Abstract
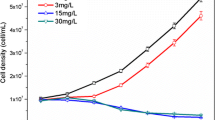
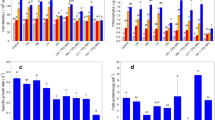
As potassium ferrate(VI) is an important kind of water treatment agent which has a high efficiency in algal removal, its effects on the cell substance are rarely discussed. The changing of the protein and enzyme was analyzed here to deeply understand the oxidation of Fe on the protein in the algae. The result of the research showed the inactivation on growth and the biochemical process of the algal cell were all inhibited by Fe, including the function of the photosynthesis system. During the process, SOD (superoxide dismutase), CAT (catalase), POD (peroxidase), and GST (glutathione S-transferase) played cooperative roles to prevent the injury on the cells from destructive oxidation stress. The lipid peroxidation strengthened the defense system. The damage was intensified with the increase of ferrate concentration.







Similar content being viewed by others
References
Alsheyab, M., Jiang, J. Q., & Stanford, C. (2009). On-line production of ferrate with an electrochemical method and its potential application for wastewater treatment - a review. Journal of Environmental Management, 90(3), 1350–1356.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1), 248–254.
Brown, M. R. (1991). The amino-acid and sugar composition of 16 species of microalgae used in mariculture. Journal of Experimental Marine Biology and Ecology, 145(1), 79–99.
Halliwell, B., Aeschbach, R., Löliger, J., & Aruoma, O. I. (1995). The characterization of antioxidants. Food and Chemical Toxicology, 33(7), 601–617.
Hrostowski, H. J., & Scott, A. B. (1950). The magnetic susceptibility of potassium ferrate. The Journal of Chemical Physics, 18(1), 105–107.
Jiang, C., Wang, R., & Ma, W. (2010). The effect of magnetic nanoparticles on Microcystis aeruginosa removal by a composite coagulant. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 369(1–3), 260–267.
Jiang, J. (2013). Advances in the development and application of ferrate(VI) for water and wastewater treatment. Journal of Chemical Technology and Biotechnology, 89(2), 165–177.
Jiang, J. Q., Lloyd, B., & Grigore, L. (2001). Preparation and evaluation of potassium ferrate as an oxidant and coagulant for potable water treatment. Environmental Engineering Science, 18(5), 323–331.
Jiang, J. Q., & Wang, S. (2006). The exploration of potassium ferrate(VI) as a disinfectant/coagulant in water and wastewater treatment. Chemosphere., 63(2), 212–219.
Jin, X. L., Xia, Q., Wang, X. Y., Yue, J. J., & Wei, D. B. (2011). Inactivation of Microcystis aeruginosa with contact glow discharge electrolysis. Plasma Chemistry and Plasma Processing, 31(5), 697–705.
Lee, Y., Cho, M., Kim, J., et al. (2004). Chemistry of ferrate (Fe(VI)) in aqueous solution and its applications as a green chemical. Journal of Industrial and Engineering Chemistry, 10(1), 161–171.
Lei, G. Y., Ma, J., Guan, X. H., Song, A. K., & Cui, Y. J. (2009). Effect of basicity on coagulation performance of polyferric chloride applied in eutrophicated raw water. Desalination., 247(1–3), 518–529.
Lim, M., & Kim, M. J. (2010). Effectiveness of potassium ferrate (K2FeO4) for simultaneous removal of heavy metals and natural organic matters from river water. Water, Air, and Soil Pollution, 211(1–4), 313–322.
Liu, L., Li, L., Wu, Z., et al. Physiological responses to ferrate(VI) stress in Microcystis aeruginosa[C]. //International Conference on Remote Sensing. Environment and Transportation Engineering, Rsete. IEEE, 2011, 5153–5156.
Ma, J., & Liu, W. (2002). Effectiveness and mechanism of potassium ferrate(VI) preoxidation for algae removal by coagulation. Wat.Res., 36(4), 871–878.
Miao, H. F., & Tao, W. Y. (2009). The mechanisms of ozonation on cyanobacteria and its toxins removal. Separation and Purification Technology, 66(1), 187–193.
Petruševski, B., Vlaški, A., Van Breeman, A., & Alaerts, G. (1993). Influence of algal species and cultivation conditions on algal removal in direct filtration. Water Science and Technology, 27, 211–220.
Pieterse, A. J. H., & Cloot, A. (1997). Algae cells and coagulation, flocculation and sedimentation process. Water Science and Technology, 36, 111–118.
Reinemer, P., Prade, L., Hof, P., et al. (1996). Three-dimensional structure of glutathione S-transferase from Arabidopsis thaliana at 2.2 Å resolution: structural characterization of herbicide-conjugating plant glutathione S-transferases and a novel active site architecture. Journal of Molecular Biology, 255(2), 289–309.
Schindler, D. W., Hecky, R. E., Findlay, D. L., Stainton, M. P., Parker, B. R., Paterson, M. J., Beaty, K. G., Lyng, M., & Kasian, S. E. M. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year wholeecosystem experiment. Proceedings of the National Academy of Science of the United States of America, 105, 11254–11258.
Shang, A. H., Ye, J., Chen, D. H., et al. (2015). Physiological effects of tetracycline antibiotic pollutants on non-target aquatic Microcystis aeruginosa. Journal of Environmental Science and Health, Part B, 50(11), 809–818.
Sharma, V. K. (2007). A review of disinfection performance of Fe(VI) in water and wastewater. Water Science and Technology, 55(1–2), 225–232.
Sharma, V. K., Jiang, J., & Kim, H. (2013). Ferrate(VI): novel compound for removal of natural organic matter in water. Functions of Natural Organic Matter in Changing Environment, 911–914.
Sharma, V. K. (2008). Oxidative transformations of environmental pharmaceuticals by Cl2, ClO2, O3, and ferrate(VI): kinetics assessment. Chemosphere., 73(9), 1379–1386.
Shiu, C. T., & Lee, T. M. (2005). Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. Journal of Experimental Botany, 56(421), 2851–2865.
Sohal, R. S., & Dubey, A. (1994). Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radical Biology and Medicine, 16(5), 621–626.
Sun, F., Pei, H. Y., Hu, W. R., & Ma, C. X. (2012). The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chemical Engineering Journal, 193, 196–202. https://doi.org/10.1016/j.cej.2012.04.043.
Teixeira, M. R., & Rosa, M. J. (2006). Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcysits aeruginosa. Part I. The key operating conditions. Separation and Purifification Technology, 52, 84–94.
Xu, J. M., Wu, J. J., & He, Y. (Eds.). (2013). Functions of natural organic matter in changing environment. Netherlands: Springer.
Yamauchi, Y., Furutera, A., Seki, K., et al. (2008). Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiology and Biochemistry, 46(8), 786–793.
Yang, M., Yu, J. W., Li, Z. L., Guo, Z. H., Burch, M., & Lin, T. F. (2008). Taihu Lake not to blame for Wuxi’s woes. Science, 319, 158–159.
Zhang, Y., Jiang, H., Liu, S., et al. (2012). Effects of dissolved inorganic carbon on competition of the bloom-forming cyanobacterium Microcystis aeruginosa with the green alga Chlamydomonas microsphaera. European Journal of Phycology, 47(1), 1–11.
Zhang, Y., Xu, Q., & Xi, B. (2013). Effect of NaCl salinity on the growth, metabolites, and antioxidant system of Microcystis aeruginosa. Journal of Freshwater Ecology, 28(4), 477–487.
Funding
This work was supported by Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (ES201910).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S. Impacts of Potassium Ferrate(VI) on the Growth, Protein, and Enzyme of the Microcystis aeruginosa. Water Air Soil Pollut 230, 173 (2019). https://doi.org/10.1007/s11270-019-4223-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4223-9