Abstract
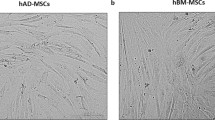
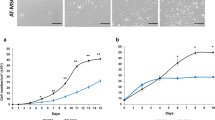
Adult Mesenchymal Stem Cells (MSC) are cells that can be defined as multipotent cells able to differentiate into diverse lineages, under appropriate conditions. These cells have been widely used in regenerative medicine, both in preclinical and clinical settings. Initially discovered in bone marrow, MSC can now be isolated from a wide spectrum of adult and foetal tissues. Studies to evaluate the therapeutic potential of these cells are based on their ability to arrive to damaged tissues. In this paper we have done a comparative study analyzing proliferation, surface markers and OCT4, SOX9, RUNX2, PPARG genes expression in MSC cells from Bone marrow (BMMSC) and Adipose tissue (ASC). We also analyzed the role of Stem Cell Factor (SCF) on MSC proliferation and on ASCs metalloproteinases MMP-2, MMP-9 secretion. Healthy dogs were used as BMMSC donors, and ASC were collected from omentum during elective ovariohysterectomy surgery. Both cell types were cultured in IMDM medium with or without SCF, 10% Dog Serum (DS), and incubated at 38 °C with 5% CO2. Growth of BMMSCs and ASCs was exponential until 25–30 days. Flow citometry of MSCs revealed positive results for CD90 and negative for CD34, CD45 and MCH-II. Genes were evaluated by RT-PCR and metalloproteinases by zymografy. Our findings indicate morphological and immunological similarities as well as expression of genes from both origins on analyzed cells. Furthermore, SCF did not affect proliferation of MSCs, however it up-regulated MMP-2 and MMP-9 secretion in ASCs. These results suggest that metalloproteinases are possibly essential molecules pivoting migration.





Similar content being viewed by others
References
Alipour F, Parham A, Kazemi Mehrjerdi H, Dehghani H (2015) Equine adipose-derived mesenchymal stem cells: phenotype and growth characteristics, gene expression profile and differentiation potentials. Cell J 16(4):456–465. https://doi.org/10.22074/cellj.2015.491
Almalki SG, Agrawal DK (2016) Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther 7(1):129. https://doi.org/10.1186/s13287-016-0393-1
Aubin JE, Liu F, Candeliere GA (2002) PCR methods for studying stem cells and progenitors. Methods Mol Biol 185:403–415
Bajek A, Gurtowska N, Olkowska J, Kazmierski L, Maj M, Drewa T (2016) Adipose-derived stem cells as a tool in cell-based therapies. Arch Immunol Ther Exp (Warsz) 64(6):443–454. https://doi.org/10.1007/s00005-016-0394-x
Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T (2003) Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253(1–2):269–285
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25(11):2739–2749
Csaki C, Matis U, Mobasheri A, Shakibaei M (2009) Co-culture of canine mesenchymal stem cells with primary bone-derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol 131(2):251–266. https://doi.org/10.1007/s00418-008-0524-6
De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever JD, Waele M, Van Riet I (2007) Migration of culture-expanded human mesenchymal stem cells hrough bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 92(4): 440–449; https://doi.org/10.3324/haematol.10475
Deruyina E, Quigley J (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Fermín ML, Gaitán S, Fragío C, Léon LG, Ostronoff LK, Kremmer E, Kolb HJ, Tejero C (2004) Canine long-term bone marrow culture neutrophil production and functionality. Acta Haematol 111(4):196–204
Frese L, Dijkman PE, Hoerstrup SP (2016) Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother 43(4):268–274
Fu Y, Karbaat L, Wu L, Leijten J, Both SK, Karperien M (2017) Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng Part B Rev 23(6):515–528. https://doi.org/10.1089/ten.TEB.2016.0365
Guercio A, Di Bella S, Casella S, Di Marco P, Russo C, Piccione G (2013) Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol Int 37(8):789–798. https://doi.org/10.1002/cbin.10090
Han SH, Jang G, Bae BK, Han SM, Koh YR, Ahn JO, Jung WS, Kang SK, Ra JC, Lee HW, Youn HY (2014) Effect of ectopic OCT4 expression on canine adipose tissue-derived mesenchymal stem cell proliferation. Cell Biol Int 38(10):1163–1173. https://doi.org/10.1002/cbin.10295
Harper E, Bloch KJ (1971) The zymogen of tadpole collagenase. Biochemistry 10(16):3055–3041
Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P, Lam PY (2009) Matrix metalloproteinase 1 is necesary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells 27(6):1366–1375
Hoffman AM, Dow SW (2016) Concise review: stem cell trials using companion animal disease models. Stem cells 34(7):1709–1729. https://doi.org/10.1002/stem.2377
Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat ML, Montaño J, Chang H, Rozmus J, Russell JA, Edwards DR, Turner AR (1999) Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34 + cells and their transmigration through reconstituted basement membrane. Blood 93(10):3379–3390
Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML (2000) Differential MMP and TIMP production by human marrow and peripheral blood CD34 + cells in response to chemokines. Exp Hematol 28(11):1274–1285
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23(10):1289–1291
Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S (2014) Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6(5):552–570. https://doi.org/10.4252/wjsc.v6.i5.552
Lapidot T, Dar A, Kollet O (2005) How do stem cells find their way home? Blood 106(6):901–910
León LG, Ostronoff LK, Fermín ML, Fragío C, Kremmer E, Tejero C (2005) In vitro generation of mature neutrophils from canine Lin- bone marrow cells. Vet Immunol Inmunopathol 107(1–2):41–50
Marx C, Silveira MD, Beyer Nardi N (2015) Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev 24(7):803–813. https://doi.org/10.1089/scd.2014.0407
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48(12):3464–3474
Neupane M, Chang C, Kiupel M, Yuzbasiyan-Gurkan V (2008) Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A 14(6):1007–1015. https://doi.org/10.1089/tea.2007.0207
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100(14):8407–8411
Ostronoff LK, Kremmer E, Fermín ML, Fragío C, Mysliwietz J, Kolb HJ, Tejero C (2008) Canine stem cell factor augments expression of matrix metalloproteinase-9 by CD34 cells. Cytotherapy 10(2):193–102. https://doi.org/10.1080/14653240701827407
Quintanilha LF, Takami T, Hirose Y, Fujisawa K, Murata Y, Yamamoto N, Goldenberg RC, Terai S, Sakaida I (2014) Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res 44(10):E206–E217. https://doi.org/10.1111/hepr.12204
Reich CM, Raabe O, Wenisch S, Bridger P, Kramer M, Arnhold S (2012) Isolation, culture and chondrogenic differentiation of canine adipose tissue and bone marrow-derived mesenchymal stem cells–a comparative study. Vet Res Commun 36(2):139–148. https://doi.org/10.1007/s11259-012-9523-0
Reid J, Nolan AM, Hughes JML, Lascelles D, Pawson P, Scott EM (2007) Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim Welf 16(S):97–104
Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P (2007) MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109(9):4055–4063
Roth V (2006) http://www.doubling-time.com/compute.php
Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC (2007) Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica 92(7):897–904
Sohni A, Verfaillie CM (2013) Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013:130763. https://doi.org/10.1155/2013/130763
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behabior. Annu Rev Cell Dev Biol 17:463–516
Trivanović D, Jauković A, Popović B, Krstić J, Mojsilović S, Okić-Djordjević I, Kukolj T, Obradović H, Santibanez JF, Bugarski D (2015) Mesenchymal stem cells of different origin: comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci 141:61–73. https://doi.org/10.1016/j.lfs.2015.09.019
Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M (2010) Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant 19(3):279–289. https://doi.org/10.3727/096368909X481764
Wang W, Cao W (2014) Treatment of osteoarthritis with mesenchymal stem cells. Sci China Life Sci 57(6):586–595. https://doi.org/10.1007/s11427-014-4673-7
Wang S, Qu X, Zhao RC (2012) Clinical applications of mesenchymal stem cells. J Hematol Oncol 5:19–28. https://doi.org/10.1186/1756-8722-5-19
Zheng Y, Watanabe N, Nagamura-Inoue T, Igura K, Nagayama H, Tojo A, Tanosaki R, Takaue Y, Okamoto S, Takahashi TA (2003) Ex vivo manipulation of umbilical cord blood-derived hematopoietic stem/progenitor cells with recombinant human stem cell factor can up-regulate levels of homing-essential molecules to increase their transmigratory potential. Exp Hematol 31(12):1237–1246
Zhu X, Shi W, Tai W, Liu F (2012) The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived mesenchymal stem cells. Cell Tissue Res 350(2):277–287. https://doi.org/10.1007/s00441-012-1453-1
Acknowledgements
We are very thankful to Dra. Carmen Fajardo for her scientific assistance. We thank Flow Cytometry, Genomics and Proteomics UCM facilities, for their technical support. Leticia G. León care Marie Curie grant. Authors would like to thank Elisabeth Salva for their technical assistance. Some studies reported in this manuscript were supported by a grant from the Deutsche José Carreras Leukämie Stiftung e.V. (R01/R13.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Additional information
Highlights
Standard phenotype from bone marrow BMMSCs and adipose tissue ASCs was confirmed.
We verified OCT4, SOX9, RUNX2, and PPARG genes expression in MSC from both origins.
ASCs constitutively express MMP-2 and MMP-9, being up-regulated by SCF.
Rights and permissions
About this article
Cite this article
Enciso, N., Ostronoff, L.L.K., Mejías, G. et al. Stem cell factor supports migration in canine mesenchymal stem cells. Vet Res Commun 42, 29–38 (2018). https://doi.org/10.1007/s11259-017-9705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-017-9705-x