011). Biological effects of anthropogenic salt – Load on the aquatic fauna: A synthesis of 17 years of biological survey on the rivers Werra and Weser. Limnologica, 41, 125–133. https://doi.org/10.1016/j.limno.2010.07.005.
Blasius, B. J., & Merritt, R. W. (2002). Field and laboratory investigations on the effects of road salt (NaCl) on stream macroinvertebrate communities. Environmental Pollution, 120, 219–231. https://doi.org/10.1016/S0269-7491(02)00142-2.
Boets, P., Lock, K., & Goethals, P. L. M. (2012). Assessing the importance of alien macro-Crustacea (Malacostraca) within macroinvertebrate assemblages in Belgian coastal harbours. Helgoland Marine Research, 66, 175–187. https://doi.org/10.1007/s10152-011-0259-y.
Bœuf, G., & Payan, P. (2001). How should salinity influence fish growth? Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology, 130(4), 411–423. https://doi.org/10.1016/S1532-0456(01)00268-X.
Braukmann, U., & Böhme, D. (2011). Salt pollution of the middle and lower sections of the river Werra (Germany) and its impact on benthic macroinvertebrates. Limnologica, 41, 113–124. https://doi.org/10.1016/j.limno.2010.09.003.
Bray, J. P., Reich, J., Nichols, S. J., Kon Kam King, G., Mac Nally, R., Thompson, R., et al. (2018). Biological interactions mediate context and species-specific sensitivities to salinity. Philosophical Transactions of the Royal Society, B: Biological Sciences, 374, 20180020. https://doi.org/10.1098/rstb.2018.0020.
Brock, M. A., Nielsen, D. L., & Crossle, K. (2005). Changes in biotic communities developing from freshwater wetland sediments under experimental salinity and water regimes. Freshwater Biology, 50, 1376–1390. https://doi.org/10.1111/j.1365-2427.2005.01408.x.
Cañedo-Argüelles, M., Kefford, B., Piscart, C., Prat, N., Schäfer, R. B., & Schulz, C. J. (2013). Salinisation of rivers: An urgent ecological issue. Environmental Pollution, 173, 157–167. https://doi.org/10.1016/j.envpol.2012.10.011.
Cañedo-Argüelles, M., Bundschuh, M., Gutiérrez-Cánovas, C., Kefford, B. J., Prat, N., Trobajo, R., & Schäfer, R. B. (2014). Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Science of the Total Environment, 476–477, 634–642. https://doi.org/10.1016/j.scitotenv.2013.12.067.
Cañedo-Argüelles, M., Sala, M., Peixoto, G., Prat, N., Faria, M., Soares, A. M. V. M., et al. (2015). Can salinity trigger cascade effects on streams? A mesocosm approach. Science of the Total Environment, 540, 3–10. https://doi.org/10.1016/j.scitotenv.2015.03.039.
Cañedo-Argüelles, M., Hawkins, C. P., Kefford, B. J., Schäfer, R. B., Dyack, B., Brucet, S., et al. (2016). Saving freshwater from salts. Science, 351, 914–916. https://doi.org/10.1126/science.aad3488.
Carver, S., Storey, A., Spafford, H., Lynas, J., Chandler, L., & Wienstein, P. (2009). Salinity as a driver of aquatic invertebrate colonization behaviour and distribution in the wheatbelt of Western Australia. Hydrobiologia, 617, 75–90. https://doi.org/10.1007/s10750-008-9527-5.
Castillo, A. M., Sharpe, D. M. T., Ghalambor, C. K., et al. (2018). Exploring the effects of salinization on trophic diversity in freshwater ecosystems: A quantitative review. Hydrobiologia, 807, 1. https://doi.org/10.1007/s10750-017-3403-0.
Chambers, D. L. (2011). Increased conductivity affects corticosterone levels and prey consumption in larval amphibians. Journal of Herpetology, 45(2), 219–223. https://doi.org/10.1670/09-211.1.
Clarke, K. R., & Warwick, R. M. (2001). Change in marine communities: An approach to statistical analysis and interpretation, 2nd edition. Plymouth: PRIMER-E, Ltd, Plymouth Marine Laboratory.
Colwell, R. K., & Lees, D. C. (2000). The mid-domain effect: Geometric constraints on the geography of species richness. Trends in Ecology & Evolution, 15, 70–76. https://doi.org/10.1016/S0169-5347(99)01767-X.
Cummins, K. W., Merritt, R. W., & Andrade, P. C. N. (2005). The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in South Brazil. Studies on Neotropical Fauna and Environment, 40, 69–89. https://doi.org/10.1080/01650520400025720.
de Nijs, M. A. J., & Pietrzak, J. D. (2012). Saltwater intrusion and ETM dynamics in a tidally-energetic stratified estuary. Ocean Modelling, 49–50, 60–85. https://doi.org/10.1016/j.ocemod.2012.03.004.
Dodélec, S., & Statzner, B. (2008). Invertebrate traits for the biomonitoring of large European rivers: An assessment of specific types of human impact. Freshwater Biology, 58, 617–634. https://doi.org/10.1111/j.1365-2427.2007.01924.x.
Dunlop, J. E., Horrigan, N., McGregor, G., Kefford, B. J., Choy, S., & Prasad, R. (2007). Effect of spatial variation on salinity tolerance of macroinvertebrates in eastern Australia and implications for ecosystem protection trigger values. Environmental Pollution, 151, 621–630. https://doi.org/10.1016/j.envpol.2007.03.020.
Echols, B. S., Currie, R. J., & Cherry, D. S. (2009). Influence of conductivity dissipation on benthic macroinvertebrates in the North Fork Holston River, Virginia downstream of a point source brine discharge during severe low-flow conditions. Human and Ecological Risk Assessment, 15(1), 170–184. https://doi.org/10.1080/10807030802615907.
Gauch Jr., H. G. (1982). Noise reduction by eigenvector ordinations. Ecology, 63(6), 1643–1649. https://doi.org/10.2307/1940105.
Golovatyuk, L. V., & Shitikov, V. K. (2016). Salinity tolerance of macrozoobenthic taxa in small Rivers of the Lake Elton Basin. Russian Journal of Ecology, 47(6), 540–545. https://doi.org/10.1134/S1067413616060059.
Golovatyuk, L. V., Zinchenko, T. D., & Nazarova, L. B. (2019). Macrozoobenthic communities of the saline Bolshaya Samoroda River (Lower Volga region, Russia): Species composition, density, biomass and production. Aquatic Ecology. https://doi.org/10.1007/s10452-019-09726-z.
Grabowski, M., Bącela, K., & Konopacka, A. (2007). How to be an invasive gammarid (Amphipoda: Gammaroidea) – Comparison on life history traits. Hydrobiologia, 590(1), 75–84. https://doi.org/10.1007/s10750-007-0759-6.
Grace, M. R., Hislop, T. M., Hart, B. T., & Beckett, R. (1997). Effect of saline groundwater on the aggregation and settling of suspended particles in a turbid Australian river. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 120(1–3), 123–141. https://doi.org/10.1016/S0927-7757(96)03863-0.
Gutiérrez-Cánovas, C., Sánchez-Fernández, D., Cañedo-Argüelles, M., Millán, A., Velasco, J., Acosta, R., et al. (2019a). Do all roads lead to Rome? Exploring community trajectories in response to anthropogenic salinization and dilution of rivers. Philosophical Transactions of the Royal Society, B: Biological Sciences, 374, 20180009. https://doi.org/10.1098/rstb.2018.0009.
Gutiérrez-Cánovas, C., Arribas, P., Naselli-Flores, L., Bennas, N., Finocchiaro, M., Millán, A., & Velasco, J. (2019b). Evaluating anthropogenic impacts on naturally stressed ecosystems: Revisiting river classifications and biomonitoring metrics along salinity gradients. Science of the Total Environment, 658, 912–921. https://doi.org/10.1016/j.scitotenv.2018.12.253.
Halabowski, D., & Lewin, I. (2020). Impact of anthropogenic transformations on the vegetation of selected abiotic types of rivers in two ecoregions (Southern Poland). Knowledge and Management of Aquatic Ecosystems, 421, 35. https://doi.org/10.1051/kmae/2020026.
Halabowski, D., Lewin, I., Buczyński, P., Krodkiewska, M., Płaska, W., Sowa, A., & Buczyńska, E. (2020). Impact of the discharge of salinised coal mine waters on the structure of the macroinvertebrate communities in an urban river (Central Europe). Water, Air, and Soil Pollution, 321(1), 5. https://doi.org/10.1007/s11270-019-4373-9.
Hammer, U. T., Sheard, J. S., & Kranabetter, J. (1990). Distribution and abundance of littoral benthic fauna in Canadian prairie saline lakes. Hydrobiologia, 197, 173–192. https://doi.org/10.1007/BF00026949.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9.
Harat, A., & Grmela, A. (2008). Impact of mine water from The Upper Silesian Coal Basin areas on change quality of water in Olza river in years 2000–2007. Monitoring Środowiska Przyrodniczego, 9, 57–62.
Hart, B. T., Bailey, P., Edwards, R., Hortle, K., & James, K. (1991). A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia, 210, 105–144. https://doi.org/10.1007/BF00014327.
Herbst, D. B. (2006). Salinity controls on trophic interactions among invertebrates and algae of solar evaporation ponds in the Majove Desert and ralation to shorebird foraging and selenium risk. Wetlands, 26, 475–485. https://doi.org/10.1672/0277-5212(2006)26[475:SCOTIA]2.0.CO;2.
Horrigan, N., Dunlop, J. E., Kefford, B. J., & Zavahir, F. (2007). Acute toxicity largely reflects the salinity sensitivity of stream macroinvertebrates derived using field distributions. Marine and Freshwater Research, 58(2), 178–186. https://doi.org/10.1071/MF05241.
James, K. R., Cant, B., & Ryan, T. (2003). Responses of freshwater biota to rising salinity levels and implications for saline water management: A review. Australian Journal of Botany, 51, 703–713. https://doi.org/10.1071/BT02110.
Jankowski, A. T., & Rzętała, M. (1999). Origin and salinity of lymnic water in the Silesian Upland and adjacent areas. In A. Choiński & J. Jańczak (Eds.), Natural and anthropogenic changes of lakes. Warszawa: IMiGW – Oddział w Poznaniu, UAM – Zakład Hydrologii i Gospodarki Wodnej IGF.
Jaruchiewicz, E. (2014). The role of anthropogenic water reservoirs within the landscapes of mining areas – A case study from the western part of the Upper Silesian Coal Basin. Environmental & Socio-economic Studies, 2, 16–26.
Johnson, B. R., Weaver, P. C., Nietch, C. T., Lazorchak, J. M., Struewing, K. A., & Funk, D. H. (2014). Elevated major ion concentrations inhibit larval mayfly growth and development. Environmental Toxicology and Chemistry, 34, 167–172. https://doi.org/10.1002/etc.2777.
Kang, S. R., & King, S. L. (2012). Influence of salinity and prey presence on the survival of aquatic macroinvertebrates of a freshwater marsh. Aquatic Ecology, 46, 411–420. https://doi.org/10.1007/s10452-012-9410-3.
Kašovská, K., Pierzchała, Ł., Sierka, E., & Stalmachová, B. (2014). Impact of the salinity gradient on the mollusc fauna in flooded mine subsidences (Karvina, Czech Republic). Archives of Environmental Protection, 40, 87–99. https://doi.org/10.2478/aep-2014-0007.
Kay, W. R., Halse, S. A., Scanlon, M. D., & Smith, M. J. (2001). Distributions and environmental tolerances of aquatic macroinvertebrate families in the agricultural zone of southwestern Australia. Journal of the North American Benthological Society, 20(2), 182–199. https://doi.org/10.2307/1468314.
Kefford, B., Papas, P., & Nugegoda, D. (2003). Relative salinity tolerance from the Barwon River, Victoria, Australia. Marine and Freshwater Research, 54, 755–765. https://doi.org/10.1071/MF02081.
Kefford, B. J., Marchant, R., Schäfer, R. B., Metzeling, L., & Dunlop, J. E. (2011). The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environmental Pollution, 159, 302–310. https://doi.org/10.1016/j.envpol.2010.08.025.
Kefford, B. J., Piscart, C., Hickey, H. L., Gasith, A., Ben-David, E., Dunlop, J. E., et al. (2012). Global scale variation in the salinity sensitivity of riverine macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS One, 7(5), e35224. https://doi.org/10.1371/journal.pone.0035224.
Kefford, B. J., Buchwalter, D., Cañedo-Argüelles, M., Davis, J., Duncan, R. P., Hoffmann, A., & Thompson, R. (2016). Salinized rivers: Degraded systems or new habitats for salt-tolerant faunas? Biology Letters, 12. https://doi.org/10.1098/rsbl.2015.1072.
Kennedy, A. J., Cherry, D. S., & Currie, R. J. (2004). Evaluation of ecologically relevant bioassays for a lotic system impacted by a coal-mine effluent, using Isonychia. Environmental Monitoring and Assessment, 95(1), 37–55. https://doi.org/10.1023/b:emas.0000029896.97074.1e.
Laceby, J. P., Kerr, J. G., Zhu, D., Chung, C., Situ, Q., Abbasi, S., & Orwin, J. F. (2019). Chloride inputs to the North Saskatchewan River watershed: The role of road salts as a potential driver of salinization downstream of North America's northern most major city (Edmonton, Canada). Science of the Total Environment, 688, 1056–1068. https://doi.org/10.1016/j.scitotenv.2019.06.208.
Ladrera, R., Cañedo-Argüelles, M., & Prat, N. (2016). Impact of potash mining in streams: The Llobregat basin (northeast Spain) as a case study. Journal of Limnology, 76(2). https://doi.org/10.4081/jlimnol.2016.1525.
Leppänen, J. J., Luoto, T. P., & Weckström, J. (2019). Spatio-temporal impact of salinated mine water on Lake Jormasjärvi, Finland. Environmental Pollution, 247, 1078–1088. https://doi.org/10.1016/j.envpol.2019.01.111.
Luek, A., & Rasmussen, J. B. (2017). Chemical, physical and biological factors shape littoral invertebrate community structure in coal-mining end-pit lakes. Environmental Management, 59, 652–664. https://doi.org/10.1007/s00267-017-0819-2.
Machowski, R. (2010). Transformation of geosystems of water bodies created in the basins of subsidence in the Katowice Upland. Katowice: Wydawnictwo Uniwersytetu Śląskiego.
Meiggs, D., & Taillefert, M. (2011). The effect of riverine discharge on biogeochemical processes in estuarine sediments. Limnology and Oceanography, 56(5), 1797–1810. https://doi.org/10.4319/lo.2011.56.5.1797.
Molenda, T. (2011). Natural and anthropogenic conditions of physical and chemical water changes in post-mining aquatic areas of Upper Silesian region and its neighbouring area. Katowice: Gnome.
Mouhoubi, D., Djenidi, R., & Bounechada, M. (2019). Contribution to the study of diversity, distribution, and abundance of insect fauna in salt wetlands of Setif region, Algeria. International Journal of Zoology, 2019(4), 1–11. https://doi.org/10.1155/2019/2128418.
Munoz, I., & Prat, N. (1994). Macroinvertebrate community in the lower Ebro River (NE Spain). Hydrobiologia, 286, 65–78. https://doi.org/10.1007/BF00008498.
Myślińska, E. (2001). Organic and laboratory land testing methods. Warszawa: Państwowe Wydawnictwo Naukowe.
Nielsen, D. L., Brock, M., Crossle, K., Harris, K., Healey, M., & Jarosinski, I. (2003). The effects of salinity on aquatic plant germination and zooplankton hatching from two wetlands sediments. Freshwater Biology, 48, 2214–2223. https://doi.org/10.1046/j.1365-2427.2003.01146.x.
Obolewski, K., Glińska-Lewczuk, K., Szymańska, M., Mrozińska, N., Bąkowska, M., Astel, A., et al. (2018). Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS One, 13(2), e0207825. https://doi.org/10.1371/journal.pone.0207825.
Olson, J. R. (2019). Predicting combined effects of land use and climate change on river and stream salinity. Philosophical Transactions of the Royal Society, B: Biological Sciences, 374(764), 20180005. https://doi.org/10.1098/rstb.2018.0005.
Palmer, M. A., Bernhardt, E. S., Schlesinger, W. H., Eshleman, K. N., Foufoula-Georgiou, E., Hendryx, M. S., et al. (2010). Mountaintop mining consequences. Science, 327, 148–149. https://doi.org/10.1126/science.1180543.
Petruck, A., & Stöffler, U. (2011). On the history of chloride concentrations in the River Lippe (Germany) and the impact on the macroinvertebrates. Limnologica, 41, 143–150. https://doi.org/10.1016/j.limno.2011.01.001.
Pinder, A. M., Halse, S. A., McRae, J. M., & Shiel, R. J. (2005). Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia, 543, 1–24. https://doi.org/10.1007/s10750-004-5712-3.
Piscart, C., Moreteau, J. C., & Beisel, J. N. (2005a). Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia, 551, 227–236. https://doi.org/10.1007/s10750-005-4463-0.
Piscart, C., Lecerf, A., Usseglio-Polatera, P., Moreteau, J., & Beisel, J. (2005b). Biodiversity patterns along a salinity gradient: The case of net-spinning caddisflies. Biodiversity and Conservation, 14, 2235–2249. https://doi.org/10.1007/s10531-004-4783-9.
Piscart, C., Moretea, J. C., & Beisel, J. N. (2006). Monitoring changes in freshwater macroinvertebrate communities along a salinity gradient using artificial substrates. Environmental Monitoring and Assessment, 116, 529–542. https://doi.org/10.1007/s10661-006-7669-3.
Piscart, C., Kefford, B. J., & Beisel, J. N. (2011). Are salinity tolerances of non-native macroinvertebrates in France an indicator of potential for their translocation in a new area? Limnologica, 41, 107–112. https://doi.org/10.1016/j.limno.2010.09.002.
Pond, G. J. (2010). Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia, 641, 185–201. https://doi.org/10.1007/s10750-009-0081-6.
Pond, G. J., Passmore, M. E., Borsuk, F. A., Reynolds, L., & Rose, C. J. (2008). Downstream effects of mountaintop coal mining: Comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. Journal of the North American Benthological Society, 27(3), 717–737. https://doi.org/10.1899/08-015.1.
Rutherford, J.C. & Kefford, B.J. (2005). Effects of salinity on stream ecosystems: Improving models for macroinvertebrate. CSIRO Land and Water, Canberra, Australia. Report: 22/05.
Rzętała, M. (2008). Functioning of water reservoirs and the course of limnic processes under conditions of varied anthropopression a case study of Upper Silesian Region. Katowice: Wydawnictwo Uniwersytetu Śląskiego.
Rzętała, M., & Jaguś, A. (2012). New lake district in Europe: Origin and hydrochemical characteristics. Water Environment Journal, 26(1), 108–117. https://doi.org/10.1111/j.1747-6593.2011.00269.x.
Sauer, F. G., Bundschuh, M., Zubrod, J. P., Schäfer, R. B., Thompson, K., & Kefford, B. J. (2016). Effects of salinity on leaf breakdown: Dryland salinity versus salinity from a coalmine. Aquatic Toxicology, 177, 425–432. https://doi.org/10.1016/j.aquatox.2016.06.014.
Schäfer, R. B., Kefford, B. J., Metzeling, L., Liess, M., Burgert, S., Marchant, R., et al. (2011). A trait database of stream invertebrates for the ecological risk assessment of single and combined effects of salinity and pesticides in South-East Australia. Science of the Total Environment, 409(11), 2055–2063. https://doi.org/10.1016/j.scitotenv.2011.01.053.
Schäfer, R. B., Bundschuh, M., Rouch, D. A., Szöcs, E., von der Ohe, P., Pettigrove, V., et al. (2012). Effects of pesticide toxicity, salinity and other environmental variables on selected ecosystem functions in streams and the relevance for ecosystem services. Science of the Total Environment, 415, 69–78. https://doi.org/10.1016/j.scitotenv.2011.05.063.
Scheibler, E. E., & Ciocco, N. F. (2011). Distribution of macroinvertebrate assemblages along a saline wetland in harsh environmental conditions from Central-West Argentina. Limnologica, 41, 37–47. https://doi.org/10.1016/j.limno.2010.03.001.
Schröder, M., Sondermann, M., Sures, B., & Hering, D. (2015). Effects of salinity gradients on benthic invertebrate and diatom communities in a German lowland river. Ecological Indicators, 57, 236–248. https://doi.org/10.1016/j.ecolind.2015.04.038.
Short, T. M., Black, J. A., & Birge, W. J. (1991). Ecology of a saline stream: Community responses to spatial gradients of environmental conditions. Hydrobiologia, 226, 167–178. https://doi.org/10.1007/BF00006858.
Smoliński, A. (2006). The economy of saline mine waters. Research Reports Mining and Environment, 1, 5–15.
Sowa, A., Tończyk, G., Halabowski, D., & Krodkiewska, M. (2018). First record of Sigara assimilis (Fieber, 1848) (Hemiptera: Heteroptera: Corixidae) in Poland. Oceanological and Hydrobiological Studies, 47(2), 211–217. https://doi.org/10.1515/ohs-2018-0020.
Strzelec, M., Spyra, A., & Krodkiewska, M. (2006). Freshwater snail of the sand-pits in Uppersilesian industrial area (Poland). TEKA Commission of Protection and Formation of Natural Environment, 3, 187–194.
Szöcs, E., Coring, E., Bäthe, J., & Schäfer, R. B. (2014). Effects of anthropogenic salinization on biological traits and community composition of stream macroinvertebrates. Science of the Total Environment, 468–469, 943–949. https://doi.org/10.1016/j.scitotenv.2013.08.058.
ter Braak, C.J.F. & Šmilauer, P. (2012). Canoco reference manual and user’s guide: Software for ordination, version 5.0. Microcomputer Power, Ithaca.
Tiwary, R. K. (2001). Environmental impact of coal mining on water regime and its management. Water, Air, and Soil Pollution, 132, 185–199. https://doi.org/10.1023/A:1012083519667.
Velasco, J., Millán, A., Hernández, J., Gutiérrez, C., Abellán, P., Sánchez, D., & Ruiz, M. (2006). Response of biotic communities to salinity changes in a Mediterranean hypersaline stream. Saline Systems, 2, 12. https://doi.org/10.1186/1746-1448-2-12.
Vineis, P., Chan, Q., & Khan, A. (2011). Climate change impacts on water salinity and health. Journal of Epidemiology and Global Health, 1, 5–10. https://doi.org/10.1016/j.jegh.2011.09.001.
Williams, W. D. (2001). Anthropogenic salinisation of inland waters. Hydrobiologia, 466, 329–337. https://doi.org/10.1023/A:1014598509028.
Williams, W. D., & Williams, N. E. (1998). Aquatic insects in an estuarine environment: Densities, distribution and salinity tolerance. Freshwater Biology, 39, 411–421. https://doi.org/10.1046/j.1365-2427.1998.00285.x.
Williams, W. D., Boulton, A. J., & Taaffe, R. G. (1990). Salinity as a determinant of salt lake fauna: A question of scale. Hydrobiologia, 197, 257–266. https://doi.org/10.1007/978-94-009-0603-7_22.
Wollheim, W. M., & Lovvorn, J. R. (1995). Salinity effect on macroinvertebrate assemblages and waterbird food webs in shallow lakes of the Wyoming High Plains. Hydrobiologia, 310, 207–223. https://doi.org/10.1007/BF00006832.
Zhao, Q., Guo, F., Zhang, Y., Yang, Z., & Ma, S. (2018). Effects of secondary salinisation on macroinvertebrate functional traits in surface mining-contaminated streams and recovery potential. Science of the Total Environment, 640–641, 1088–1097. https://doi.org/10.1016/j.scitotenv.2018.05.347.
Zinchenko, T. D., & Golovatyuk, L. V. (2013). Salinity tolerance of macroinvertebrates in stream waters (review). Arid Ecosystems, 3(3), 113–121. https://doi.org/10.1134/S207909611303011.
Acknowledgments
The authors would like to thank Ms. Michele L. Simmons, BA from the English Language Centre (ELC) for corrections and improving the language style of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sowa, A., Krodkiewska, M. & Halabowski, D. How Does Mining Salinisation Gradient Affect the Structure and Functioning of Macroinvertebrate Communities?. Water Air Soil Pollut 231, 453 (2020). https://doi.org/10.1007/s11270-020-04823-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04823-4