Abstract
Filaroid nematodes Setaria tundra (Issaitshikoff & Rajewskaya, 1928) and Setaria cervi (Rudolphi, 1819) are internal parasites from family Onchocercidae with occurrence in the northern hemisphere. They have a considerably wide range of final host, including many species of family Cervidae. Intermediate hosts and vectors at the same time, are represented by the several mosquito species, mostly of genus Aedes. Infection of Setaria is relatively harmless and especially in wild cervids usually pass unnoticed. Although in some cases it can induce peritonitis which might be a life threatening condition.
This study was determined to reveal the presence of helminths Setaria tundra and Setaria cervi in red deer (Cervus elaphus) in Slovakia. The parasites were identified morphologically and genetically, based on the sequences of a fragment of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene. For this purpose we used partial results of our longer parasitological monitoring realized in one particular hunting area located in eastern Slovakia, near the city of Košice. A total of 60 red deer individuals were tested, of which one was found to be infected with Setaria tundra (prevalence of 1.7%) and four were detected to be infected with Setaria cervi (prevalence 6.7%). The intensity of infection was very low, only one specimen of Setaria spp. in each positive animal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filaroid nematodes Setaria tundra (Issaitshikoff & Rajewskaya, 1928) and Setaria cervi (Rudolphi, 1819) are internal parasites from family Onchocercidae. These parasites have an indirect life cycle that includes mosquitoes as an intermediate host and final hosts are represented by animals of the Cervidae and Bovidae families (Taylor et al. 2007; Laaksonen and Paulsen 2015). Mosquitoes play an important role not only as intermediate hosts but also as a vector of this parasitic infection. Most competent species for transmission S. tundra are supposed to be mosquitoes of genus Aedes, especially Ae. vexans. Besides the genus Aedes, suitable vectors are also mosquito species of genera Anopheles, Ochlerotatus, Culex and Coquillettidia (Oloś et al. 2021). Demiaszkiewicz et al. 2015 in Poland found that the intermediate host of S. cervi is Haematobia stimulans.
Both species are long, thread-like nematodes, milk-white in color (Nikander et al. 2007). Presently, the genus Setaria, Viborg, 1795, contains 46 species that parasitize in the peritoneal cavity of Artiodactyla, Perissodactyla and Hyracoidea. The adult parasites dwell in the peritoneal cavity, where the females produce first-stage larvae (microfilariae) that enter the host’s bloodstream. First-stage larvae are then uptake by mosquitoes (intermediate host/vector) during their blood feeding, so parasites can subsequently reach the infectious stage (Laaksonen and Paulsen 2015).
Infection of Setaria spp. is usually harmless and only occasionally inducing fibrinous peritonitis associated with poor body condition, especially in young animals (Laaksonen et al. 2007), this phenomenon was recorded in domestic and semi-domestic reindeer (Rangifer tarrandus) in Finland, which caused massive economic losses. The outbreak of serofibrinous peritonitis was noticed by meat-inspecting veterinarians in slaughterhouses (Laaksonen et al. 2007; Enemark et al. 2017).
There have been a multiple reports of S. tundra occurrence in roe deer (Capreolus capreolus) in many countries across the Europe, most importantly in countries neighboring with Slovakia such as Austria (Kutzer and Hinaidy 1969), Hungary (Kemenesi et al. 2015) and Poland (Kowal et al. 2013; Tomczuk et al. 2017) moreover in elk (Alces alces) in Poland (Demiaszkiewicz et al. 2015). But there is not as many reports of S.cervi in red deer (Alasaad et al. 2012; Oloś et al. 2019; Lanková et al. 2021).
Therefore the aim of this study was to confirm the presence helminths of genus Setaria Viborg, 1795 (Filarioidea, Onchocercidae) in red deer (Cervus elaphus) in Slovakia by using molecular methods, which could be considered as a follow-up work to previous study about S. tundra in roe deer, done by Čurlík et al. (2023).
Materials and methods
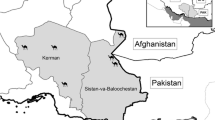
For the purpose of this study we were focused on red deer (Cervus elaphus) Linnaeus, 1758. Study was conducted in the one particular hunting area which is located in a range of mountains in eastern Slovakia (in Slovak: Slanské vrchy), approximately 30 km far from the city of Košice. The executive organ of hunting management in mentioned hunting area is University of Veterinary Medicine and Pharmacy in Košice. The size of hunting area is 2 300 hectares of land, mostly covered by deciduous forest with a few meadows located in lower parts. This region has moderate climate and altitude in hunting area ranging from (400 m asl) in lower parts to highest point Makovica Hill (981 m asl).
All animals in the presented paper have been killed during three consecutive hunting seasons (2020/2021, 2021/2022 and 2022/2023). In total, we managed to perform a post mortem examination in 60 red deer individuals (17 males, 25 females and 18 fawns). According to valid Slovak hunting regulations, is the date of hunt for males and fawns from 1 August to 15 January and females from 1 August to 31 December, exceptions may occur if there is a valid reason such as an unfilled annual quota for young males or fawns. The positive animals were culled– fawns in February 2022, first young male in January 2021 and second young male in March 2022, and old male in February 2023.
The Setaria nematodes were found either during the procedure of evisceration in the field conditions soon after the animal has been killed or during examination of internal organs at the necropsy room. The Setaria nematodes which were found within the infected host animals were located on the surface of the intestines and rumen. Other parts of the animal (spinal cord, brain) were not dissected. If it was possible, we additionally performed basic parasitological examination of internal organs such as lungs, liver and digestive tract (abomasum, small intestine, and large intestine).
Collected nematodes Setaria spp. were rinsed in saline solution, and their morphological structures were analyzed under the light microscope according to recent morphological keys (Nikander et al. 2007; Oloś et al. 2019; Lanková et al. 2021). Helminths were subsequently stored in 96% ethyl alcohol for further molecular analyses. Species diagnostics were confirmed by using molecular methods.
Extraction, amplification of DNA and sequence analysis
Genomic DNA was extracted from adult nematode identified morphologically as S. tundra, using the QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Amplification of a partial sequence of the mitochondrial cytochrome oxidase subunit I (COI) gene was performed using the forward primer cox1int F (5’-TGATTGGTGGTTTTGGTAA-3’) and reverse primer cox1int R (5’-ATAAGTACGAGTATCAATATC-3’) (Casiraghi et al. 2001). The resulting fragment was approximately 690 bp in length. For PCR reaction to amplify the COI fragment was used total volume of 25 µl consisted 5 µl of extracted parasitic DNA, 1X DreamTaq Green Buffer, 1 mM MgCl2, 200 µM of each dNTP, 500 nM of each of the primers, 2.5 U of HotStarTaq DNA Polymerase (Qiagen, Germany).
PCR was performed as follows: initial denaturation was performed at 95 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 1 min and elongation at 72 °C. Final extension was performed at 72 °C for 5 min in the MyCycler™ Thermal Cycler System (Bio-Rad Laboratories, Berkeley, CA, USA). Amplicons were separated on a 1.5% agarose gel stained with Goodview Nucleic Acid Stain (SBS Genetech Co., China) and TAE buffer (40 mM Tris, pH 7.8, 20 mM acetic acid, 2 mM EDTA).
Positive PCR products were purified using the ISOLATE II PCR and Gel Kit (Bioline, UK). DNA sequencing was performed on an automated DNA sequencer ABI prism 3700 at the University of Veterinary Medicine and Pharmacy, Košice, Slovakia. The obtained DNA sequences were compared with the reference sequences of in the GenBank database by the nucleotide BLASTn (https://blast.ncbi.nlm.nih.gov) program and were grouped by similarity and aligned using the MEGA 11 (Tamura et al. 2021). The Tamura-Nei model was chosen as the most appropriate model for the data analysed. All positions containing gaps and missing data were eliminated. Thelazia callipaeda was chosen as an outgroup. Bootstrap analysis was performed with 1,000 replicates to test robustness of the phylogeny.
Results
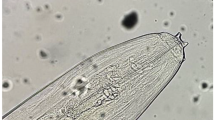
By the available descriptions of morphological features in the literature, all nematodes found in the abdominal cavity were classified as adults of genus Setaria spp. This species diagnostic was based on specific localization in host (peritoneal cavity) and on the structure of the peribuccal crown on the cephalic part of the parasite as well as on the morphology of their caudal end (Nikander et al. 2007; Čurlík et al. 2019; Oloś et al. 2019; Lanková et al. 2021; Oehm et al. 2022).
Individuals of Setaria spp. were found in 5 out of 60 (prevalence 8.3%) examined red deer (Cervidae, Cervus elaphus) individuals (in 2 fawns - females; in 2 males of age 2–3 years and in 1 male of age 7–8 years). All five positive animals were in adequate body condition except for one young male (killed on 4. February 2023 with bodyweight only 60 kg) which was in poor body condition. No apparent clinical signs or pathological lesions associated with Setaria infection were observed before animals were killed or during post mortem examination. The intensity of infection was very low, only one specimen of Setaria spp. in each positive animal. We did not observe any difference in prevalence between individual years.
Based on morphology (Fig. 1.) and molecular diagnostic, one of the five helminths was identify as S. tundra (in the oldest male) and remaining four of the Setaria helminths were identify as a S. cervi. All collected nematodes were adult females (S. tundra length– 51 mm, S. cervi average length 121 mm) which were localised on surface of intestine (Fig. 2) or rumen (Fig. 3). In addition, in 4/5 individuals with Setaria infection there were also present infection of gastrointestinal nematodes, in 2/5 there were present infections of pulmonary nematodes.
Genomic DNA was successfully extracted from 5 adult individuals of nematodes species Setaria. Amplification of COI gene regions was successful in five isolates of the parasitic DNA. The final amplified fragment was approximately 690 bp. Morphological analyses supported by molecular analyses are the best approach to identify Setaria nematodes. Both analyses were applied to all nematode individuals in our study.
Accession numbers of the sequences obtained were stored in GenBank. Individual of S. tundra was deposited under accession number: PP196346 and S. cervi under accession numbers: PP198315; PP198852; PP198854; PP208633.
To illustrate the genetic relationships between our isolates and the reference isolates, a phylogram was constructed using the Neighbor-Joining (NJ) method (Fig. 4). All isolates of the two Setaria species studied were grouped into separate clusters with the highest support values confirming the identification of the species examined. Based on the analysed COI fragment, the intraspecific genetic variation was observed only for S. tundra.
Setaria spp. COI phylogenetic tree inferred by the NJ method. The construction of the phylogenetic tree was conducted utilizing the best model Tamura-Nei. Bootstrap analysis was performed with 1.000 replicates; numbers on the tree nodes indicate bootstrap values > 85%. Thelazia callipaeda was used as an outgroup. Accession numbers of sequences retrieved from GenBank are indicated together with their geographic origin. Evolutionary analyses were conducted in MEGA 11
Discussion
Epidemiology of filarial infection especially in red deer has received very little attention. Not many molecular studies have been carried out to describe parasitic infection of Setaria cervi in red deer, as far as we know, only a few of them address this topic (Alasaad et al. 2012; Oloś et al. 2019; Lanková et al. 2021). Although in the last century there has been some studies dedicated to morphological descriptions of S. cervi such as (Kotrlá et al. 1984; Rajsky 2015).
However, there have been some reports of S. tundra occurrence from European countries Germany (Oehm et al. 2022), Denmark (Enemark et al. 2017), Spain (Angelone-Alasaad et al. 2016), Croatia (Čurlík et al. 2019), Austria (Kutzer and Hinaidy 1969), Hungary (Kemenesi et al. 2015), Poland (Tomczuk et al. 2017) and Slovakia (Čurlík et al. 2023), and most importantly there is a recent report of S. tundra in red deer (Cervus elaphus) in Poland (Oloś et al. 2019). The purpose of our study was to confirm the occurrence of nematode of genus Setaria in red deer (Cervus elaphus) in Slovakia by using molecular methods. To reach this aim, amplification of the COI gene region was performed, in accordance with similar works (Čurlík et al. 2019, 2023). Consequently, comparison with sequences archived in the GenBank revealed that the analyzed nematodes belong to the species Setaria tundra and Setaria cervi. Our Setaria tundra sequence was compared with sequences in GenBank and revealed the highest sequence similarity (100%-99.42%) with the S. tundra sequence (KF692103; PP158773; MK360915) isolated from mosquitoes in Germany, from wolf blood in Australia and in red deer blood in Poland. The final sequences deposited in GenBank showed the highest (100%) sequence similarity to S. cervi isolated in Spain from adult nematode and S. cervi from red deer in Poland and from Cervus Nippon in the Czech Republic (JF800924; MK360913; MT977072).
Infection of Setaria is usually harmless, occasionally inducing a mild fibrinous peritonitis which only can be seen at necropsy. Pathological changes may also include dead and partly calcified Setaria helminths on the liver surface (Laaksonen and Paulsen 2015). There are no clinical signs when the parasites are in their normal site, although if nervous tissue is involved there is locomotor disturbance which might lead to paraplegia (Taylor et al. 2007), however setariosis with recognizable clinical signs and pathological features can be spotted. The most common findings in reindeer during ante-mortem examination at the slaughterhouses in Finland 2003 were poor body condition, dry fur and underdeveloped winter coat and slightly distended abdomen (Laaksonen et al. 2007). Very typical pathological signs associated with Setaria infection during outbreak in Finland 2003 were granulomatous inflammation (greenish or greyish fibrinous membranes covering peritoneum and visceral organs). The surfaces of the livers were typically covered by a thin layer of fibrin and in some severe cases were fibrinous layers presented between all abdominal organs (Laaksonen et al. 2007). Yet, there has not been any report of severe peritonitis associated with Setaria infection in roe deer neither red deer. Likewise in this study, no gross lesions of internal organs or any neurological signs associated with Setaria infection were observed.
Four out of five positive animals were in good body condition, despite other parasitic infections being presented. We can sum up there was only one individual of red deer (young male/yearling, killed on 04.02.2023) in very poor body condition. Although this particular animal had suffered from multiple parasitic infections such as infection of Setaria tundra, gastrointestinal nematodes, larvae of nose botflies, larvae of warble flies and severe Onchocerca spp. infection.
All of the Setaria helminths were localized in the abdominal cavity which is very typical for this genus (Laaksonen and Paulsen 2015). The prevalence of Setaria infection in the present paper was 8.3% which is less in comparison with similar studies conducted in red deer in Poland (72.7%) or Czech Republic (38.5%) (Oloś et al. 2019; Lanková et al. 2021). The intensity of infection was very low, only one specimen of S. tundra in positive animal. Compared to the outbreak in Finland 2003 (mean intensity in calves was 8 − 5, with range 0 to 30; mean intensity in adults was 1–5, with range 0 to 84 of Setaria) (Laaksonen et al. 2007).
Conclusion
Infection of Setaria spp. has been observed in cervids in several European countries, however this is only the second report of S. tundra occurrence in red deer in general and the first report of S. tundra and S. cervi in red deer in Slovakia. It is only questionable if infections of S. tundra and S. cervi have started spreading in red deer in recent years or if it has not been objective of any previous studies. The differences of parasitic load of S. tundra and S. cervi might be caused by unequal biotic and abiotic influence on their life cycle.
Data availability
No datasets were generated or analysed during the current study.
References
Alasaad S, Pascucci I, Jowers MJ, Soriguer RC, Zhu XQ, Rossi L (2012) Phylogenetic study of Setaria Cervi based on mitochondrial cox 1 gene sequences. Parasitol Res 110:281–285. https://doi.org/10.1007/s00436-011-2486-1
Angelone-Alasaad S, Jowers MJ, Panadero R, Pérez-Creo A, Pajares G, Díez-Baños P, Soriguer RC, Morrondo P (2016) First report of Setaria tundra in roe deer (Capreolus capreolus) from the Iberian Peninsula inferred from molecular data: epidemiological implications. Parasit Vectors 9(1):521. https://doi.org/10.1186/s13071-016-1793-x
Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C (2001) A phylogenetic analysis of flarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology 122:93–103. https://doi.org/10.1017/S0031182000007149
Čurlík J, Konjević D, Bujanić M, Sabol Ž, Martinković F, Sindičić M (2019) The first description of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in roe deer from Croatia. Helminthologia 1(3):252–255. https://doi.org/10.2478/helm-2019-0015
Čurlík J, Šmigová J, Šmiga Ľ, Lazár J, Lazár P, Konjević D, Papajová I (2023) The first report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Slovakia by using of molecular methods. Vet Res Commun 47(4):2247–2251. https://doi.org/10.1007/s11259-023-10127-9
Demiaszkiewicz AW, Kuligowska I, Pyziel AM, Lachowicz J (2015) Pierwsze w Polsce przypadki inwazji Nicieni Setaria tundra u łosi. Med Weter 71(8):510–512
Enemark HL, Oksanen A, Chriél M, le Fèvre Harslund J, Woolsey ID, Al-Sabi MN (2017) Detection and molecular characterization of the mosquito-borne filarial nematode Setaria tundra in Danish roe deer (Capreolus capreolus). Int J Parasitol Parasites Wildl 3(1):16–21. https://doi.org/10.1016/j.ijppaw.2017.01.002
Kemenesi G, Kurucz K, Kepner A, Dallos B, Oldal M, Herczeg R, Vajdovics P, Bányai K, Jakab F (2015) Circulation of Dirofilaria repens, Setaria tundra, and Onchocercidae species in Hungary during the period 2011–2013. Vet Parasitol 214:108–113. https://doi.org/10.1016/j.vetpar.2015.09.010
Kotrlá B, Černý V, Kotrlý A, Minář J, Ryšavý B, Šebek Z (1984) Parazitózy zvěře. Nakladatelství Československé akademie vied. Polygrafia n.p., Praha8 Libeň
Kowal J, Kornaś S, Nosal P, Basiaga M, Lesiak M (2013) Setaria tundra in roe deer (Capreolus capreolus)–new findings in Poland. Ann Parasitol 59:179–182
Kutzer E, Hinaidy HK (1969) Die Parasiten Der Wildlebenden Wiederkäuer Österreichs. (in German with English summary). Z Parasitenk 32:354–368
Laaksonen S, Paulsen P (2015) Hunting hygiene. Wageningen Academic Publishers. The Nederlands. ISBN: 978-90-8686-249-8
Laaksonen S, Kuusela J, Nikander S, Nylund M, Oksanen A (2007) Outbreak of parasitic peritonitis in reindeer in Finland. Vet Rec 160(24):835–841. https://doi.org/10.1136/vr.160.24.835
Lanková S, Vejl P, Melounová M, Čílová D, Vadlejch J, Miklisová D, Langrová I (2021) Setaria Cervi (Filarioidea, Onchocercidae) undressing in ungulates: altered morphology of developmental stages, their molecular detection and complete sequence cox1 gene. Parasitology 148(5):598–611. https://doi.org/10.1017/S0031182020002449
Nikander S, Laaksonen S, Saari S, Oksanen A (2007) The morphology of the filaroid nematode Setaria tundra, the cause of peritonitis in reindeer Rangifer tarandus. J Helminthol 81:49–55. https://doi.org/10.1017/S0022149X07214099
Oehm AW, Jaeger L, Stoll A, Knubben-Schweizer G, Jaeger-Scheuerle MC (2022) Setaria tundra in a roe deer (Capreolus capreolus) in the Donau-Ries district of Bavaria. Ger Schwei Arch Tierheilk 164(1):107–111. https://doi.org/10.17236/sat00341
Oloś G, Nowakowska J, Rojewska S, Welc-Falęciak R (2019) New findings of Setaria tundra and Setaria Cervi in the red deer (Cervus elaphus) in Poland. Parasitology 146(10):1333–1337. https://doi.org/10.1017/S0031182019000568
Oloś G, Nowakowska J, Welc-Faleciak R (2021) Setaria tundra, what do we know, what is still to be discovered? Annals Parasitol 67(1). https://doi.org/10.17420/ap6701.306
Rajsky D (2015) Setariosis in cervids. Vojenské Lesy 9:13 (in Czech language)
Tamura K, Stecher G, Kumar S (2021) MEGA 11: molecular evolutionary genetics analysis version. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Taylor MA, Coop RL, Wall RL (2007) Veterinary parasitology third edition. UK: Blackwell Publishing, ISBN 978-1-4051-1965-1
Tomczuk K, Szczepaniak K, Grzybek M, Studzińska M, Demkowska, Kutrzepa M, Roczeń-Karczmarz M, Łopuszyński W, Junkuszew A, Gruszecki T, Dudko P, Bojar W (2017) Internal parasites in roe deer of the Lubartów Forest Division in postmortem studies. Medycyna Weterynaryjna 73:726–730
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Contributions
Conceptualization– Jozef Lazár, Ľubomír Šmiga; methodology– Júlia Šmigová, Federica Berrilli; formal analysis– Jozef Lazár, Ľubomír Šmiga, Júlia Šmigová, Peter Lazár, Ingrid Papajová; investigation– Jozef Lazár, Ľubomír Šmiga, Júlia Šmigová; resources - Jozef Lazár, Ľubomír Šmiga; data duration– Jozef Lazár, Ľubomír Šmiga, Júlia Šmigová, Federica Berrilli; original draft preparation– Jozef Lazár, Ľubomír Šmiga; review and editing - Ľubomír Šmiga, Júlia Šmigová; supervision– Peter Lazár, Ingrid Papajová, Ján Čurlík; project administration - Peter Lazár, Ingrid Papajová; funding acquisition - 0. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to publish
Yes.
Ethical standards
The study reported here was conducted in compliance with the relevant local laws and regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lazár, J., Šmigová, J., Šmiga, Ľ. et al. Molecular detection of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) and Setaria cervi (Rudolphi, 1819) in red deer in Slovakia. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10394-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-024-10394-0