Abstract
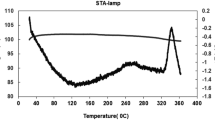
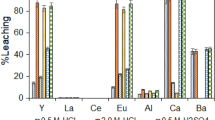
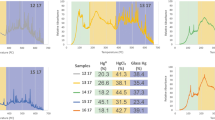
In this work, the recovery of mercury from spent fluorescent lamps by oxidative leaching followed by cementation process was studied. Two different reactive solutions (NaOCl/NaCl and KI/I2) during oxidative leaching were investigated whereas at the cementation process, metallic powders of iron (Fe), copper (Cu), and zinc (Zn) were used as reducing agents to capture mercury in solid phase. Mercury could be transferred to the solution with an efficiency of 96 % from the spent lamp samples through the NaOCl/NaCl reagent. The optimal leaching conditions were determined as 2-h contact time, 120 rpm agitation speed, pH 7.5, and 50 °C of temperature. The reducing agent, Zn, provided 99 % of the cementation. The optimal process conditions were observed to be as 5-min contact time, pH 1, and 5 g L−1 of reducing agent concentration. This combined approach appears to be technically effective for the recovery of mercury from spent fluorescent lamps.







Similar content being viewed by others
References
Anacleto, A. L., & Carvalho, J. R. (1996). Mercury cementation from chloride solutions using iron, zinc and aluminium. Minerals Engineering, 9, 385–397.
Bussi, J., Cabrera, M. N., Chiazzaro, J., Canel, C., Veiga, S., & Florencio, C. (2010). The recovery and recycling of mercury from fluorescent lamps using photocatalytic techniques. Journal of Chemical Technology and Biotechnology, 85, 478–484.
Chang, T. C., You, S. J., Yu, B. S., Chen, C. M., & Chiu, Y. C. (2009). Treating high-mercury-containing lamps using full-scale thermal desorption technology. Journal of Hazardous Materials, 162, 967–972.
Chaturabul, S., Srirachat, W., Wannachod, T., Ramakul, P., Pancharoen, U., & Kheawhom, S. (2015). Separation of mercury(II) from petroleum produced water via hollow fiber supported liquid membrane and mass transfer modeling. Chemical Engineering Journal, 265, 34–36.
Coskun, S., & Civelekoglu, G. (2014). Characterisation of waste fluorescent lamps to investigate their potential recovery in Turkey. International Journal of Global Warming, 6(2/3), 140–148.
Djokic, S. S. (1996). Cementation of copper by aluminum in alkaline solution. Journal of Electrochemical Society, 143, 1300–1305.
Durão, W. A., de Castro, C. A., & Windmöller, C. C. (2008). Mercury reduction studies to facilitate the thermal decontamination of phosphor powder residues from spent fluorescent lamps. Waste Management, 28, 2311–2319.
Fujiwara, K., & Fujinami, K. (2004). Mercury recovery method. U.S. Patent 2004/6800112 B2.
Hummer, H., Gonzala, L. S. S., Fernandes, A. L. V., & Yallouz, A. V. (2006). Treatment of mercury bearing fluorescent lamps by using an electrochemical process. Thesis, Centre Tecn. Miner. Leoben.
Jang, M., Hong, S. M., & Park, J. K. (2005). Characterization and recovery of mercury from spent fluorescent lamps. Waste Management, 25, 5–14.
Kalb, P. D., Adams, J. W., Milian, L. W., Penny, G., Brower, J., & Lockwood, A. (1999). Mercury bakeoff: technology comparison for the treatment of mixed waste mercury contaminated soils at BNL. Tucson: Waste Management Congress.
Klasson, K. T., Koran, L. J., Gates, D. D., & Cameron, P. A. (1998). Removal of mercury from solids using potassium iodide/iodine leaching process. The Scientific Report. Tennessee: OAK Ridge National Laboratory.
Ku, Y., Wu, M. H., & Shen, Y. S. (2002). Mercury removal from aqueous solutions by zinc cementation. Waste Management, 22, 721–726.
Montgomery, D. C. (1991). Design and analysis of experiments (3rd ed.). New York: Wiley.
Nosier, S. A., & Sallam, S. A. (2000). Removal of lead ions from wastewaters by cementation on a gas-sparged zinc cylinder. Separation and Purification Technology, 18, 93–101.
Noubactep, C. (2010). Elemental metals for environmental remediation: learning from cementation process. Journal of Hazardous Materials, 181, 1170–1174.
Piao, H., & Bishop, P. L. (2006). Stabilization of mercury-containing wastes using sulfides. Environmental Pollution, 139, 498–506.
Raap, N. R., & Gallardo, A. (2013). Removal of mercury bonded in residual glass from spent fluorescent lamps. Journal of Environmental Management, 115, 175–178.
Rhee, S. W., Choi, H. H., & Park, H. S. (2014). Characteristics of mercury emission from linear type of spent fluorescent lamp. Waste Management, 34(6), 1066–1071.
Tunsu, C., Ekberg, C., & Retegan, T. (2014). Characterization and leaching of real fluorescent lamp waste for the recovery of rare earth metals and mercury. Hydrometallurgy, 144–145, 91–98.
Twidwell, L. G., & Thompson, R. J. (2001). Recovering and recycling Hg from chlor-alkali plant wastewater sludge. Journal of The Minerals Metals & Materials Society, 53, 15–17.
US EPA. (1998). Comments related to industry source reduction efforts. In Response to comments document, final rule on hazardous waste lamps. Washington, DC: United States Environmental Protection Agency, DCN FLEP-00156.
US EPA. (2007). Treatment technologies for mercury in soil, waste, and water, final report. Washington, DC: United States Environmental Protection Agency.
Wang, J., Feng, X., Anderson, C. W. N., Xing, Y., & Shang, L. (2012). Remediation of mercury contaminated sites—a review. Journal of Hazardous Materials, 221, 1–18.
Acknowledgments
This work was funded by a research grant from the Scientific and Technical Research Council of Turkey (TUBITAK) (project no. 110Y264) and Research Projects Funding Unit of the Suleyman Demirel University (project no. BAP 2376-D-10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coskun, S., Civelekoglu, G. Recovery of Mercury from Spent Fluorescent Lamps via Oxidative Leaching and Cementation. Water Air Soil Pollut 226, 196 (2015). https://doi.org/10.1007/s11270-015-2391-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2391-9