Abstract
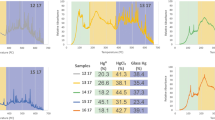
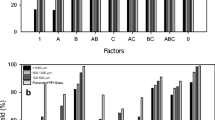
Mercury is one of the main components of fluorescent lamps. Considering the adverse effects of mercury on human health and the environment, recovery of mercury-containing fluorescent lamps is very important in developed countries. The glass parts of used fluorescent lamps are among the dangerous wastes whose mercury content should be reduced to the lowest possible level according to international standards. The aim of this research is to achieve a systematic approach to minimize the amount of mercury present in fluorescent lamp glass residues according to the European Commission EC95/2002 regulations. In order to extract mercury from glasses, glass pieces were washed with deionized water, using stirring to increase washing efficiency. In order to achieve the maximum amount of extraction, parameters such as ratio of glass to deionized water (S/L), stirring time, temperature and pH were changed. The results showed that, the highest mercury extraction rate is about 98% and in the conditions S/L = 0.1, stirring time of 12 h, temperature of 60 °C and pH 1, which is using a combination of HCl and H3PO4 acid 5% with a ratio of 1:4 has been obtained. The success of this method not only increases environmental sustainability, but also classifies the resulting glass waste as non-hazardous.







Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Rodríguez O, et al. Concerns on liquid mercury and mercury-containing wastes: a review of the treatment technologies for the safe storage. J Environ Manage. 2012;101:197–205.
Viana LN, et al. Fluorescent lamps: a review on environmental concerns and current recycling perspectives highlighting hg and rare earth elements. J Environ Chem Eng. 2022;10(6):108915.
Ali HS, Jaber HA, Farid SBH. Investigation of fluorescent lamp glass waste as a fluxing agent in porcelain bodies Materials Today: Proceedings, 2021. 42: pp. 2381–2386.
Aucott M, McLinden M, Winka M. Release of mercury from broken fluorescent bulbs. J Air Waste Manag Assoc. 2003;53(2):143–51.
Hildenbrand VD, et al. Interactions of thin oxide films with a low-pressure mercury discharge. Thin Solid Films. 2000;371(1):295–302.
Rey-Raap N, Gallardo A. Determination of mercury distribution inside spent compact fluorescent lamps by atomic absorption spectrometry. Waste Manag. 2012;32(5):944–8.
Kadam AR, Nair GB, Dhoble SJ. Insights into the extraction of mercury from fluorescent lamps: a review. J Environ Chem Eng. 2019;7(4):103279.
Ali HA, et al. Valorization of spent fluorescent lamp waste glass powder as an activator for eco-efficient binder materials. Constr Build Mater. 2022;352:129020.
Wijesekara RG, Navarro R, Matsumura M. Removal and recovery of mercury from used fluorescent lamp glass by pyrolysis. J Natl Sci Foundation Sri Lanka, 2011. 39.
Ozgur C, et al. Combined oxidative leaching and electrowinning process for mercury recovery from spent fluorescent lamps. Waste Manag. 2016;57:215–9.
Jang M, Hong SM, Park JK. Characterization and recovery of mercury from spent fluorescent lamps. Waste Manag. 2005;25(1):5–14.
Chang TC, et al. The fate and management of high mercury-containing lamps from high technology industry. J Hazard Mater. 2007;141(3):784–92.
Rey-Raap N, Gallardo A. Removal of mercury bonded in residual glass from spent fluorescent lamps. J Environ Manage. 2013;115:175–8.
Fernández-Martínez R, Rucandio MI. Study of the suitability of HNO3 and HCl as extracting agents of mercury species in soils from cinnabar mines. Anal Bioanal Chem. 2005;381(8):1499–506.
Hobohm J, et al. Recycling oriented comparison of mercury distribution in new and spent fluorescent lamps and their potential risk. Chemosphere. 2017;169:618–26.
Tunsu C, Ekberg C, Retegan T. Characterization and leaching of real fluorescent lamp waste for the recovery of rare earth metals and mercury. Hydrometallurgy. 2014;144–145:91–8.
Lu G, et al. High mercury leachate containing HgS22– complex ion: detoxifying solidification and high efficiency hg extraction. J Environ Sci. 2018;73:177–84.
Ling T-C, Poon CS. Spent fluorescent lamp glass as a substitute for fine aggregate in cement mortar. J Clean Prod. 2017;161:646–54.
Bawab J, et al. Structural performance of Reinforced concrete beams incorporating cathode-Ray Tube (CRT) Glass Waste. Buildings. 2021;11. https://doi.org/10.3390/buildings11020067.
Małek M, et al. Physical and Mechanical properties of Polypropylene Fibre-Reinforced cement–glass composite. Materials. 2021;14. https://doi.org/10.3390/ma14030637.
HepsaĞ F, Kizildeniz T. Validation of determination by Icp-Oes Method of Mercury residual levels in meat of canned fish sold in Turkey. Hacettepe J Biology Chem. 2022;50(1):45–54.
Li Z, et al. Mercury Pollution, Treatment and Solutions in Spent fluorescent lamps in Mainland China. Int J Environ Res Public Health. 2018;15. https://doi.org/10.3390/ijerph15122766.
Raposo C, Windmöller CC, Durão WA, Júnior. Mercury speciation in fluorescent lamps by thermal release analysis. Waste Manag. 2003;23(10):879–86.
Kantarcı S, Alp İ. Removal of mercury from cyanide leach solution using potassium amyl xanthate (PAX). Sep Purif Technol. 2023;309:123036.
Sverdrup HU, Olafsdottir AH. System Dynamics Modelling of the global extraction, supply, price, reserves, resources and environmental losses of Mercury. Water Air Soil Pollut. 2020;231(8):439.
Fábrega FdM, Mansur MB. Liquid–liquid extraction of mercury (II) from hydrochloric acid solutions by Aliquat 336. Hydrometallurgy. 2007;87(3):83–90.
Steinhaus J, et al. Mercury Adsorption on Phosphoric Acid- and nitric acid-modified activated Carbon. Industrial & Engineering Chemistry Research; 2024.
Salem M, et al. Phosphoric acid purification sludge: potential in heavy metals and rare earth elements. Waste Manag. 2019;83:46–56.
Cervini-Silva J, et al. Natural incorporation of mercury in bone. J Trace Elem Med Biol. 2021;67:126797.
Gabriel MC, Williamson DG. Principal biogeochemical factors affecting the speciation and transport of Mercury through the terrestrial environment. Environ Geochem Health. 2004;26(3):421–34.
Mitali J, Dhinakaran S, Mohamad AA. Energy storage systems: a review. Energy Storage Sav. 2022;1(3):166–216.
Acknowledgements
This research was supported by Material and Energy Research Center for Doctoral Research in Materials sience.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karamzadeh, L., Salahi, E., Mobasherpour, I. et al. Improved rare mercury recovery from fluorescent lamp wastes through simultaneous leaching and heating. J Environ Health Sci Engineer (2024). https://doi.org/10.1007/s40201-024-00901-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40201-024-00901-5