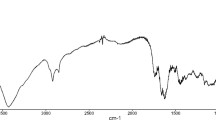
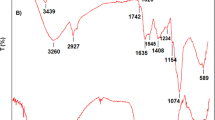
Abstract
The textile industry is responsible for the disposal of a large volume of effluents containing synthetic dyes, which are considered to be highly toxic compounds for both human health and the environment. The aim of the present study was to test potential use of a renewable, low-cost product—Luffa cylindrica in disk and powder form—as adsorbent material for the treatment of textile effluents containing dyes. Saccharomyces cerevisiae cells were also immobilized on L. cylindrica to increase the adsorbent capacity. Batch experiments were conducted for the evaluation of the removal of the azo dye Direct Red 23. The Langmuir, Freundlich, and Temkin isotherms were used for a better interpretation of the data. The results showed that adsorption is more efficient at acidic pH and all adsorbent materials best fit the Langmuir model, indicating the formation of a monolayer. The isotherm results also demonstrated that the materials immobilized with the yeast had a greater sorption rate, but the cell-free L. cylindrica powder had a higher adsorbate/adsorbent interaction. The comparison with a spectrophotometrically defined standard revealed that the powder without and with yeast cells was able to achieve an acceptable removal rate of the dye from the solution. Moreover, the difference in adsorption between the powder without and with yeast cells was very small. Thus, the application of the cell-free L. cylindrica powder is economically more feasible. The findings demonstrate the potential use of L. cylindrica powder as an adsorbent for the treatment of effluents containing textile dyes.









Similar content being viewed by others
References
Aljeboree, A. M., Alkaim, A. F., & Al-Dujaili, A. H. (2015). Adsorption isotherm, kinetic modeling and thermodynamics of crystal violet dye on coconut husk-based activated carbon. Desalination and Water Treatment, 53, 3656–3667.
Aravindhan, R., Rao, J. R., & Nair, B. U. (2007). Removal of basic yellow dye from aqueous solution by sorption on green algae Caulerpa scalpelliformis. Journal of Hazardous Materials, 142, 68–76.
Behera, S., Mohanty, R. C., & Ray, R. C. (2011). Ethanol production from mahula (Madhuca latifolia L.) flowers with immobilized cells of Saccharomyces cerevisiae in Luffa cylindrica L. sponge discs. Applied Energy, 88, 212–215.
Bhatnagar, A., Vilar, V. J. P., Botelho, C. M. S., & Boaventura, R. A. R. (2011). A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environmental Technology, 32, 231–249.
Choy, K. K. H., Porter, J. F., & McKay, G. (2000). Langmuir, isotherms models applied to the multicomponent sorption of acid dyes from effluent onto activated carbon. Journal of Chemical & Engineering Data, 45, 575–584.
Cooper, P. (1993). Removing colour from dye house waste waters—a critical review of technology available. Journal of the Society of Dyes and Colourists Coloration Technology, 109, 97–100.
D’Almeida, A. L. F. S., Calado, V., Barreto, D. W., & D’Almeida, J. R. M. (2005). Acetylation of loofah fiber (Luffa cylindrica). Polímeros, 15, 59–62.
Dilarri, G., Almeida, E. J. R., Pecora, H. B., & Corso, C. R. (2016). Removal of dye toxicity from an aqueous solution using an industrial strain of Saccharomyces cerevisiae (Meyen). Water, Air, & Soil Pollution, 227, 269.
Farah, J. Y., El-Gendy, N. S., & Farahat, L. A. (2007). Biosorption of astrazone blue basic dye from an aqueous solution using dried biomass of Baker’s yeast. Journal of Hazardous Materials, 148, 402–408.
Freundlich, H. (1906). Adsorption in solution. Zeitschrift für Physikalische Chemie, 40, 1361–1368.
Ghazi Mokri, H. S., Modirshahla, N., Behnajady, M. A., & Vahid, B. (2015). Adsorption of C.I. Acid Red 97 dye from aqueous solution onto walnut shell: kinetics, thermodynamics parameters, isotherms. International journal of Environmental Science and Technology, 12, 1401–1408.
Lalnunhlimi, S., & Krishnaswamy, V. (2016). Decolorization of azo dyes (Direct Blue 151 and Direct Red 31) by moderately alkaliphilic bacterial consortium. Brazilian Journal of Microbiology, 47, 39–46.
Langmuir, I. (1918). The adsorption of gases on plane surface of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361–1368.
Li, G., Jiang, Y., Huang, K., Ding, P., & Yao, L. (2008). Kinetics of adsorption of Saccharomyces cerevisiae mandelated dehydrogenase on magnetic Fe3O4—chitosan nanoparticles. Colloids and Surfaces A, 320, 11–18.
McKay, G., Blair, H. S., & Gardner, J. R. (1982). Adsorption of dyes on chitin: equilibrium studies. Journal of Applied Polymer Science, 27, 3043–3043.
Mendes, C. R., Dilarri, G., & Pelegrini, R. T. (2015). Application of biomass Saccharomyces cerevisiae as adsorption agent of dye Direct Orange 2GL and possible interactions mechanisms adsorbate/adsorbent. Matéria (Rio de Janeiro), 20, 898–908.
Raval, N. P., Shah, P. U., & Shah, N. K. (2016). Adsorptive amputation of hazardous azo dye Congo red from wastewater: a critical review. Environmental Science and Pollution Research, 23, 14810–14853.
Regan, F., Chapman, J., & Sullivan, T. (2012). Nanoparticles in anti-microbial materials: use and characterisation. Cambridge: East of England.
Saber-Samandari, S., & Heydaripour, J. (2015). Onion membrane: an efficient adsorbent for decoloring of wastewater. Journal of Environmental Health Science & Engineering, 13, 16.
Sharma, P., Kaur, H., Sharma, M., & Sahore, V. (2011). A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environmental Monitoring and Assessment, 183, 151–195.
Souza, C. R. L., & Peralta-Zamora, P. (2005). Degradation of reactive dyes by the metallic iron/hydrogen peroxide system. Química Nova, 28, 226–228.
Sumbu, Z. L., Thonart, P., & Bechet, J. (1983). Action of patulin on a yeast. Applied and Environmental Microbiology, 45, 110–115.
Suteu, D., Blaga, A. C., Diaconu, M., & Malutan, T. (2013). Biosorption of reactive dye from aqueous media using Saccharomyces cerevisiae biomass. Equilibrium and kinetic study. Central European Journal of Chemistry, 11, 2048–2057.
Temkin, M. J., & Pyzhev, V. (1940). Kinetics of ammonia synthesis on promoted iron catalysis. Acta Physicochimica URSS, 12, 327–356.
Vignoli, J. A., Celligoi, M. A. P. C., & Silva, R. S. F. (2006). Development of a statistical model for sorbitol production by free and immobilized Zymomonas mobilis in loofa sponge Luffa cylindrica. Process Biochemistry, 41, 240–243.
Yousef, R. I., El-Eswed, B., & Al-Muhtaseb, A. H. (2011). Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism, and thermodynamics studies. Chemical Engineering Journal, 171, 1143–1149.
Acknowledgments
This study received support from the Brazilian National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morão, L.G., Dilarri, G. & Corso, C.R. Immobilization of Saccharomyces cerevisiae Cells on Luffa cylindrica: a Study of a Novel Material for the Adsorption of Textile Dye. Water Air Soil Pollut 228, 248 (2017). https://doi.org/10.1007/s11270-017-3433-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3433-2