Abstract
Acacia longifolia (Andrews) Willd. is a legume native to southeast mainland Australia and Tasmania and has two described subspecies: A. l. subsp. longifolia and A. l. subsp. sophorae. The species has been introduced around the world and is considered invasive in several Mediterranean-type climate regions, including in South America, South Africa, and southern Europe. Previous studies comparing native and invasive populations of A. longifolia have focused on its reproductive ecology and population genetics, and little information exists on the species’ early life development and how abiotic factors influence it. Here, we performed a glasshouse experiment to compare the phenotypic responses of native and invasive (in Portugal) A. longifolia seedlings to different levels of water and nutrient availability. We found that seedlings of both subspecies responded similarly to different water and nutrient availability conditions in terms of biomass accumulation, root length, the number of phyllodes produced, phyllode water content, and root-to-shoot ratio. However, compared to native seedlings, invasive seedlings had limited capacity for stress responses. We found that invasive seedlings had lower drought tolerance than native seedlings, and thus the speed of invasion by A. longifolia into drier parts of Portugal may be hindered. Our results also hint of a possible role of seed “imprinting” in this species’ early growth responses, resulting in different resource allocation strategies such as favouring early growth and development over drought resistance in the invaded range. Further studies are required to better understand the species’ abiotic stress responses at the intraspecific level and their relation to its invasiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human-mediated introductions of species outside their native ranges have in many instances led to the establishment of invasive populations. Invasive species are now widely recognised as one of the leading causes of habitat degradation and biodiversity loss (Simberloff et al. 2013; IPBES 2023). Globally, the number of invasive species is increasing, and the extent of their impacts are likely to increase under global change conditions (Pyšek et al. 2020). Australian acacias (genus Acacia sensu stricto) are among the most problematic invasive plants globally (Richardson et al. 2011; 2023). More than 400 Acacia species have been introduced around the world for various purposes (Botella et al. 2023) and at least 28 are considered invasive (Richardson et al. 2023; Magona et al. 2018).
Acacia longifolia (Andrews) Willd., also known as the Sydney Golden Wattle, is one of the most widespread invasive acacias. This species is native to southeast mainland Australia and Tasmania and has been introduced to many regions of the world for coastal dune stabilisation and as an ornamental plant, subsequently becoming invasive, especially in Mediterranean climate regions (Kull et al. 2011; Richardson et al. 2011; Souza-Alonso et al. 2017). This species has also been introduced to parts of Australia outside of its native range, such as the state of Western Australia, where it has also become invasive (Richardson et al. 2011; Harris et al. 2012). Two subspecies have been described for A. longifolia based on morphological characters such as phyllode shape, seed pod size and shape, as well as slightly different, but overlapping, distributions: subsp. longifolia and subsp. sophorae (Whibley and Symon 1992; Flora of Australia 11B, 2001; Vicente et al. 2023a). Some authors even consider them to be two distinct species (Flora of Australia 11B, 2001). However, recent niche modelling and genetic studies do not support this intraspecific subdivision, suggesting that the classification of these two subspecies requires revision (Vicente et al. 2023a, b). Acacia longifolia has long-lasting flowering periods characterised by massive investments in flower and pollen production (Correia et al. 2014; 2015), resulting in high seasonal seed crops (Gibson et al. 2011) and the formation of massive seeds banks (Marchante et al. 2010; Le Maitre et al. 2011).
In its invasive range, A. longifolia is considered to be an “ecosystem engineer”, actively transforming ecosystems and causing significant impacts on both the above- and belowground components of ecosystems (Yelenik et al. 2007; Ulm et al. 2017a; Jesus et al. 2020). It forms dense canopies which limit light availability to native understory plants (Rascher et al. 2011) and accumulate high amounts of biomass and litter (Zhang et al. 2020), which increases the intensity of wildfires—an important feature of Mediterranean ecosystems especially during hot summer months (Souza-Alonso et al. 2017). In turn, wildfires promote the germination of acacia seeds, which can remain dormant and viable in soils for several years (Marchante et al. 2010; Le Maitre et al. 2011), while reducing the viability of the seeds of native plants (Richardson and Kluge 2008; Le Maitre et al. 2011; Souza-Alonso et al. 2017). The accumulation of leaf litter also leads to changes in soil chemical composition and nutrient cycles, reduces water availability, and increases soil organic matter content (Lorenzo and Rodríguez-Echeverría 2015; Ulm et al. 2017b; Hamad-Sheip et al. 2021). Even young A. longifolia plants, that have not yet accumulated litter, cause several changes in soil functioning (Ulm et al. 2017a), highlighting that the species can affect ecosystem properties even during the early stages of invasion.
Several hypotheses have been put forward to explain plant invasiveness based on functional and life-history traits related to growth, reproduction, dispersal, and resource allocation responses (Catford et al. 2009; Matzek 2011, 2012; Skálová et al. 2012; Enders et al. 2020; Guo et al. 2022; Gioria et al. 2023). For example, phenotypic plasticity, i.e., the expression of different phenotypes in response to environmental cues by the same genotype (Bradshaw 1965), is an important strategy that allows introduced species to rapidly respond to changes in environmental conditions (Xue and Leibler 2018) and has been linked with invasiveness (Bradshaw 1965; Richards et al. 2005, 2006; Skálová et al. 2012). Phenotypic plasticity provides organisms with ecological flexibility, even when levels of standing genetic diversity are low. In plants, such plasticity can stem from direct interactions with the environment or from “imprinting” of the seeds (i.e., maternal effects), creating semi-heritable trait variation (Bai and Settles 2015; Ortmans et al. 2016; Mounger et al. 2021).
Growth experiments under controlled environmental conditions provide opportunities to understand the roles of functional traits, phenotypic plasticity, and local adaptation in facilitating plant invasion (de Villemereuil et al. 2016). For example, a glasshouse experiment showed that invasive A. longifolia seedlings in Portugal have higher tolerance to saltwater stress than seedlings from a co-occurring native species, a clear advantage to outcompete natives during early stages of invasion in coastal areas (Morais et al. 2012). A recent glasshouse study also showed that soil origin influenced the growth of invasive A. longifolia seedings, as well as the bacterial diversity found in the species’ root nodules, highlighting the importance of above- and belowground interactions in its invasiveness (Sampaio et al. 2023). Harris et al. (2012) performed a glasshouse experiment and genetic analyses of native and invasive seedlings of several acacias in Australia, including A. longifolia. They found traits to vary between native and non-native ranges, but not in the same direction, for different species. Two acacias (A. longifolia and A. saligna) showed evidence for genetic bottlenecks in their non-native ranges, a finding that is contrary to what is known for introduced Acacia species in general, including invasive A. longifolia populations outside of Australia, that have retained high levels of genetic diversity (Vicente et al. 2021; 2023b). However, the lower genetic diversity in invasive A. longifolia and A. saligna populations in Australia did not affect their growth responses or invasiveness (Harris et al. 2012).
Comparative studies of native and invasive A. longifolia populations have focused on the reproductive ecology of the species (e.g., Correia et al. 2014; 2015; 2016), and early development and growth (e.g., Welgama et al. 2019). Here, we conducted a glasshouse experiment to compare the phenotypic responses of Australian and Portuguese A. longifolia seedlings to different treatments of water and nutrient availability. Our aims were to: (1) evaluate whether differences exist in seedling survival between native and invasive ranges under stress conditions (i.e., low water and nutrient availability) and (2) determine whether the two A. longifolia subspecies differ in their responses to different levels of water and nutrient stress. We hypothesised that invasive A. longifolia seedlings will have higher tolerance to water and nutrient stress compared to native range seedlings and that no differences in stress responses exist between the two subspecies.
Materials and methods
Seed origins, characterisation, and germination
Seeds from both A. longifolia subspecies (i.e., subsp. longifolia and subsp. sophorae) from Australia, and from invasive populations in Portugal (subspecies identity unknown), were obtained from three different geographically distinct provenances in each country. Seeds from the native range were bought at three nurseries: AustraHort (AH; https://www.austrahort.com.au), Indigo Native Nursery (IN; https://indigonursery.com.au) and Royston Petrie Seeds (RP; https://rpseeds.com.au). These seeds were harvested in the field by nursery staff. Seeds from the invaded range were collected in January 2021 from soils seed banks in three areas in Portugal: Vila do Conde (VC), Costa da Caparica (CC) and Monte Gordo (MG). In these areas, only one collection site was visited, and 100–300 seeds were collected directly under the canopy of three to four separate plants from the upper layer of soil. See Table 1 for further details.
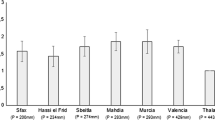
We measured seed weight (mg) of 50 seeds from each provenance as a proxy for assessing maternal effects (see Results; Fig. 1) using an Acculab ALC-210.4 analytical balance (Acculab, NY, USA). When present, elaiosomes (i.e., fleshy structures rich in lipids and proteins attached to the top of the seeds) were removed before weighing. Seeds with weights within the range of mean weight ± 1 SD from their corresponding provenance were then selected to minimise maternal effects in the experiment (Harris et al. 2012). We selected 50–90 seeds per provenance for germination, and, since seed viability was unknown, available seeds from other provenances were also germinated as a precaution. Seeds were surface sterilised with 5% commercial bleach for 5 min and then rinsed with distilled water. Immediately following the sterilisation, seeds were transferred to a water bath at 100 °C for 1 min to promote germination, then placed into Petri dishes lined with paper filter saturated with distilled water (maximum 10 seeds per dish). Petri dishes were sealed and placed in a germination room with a 16 h/8 h photoperiod and temperature ranging from 23 to 25 °C.
Glasshouse experiment
Following germination, seedlings were transferred to black plastic pots in a glasshouse (Faculty of Sciences, University of Lisbon, Lisbon, Portugal) containing 0.5 L of commercial garden soil mixed with sand in a 3:2 ratio, following Harris et al. (2012). We placed 1–3 seedlings in each pot to account for variation in seedling survival. Seedling transfers between pots were done over the course of 12 days due to variability in germination speed and germinability (i.e., percentage of seeds that germinated; Lozano-Isla et al. 2019) between seeds (Supplementary Information, Fig. S1, Lozano-Isla et al. 2019). Water-holding capacity of the soil was estimated by fully saturating 10 pots with 100 or 200 mL of water and measuring the excess water that leaked from the bottom of the pots, resulting in a mean estimate of 100 mL. After an acclimation period of 3 weeks, we randomly removed any extra seedlings so that only one remained per pot. Shoot length (cm) was measured for each retained seedling. Seedlings were then subjected to different water and nutrient treatments for 13 weeks, with pots randomly repositioned in the glasshouse every 2 weeks. We applied different water and nutrient treatments in a fully factorial design, consisting of the following treatment combinations: high water and nutrients (hereafter W + N +), irrigation at 70% field capacity (70 mL) with nutrient supplementation; high water and low nutrients (hereafter W + N−), irrigation at 70% field capacity (70 mL) without nutrient supplementation; low water and high nutrients (hereafter W–N +), irrigation at 20% field capacity (20 mL) with nutrient supplementation; and low water and nutrients (hereafter W–N−), irrigation at 20% field capacity (20 mL) without nutrient supplementation. Nutrient supplementation involved watering the seedlings with a modified Hoagland solution (see Supplementary Information, Table S1; Hoagland 1932) every 4 weeks, starting on the first day of treatment application. Each unique provenance by treatment combination was replicated 12 times, except for the RP16 provenance for which only 33 seedlings were available, and thus nine seedlings for the W + N + treatment and eight seedlings for the remaining treatments were used. In total, our experiment consisted of 417 potted seedlings. As we had no control over temperature and relative humidity in the glasshouse and an hourly registry of these variables was kept for the duration of the experiment (see Supplementary Information, Fig. S2) using a HOBO Data Logger (Onset, MA, USA). During this period, temperature ranged between 8.52 and 41.62 °C, with the mean being 22.32 °C, and relative humidity ranged between 18.10 and 88.06%, with the mean being 58.28%. Regardless of treatments, seedlings were watered twice a week with the appropriate amount of water. Towards the end of the experiment, and during the onset of summer, seedlings were watered three times a week to ensure that plants under stress conditions remained alive and that no water stress was induced in well-watered plants.
Data collection
After the 13-week growth period, plants were harvested and the following measurements taken: total plant length (cm), shoot length (cm), root length (cm; total plant length—shoot length), shoot increment (cm; initial shoot length—final shoot length), total number of phyllodes, total phyllode area (cm2; Li-3000C Portable Leaf Area Meter, LI-COR, NE, USA), and total phyllode wet weight (g; Acculab ALC-210.4 analytical balance, Acculab, NY, USA). After taking measurements, shoots and roots were separated into paper envelopes and placed in an oven at 60 °C for 72 h to dry. Once dried, we measured phyllode biomass (g), shoot biomass (g), total biomass (g), and root biomass (g; total biomass—shoot biomass) using an Acculab ALC-210.4 analytical balance (Acculab, NY, USA), and calculated the root-to-shoot biomass ratio (RSR) and total phyllode water content (%) using the formula [(total phyllode wet weight–total phyllode biomass)/total phyllode wet weight] × 100. All wet weight/biomass measurements were converted to mg for statistical analyses.
Statistical analyses
All statistical analyses were performed in the R statistical environment (R Core Team 2016). To assess if seed mass was significantly different between subspecies of A. longifolia in Australia and invasive populations, we used a Kruskal–Wallis test followed by a pairwise Mann–Whitney U Post-Hoc test (n = 150 seeds per range, 50 seeds per provenance), as our data failed the assumptions of normality and homoscedasticity of variances.
We calculated a Pearson correlation matrix for all trait/growth measurements (Hmisc R package; Harrell Jr. and Dupont 2023) to eliminate highly correlated variables from the dataset. Using a threshold of r = 0.8, we selected five variables for further analyses: total biomass, root length, number of phyllodes, total phyllode water content, and RSR (Supplementary Information, Fig. S3, but see also Fig. S4A). To explore whether the two subspecies of A. longifolia have different responses to water and nutrient availability, we performed a Principal Components Analysis (PCA) using the selected traits (FactoMineR and factoextra R packages, Kassambara and Mundt 2020; Husson et al. 2023). Since both seed mass and the PCA revealed no significant differences between subspecies (see Results), data from the native range were pooled for further analyses.
For the five selected traits, we determined the distribution model that best fit each dataset (e.g., log-normal, Poisson, gamma), as these data were not normally distributed. For total biomass, root length, and RSR we selected a gamma distribution, while for the number of phyllodes we selected a Poisson distribution, and for total phyllode water content we selected a log-normal distribution. We then fitted a generalised linear model (GLM; lme4 R package, Bates et al. 2023) using each trait as response and treatment, range, and their interaction as fixed terms, followed by a Type III Likelihood Ratio test (ANOVA type III; car R package, Fox et al. 2023) and a post-hoc test via computation of estimated marginal means (EMMs; emmeans R package; Lenth et al. 2023) and a p-value Šidák correction for multiple comparisons only for significant terms. We also fitted a GLM for percentage of plant survival using the same procedure (gamma distribution).
Results
Differences between Acacia longifolia subspecies
Seed mass did not differ significantly between native A. longifolia subsp. longifolia and A. l. subsp. sophorae (Fig. 1). However, seeds from the invaded range were significantly heavier than seeds from the native range (Fig. 1; Kruskal–Wallis test χ2 = 71.500, df = 2, p < 0.001). Mean seed mass was 14.79 mg (± 3.104 SD) for subsp. longifolia, 14.64 mg (± 4.880 SD) for subsp. sophorae, and 18.46 mg (± 4.214 SD) for invasive range seeds.
PCA analysis based on the five selected traits revealed no obvious grouping by subspecies (Fig. 2). The selected traits showed low multicollinearity (Supplementary Information, Fig. S4A), and all data variability was explained by five dimensions, with the first two dimensions contributing to 46.0 and 22.2% of the observed variation (Supplementary Information, Fig. S4B). Considering these results, data from the native range of both subspecies were pooled for further analyses.
Seedling responses to water and nutrient availability
Seedling responses differed significantly among water and nutrient treatments, range (native vs. invasive), and their interaction (Table 2). The exceptions were: phyllode water content, which had no significant differences among ranges, yet the interaction between range and treatment did show significant differences for this trait; and RSR and percentage of plant survival, which were only significantly impacted by water and nutrient treatments (Table 2). Bar plots of mean values of each trait, grouped by treatment and range, and the results of the post-hoc tests are shown in Fig. 3. See Supplementary Information, Table S2 for trait means and standard error (SE).
Bar plots showing the mean values of a total biomass, b root length, c number of phyllodes, d phyllode water content, and e root:shoot ratio (RSR) of A. longifolia, in response to water and nutrient availability treatments. W +/- - well-watered/drought conditions; N +/- - Nutrient supplementation/no supplementation. Error bars represent standard error. Letters show significant differences identified by a GLM followed by an ANOVA type III analysis and a post-hoc test via computation of EEMs. For RSR, because only treatment showed to have an effect, post-hoc analysis was averaged by range for each treatment
Overall, the total biomass of seedlings decreased significantly in response to increased water and nutrient stress conditions (Fig. 3a). The total biomass of seedlings under the W + N + treatment was higher than that of seedlings under all other treatment combinations, irrespective of range. Conversely, total biomass of invasive seedlings under the W–N− treatment was significantly lower than that of seedlings under other treatment combinations (p < 0.001 for all comparisons), while native seedlings under this treatment did not significantly differ in biomass from that of seedlings under intermediate treatments (i.e., W + N− and W–N +). Seedlings under intermediate treatments produced similar amounts of total biomass, irrespective of range.
Within ranges, invasive seedlings had significantly higher biomass under the W + N + treatment and significantly lower biomass under the W–N− treatment (p < 0.001) when compared with seedlings under other treatments (i.e., W + N− and W–N +), which resulted into similar and intermediate levels of biomass. Native seedlings, however, had similar biomass under well-watered conditions (W +) regardless of the addition of nutrients, that was significantly higher than the biomass produced by seedlings under water stress conditions (W−), also regardless of additional nutrients (p < 0.001 for all W + /W− treatment comparisons).
In general, the root lengths of seedlings under W + treatments were significantly higher than that of seedlings under W− treatments (except for invasive W–N + seedlings), but no significant differences among ranges were found (Fig. 3b). Conversely, several differences were found within each range. Invasive seedlings had significantly longer roots under the W + N + treatment than under the W–N− treatment (p < 0.001), yet, similar and intermediate root lengths under intermediate treatments. Water stress also significantly impacted the root length of invasive seedlings (p < 0.01 for all W + /W− treatment comparisons except the W–N + /W + N− comparison, which was non-significant), regardless of nutrition. For native seedlings, seedlings under both W + treatments (i.e., W + N + and W + N−) had significantly longer roots than seedlings under both W− treatments (i.e., W–N + and W–N−, p < 0.01 for all comparisons).
In contrast with other traits, there was no identifiable trend for the number of phyllodes produced with increased stress conditions, and there were no significant differences in the number of phyllodes produced among ranges under any treatment (Fig. 3c). However, when comparing seedlings within each range, invasive seedlings produced a significantly lower number of phyllodes under the W–N− treatment than under the W + N + treatment (p < 0.001), and a similar number of phyllodes under intermediate treatments. Curiously, for native seedlings, those under the W–N + treatment had less phyllodes compared to all other treatments and seedlings under the W + N + treatment had more phyllodes, while seedlings from the W + N− and W–N− had similar, and intermediate, numbers of phyllodes, indicating a combination effect of water and nutrition on the number of phyllodes formed by native seedlings.
Regarding phyllode water content, it is important to point out that the variation in this trait was very low compared to the other traits analysed, both among ranges and among treatments (Fig. 3d). Despite this, and contrary to other traits, there was an overall increase in phyllode water content as stress conditions increased, while no significant differences were found among ranges under any treatment. Within ranges, phyllodes of both invasive and native seedlings had lower water content under the W + N + treatment compared to all other treatments. Additionally, for seedlings from the native range, phyllode water content was higher under the W–N + treatment compared to other treatments, highlighting the effects of water stress.
Post-hoc analysis of RSR was averaged over ranges, as only treatment significantly affected this trait (Fig. 3e; Table 2). Seedlings grown under nutrient supplementation (N +) had significantly lower RSR when compared with seedlings under treatments without nutrient supplementation (N−, p < 0.001 for all comparisons), indicating that the latter tend to invest more into roots than aboveground biomass.
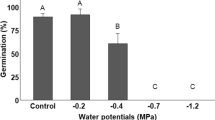
Lastly, we analysed percentage survival, which was also averaged over ranges since these did not have a significant effect on plant survival (Fig. 4). As expected, seedlings under the highest stress conditions (i.e., W–N− treatment) had significantly lower survival than those under all other treatments (p < 0.05 for all comparisons).
Box plots showing the comparison of A. longifolia seedling survival (%) among treatments. Because only treatment showed to have an effect, post-hoc analysis was averaged by range for each treatment. Letters show significant differences identified by a GLM followed by an ANOVA type III analysis and a post-hoc test via computation of EEMs. W +/- – Well-watered/drought conditions; N +/- – Nutrient supplementation/no supplementation
Discussion
In this study, we assessed seedling growth responses and survival to water and nutrient availability of one of the most problematic plant invaders in Mediterranean areas of the world, Acacia longifolia (Kull et al. 2011; Richardson et al. 2011), by comparing the performance of seedlings from native and invasive ranges. The inclusion of seedlings from the two described A. longifolia subspecies also enabled us to determine whether stress responses differ between them. Contrary to our initial hypothesis, our results revealed that invasive seedlings have limited capacity for stress resistance compared to their native counterparts, probably related to lower drought tolerance, highlighting that the speed of invasion by A. longifolia is likely dependent on local conditions. In the case of Portugal, this means that the drier parts in the south of the country may be less likely to be invaded or, if A. longifolia is introduced into these areas, its invasiveness may be lower. However, it is important to note that while seeds from the invasive range used in this study were collected in the field from soil seed banks, the seeds from the native range were obtained from nurseries, and information on how long ago these seeds were collected, from how many different individuals they originated, their genetic relationships (e.g., sibling trees), or their exact geographic origins, are unknown. These nursery seeds may have also been collected from fully mature pods of the best performing plants, while seeds collected from soil seedbanks in Portugal be of any quality and age (e.g., seeds may have remained dormant in the soil for multiple years). Further, we have not performed any tests to identify potential differences in germination and the effects of scarification in nursery vs. soil seed bank origins. Nevertheless, we found the mean germination time and germinability of seeds to be unrelated to their origin (Supplementary Information, Fig. S1). We found support for our second hypothesis, by showing that both A. longifolia subspecies responded similarly to different conditions of water and nutrient availability, which agrees with other studies suggesting that the species’ intraspecific taxonomic subdivision requires revision (see Vicente et al. 2023a, b).
We found that A. longifolia seeds from the invaded range were significantly heavier than those from the native range (also see Correia et al. 2015; 2016; Fig. 1). Like most other traits we measured, seed mass was also similar between the two subspecies of A. longifolia (Fig. 2). Thus, any discussion regarding range will focus only on native versus invasive range only and not subspecies identity.
We found stressful abiotic conditions (i.e., water stress without nutrient supplementation) to significantly reduce plant survival (Fig. 4), as well as the production of total biomass, root length, and the number of phyllodes (Fig. 3a, b, c), while intermediate stress conditions (i.e., W + N− and W–N +) had less severe effects on these traits compared to the low stress conditions (W + N + treatment). Moreover, water stress (W−) seemed to impact seedling growth performances more negatively than nutrient stress, as was seen by the effects of the W + N− and W–N + treatments. This is in contrast with the results reported by Sampaio et al. (2023), which showed that water stress by itself had minor effects on invasive A. longifolia seedling development while its interaction with added nutrition had stronger effects, although these authors did note that the water stress regime applied in their experiment was mild. Conversely, in our study, water stress was the determinant factor when nutrients were added (N +) in some instances, as observed by its effects on the number of phyllodes and phyllode water content (Fig. 3c and d, respectively). This is likely due to the capacity for A. longifolia to successfully form symbioses with nitrogen-fixing rhizobia (e.g., Rodríguez-Echeverría et al. 2009; Jesus et al. 2020), making a lack of nutrients perhaps an easier barrier to overcome during early stages of development compared to water stress. Australian acacias are generally also well-adapted to nutrient-poor soils (Young and Young 2001; Morris et al. 2011). Several studies have also found that A. longifolia has been co-introduced from Australia with its compatible rhizobia into countries such as New Zealand, Portugal, and South Africa (Rodríguez-Echeverría 2010; Crisóstomo et al. 2013; Warrington et al. 2019; Le Roux et al. 2021), which likely contributes to the success of this species in nutrient-poor soils, such as coastal dune systems. Given the short duration of our experiment, we did not quantify nodulation. Similar to our findings, Morais and Freitas (2012) found A. longifolia seedlings to persist under moderate water stress conditions, regardless of seed origin within the invasive range (wet or dry regions of Portugal), but that prolonged water stress severely limited seedling development. We found seedling growth responses to be dependent on range, with native seedlings showing higher tolerance to drought than invasive seedlings. Interestingly, it is generally thought that larger seeds supply more provisions during early seedling life compared to smaller seeds (e.g., Westoby et al. 2002), resulting in higher tolerance to abiotic stresses. Thus, it is reasonable to expect that invasive A. longifolia seedlings have an advantage over native seedlings under abiotic stress conditions since they originate from heavier seeds (Fig. 1). However, our results show that this is not the case for water and nutrient stress. One possible explanation for this result may be the effects of seed imprinting that result in different resource allocation strategies (Skálová et al. 2012). That is, invasive seedlings might have inherited maternal allocation strategies favouring traits such as early growth and development (Matzek 2011; 2012) instead of drought resistance. Whether such imprinting benefits successful colonisation of new environments deserves further research attention. Similarly, Dlugosch et al. (2015) found that invasive Centaurea solstitialis was less drought tolerant than its native-range counterparts, and that this tolerance was strongly negatively correlated with plant size. These authors argued that invasive C. solstitialis evolved strategies for better resource (i.e., water) exploitation, rather than resource allocation, which is a possible explanation for our observations in A. longifolia.
The trait most affected by stress conditions in our experiment was the amount of biomass produced by A. longifolia seedlings, which was drastically reduced under the most stressful conditions in invasive seedlings, supporting the idea that invasive seedlings have lower drought tolerance than native seedlings. Similarly, Harris et al. (2012) found invasive A. longifolia seedlings in Australia to produce more biomass than native range seedlings. However, our results also indicated that this is the opposite under stress conditions, i.e., invasive seedlings produce significantly lower total biomass compared to native seedlings (Fig. 3a). This result suggests, from a plant economics spectrum perspective, that invasive seedlings might have better strategies to utilise resources when these are available, thus displaying faster growth under favourable conditions (Montesinos 2022). On the other hand, under increased stress conditions, we found both phyllode water content and RSR to increase. The former was significantly lower under high water and nutrient availability (W + N +) in invasive seedlings (Fig. 3d), while RSR was significantly higher in the absence of nutrient supplementation (N−), regardless of water availability (Fig. 3e). We suggest that the general increase in phyllode water content under stress conditions is the result of higher water allocation into phyllodes to sustain their effective functioning, such as photosynthesis and evapotranspiration regulation. Regarding RSR, as expected, seedlings under treatments with nutrient supplementation (N +) had higher RSR in comparison with seedlings under nutrient stress (N−), irrespective of water availability. While differences in shoot biomass also influence RSR, it is more likely that this result is related to differences in root biomass; under nutrient and water stress (W–N−), seedlings invested more resources into roots in order to maximise their access to potential soil water, while seedlings under well-watered and nutrient stress conditions (W + N−) invested more resources into roots in order to start the nodulation process and compensate for the lack of nutrients, a known strategy of A. longifolia (e.g., see Rodríguez-Echeverría et al. 2009; Ulm et al. 2017b). In fact, studies have found Acacia roots to be more plastic in response to nutrient availability compared to other species (Morris et al. 2011), and the RSR of A. longifolia under low nutrient concentrations can be double that of native Mediterranean dune species (Peperkorn et al. 2005). Here, we observed the same pattern at the intra-specific level, although the difference in RSR was not as severe. Interestingly, a previous study showed that seedlings of invasive species tend to have faster root growth compared to seedlings of native species, leading to competitive superiority (Ni et al. 2018). However, this was not observed at the intra-specific level of this study (W + N + treatment), either for RSR or root length measurements.
In conclusion, our data suggest that native range A. longifolia seedlings have higher drought tolerance than invasive seedlings, which might limit the spread of invasive populations in Portugal into drier areas in the country. This result is also relevant in the context of ongoing climate change, as it indicates that some environments that are currently invaded by the species might become unsuitable in the future while others might become more suitable (see Vicente et al. 2020 for an example in South America). We also found no differences in stress responses among the described native subspecies of A. longifolia, an important result for the implementation of efficient management strategies. In addition, our results also hint at a possible role of seed “imprinting” in the early growth responses of A. longifolia to water and nutrient availability. That is, from a plant economics spectrum perspective, invasive populations may invest more resources into seeds (i.e., quality) than native-range populations, and this translates into seedling performance. Further studies are required to understand the differences between these responses at the intraspecific level, and how these relate to different environmental conditions and the species’ invasive capacity.
Data Availability Statement
Data originated in this study are available from the authors upon request.
References
Bai F, Settles AM (2015) Imprinting in plants as a mechanism to generate seed phenotypic diversity. Front Plant Sci 5:780. https://doi.org/10.3389/fpls.2014.00780
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G, Green P, Fox J, Bauer A, Krivitsky PN (2023) lme4: linear mixed-effects models using “Eigen” and S4. https://CRAN.R-project.org/package=lme4 Accessed 09 June 2023
Botella C, Marchante H, Celesti-Grapow L, Brundu G, Geerts S, Ramirez-Albores JE, Gonzáles-Moreno P, Ritter M, Richardson DM (2023) The global distribution of Acacia. In: Richardson DM, Le Roux JJ, Marchante E (eds) Wattles: Australian Acacia species around the world. CABI, UK, pp 131–147
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. In: Caspari EW, Thoday JM (eds) Advances in genetics. Academic Press, Cambridge, pp 115–155
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40. https://doi.org/10.1111/j.1472-4642.2008.00521.x
Correia M, Castro S, Ferrero V, Crisóstomo JA, Rodríguez-Echeverría S (2014) Reproductive biology and success of invasive Australian acacias in Portugal. Bot J Linn Soc 174:574–588. https://doi.org/10.1111/boj.12155
Correia M, Castro S, Rodríguez-Echeverría S (2015) Reproductive success of Acacia longifolia (Fabaceae, Mimosoideae) in native and invasive populations. Aust J Bot 63:387–391. https://doi.org/10.1071/BT14318
Correia M, Montesinos D, French K, Rodríguez-Echeverría S (2016) Evidence for enemy release and increased seed production and size for two invasive Australian acacias. J Ecol 104:1391–1399. https://doi.org/10.1111/1365-2745.12612
Crisóstomo JA, Rodríguez-Echeverría S, Freitas H (2013) Co-introduction of exotic rhizobia to the rhizosphere of the invasive legume Acacia saligna, an intercontinental study. Appl Soil Ecol 64:118–126. https://doi.org/10.1016/j.apsoil.2012.10.005
de Jesus JG, Tenreiro R, Máguas C, Trindade H (2020) Acacia longifolia: a host of many guests even after fire. Diversity 12:250. https://doi.org/10.3390/d12060250
de Villemereuil P, Gaggiotti OE, Mouterde M, Till-Bottraud I (2016) Common Garden experiments in the genomic era: new perspectives and opportunities. Heredity 116:249–254. https://doi.org/10.1038/hdy.2015.93
Dlugosch KM, Alice Cang F, Barker BS, Andonian K, Swope SM, Rieseberg LH (2015) Evolution of invasiveness through increased resource use in a vacant niche. Nat Plants 1:1–5. https://doi.org/10.1038/nplants.2015.66
Enders M, Havemann F, Ruland F, Bernard-Verdier M, Catford JA, Gómez-Aparicio L, Haider S, Heger T, Kueffer C, Kühn I, Meyerson LA, Musseau C, Novoa A, Ricciardi A, Sagouis A, Schittko C, Strayer DL, Vilà M, Essl F, Hulme PE, van Kleunen M, Kumschick S, Lockwood JL, Mabey AL, McGeoch MA, Palma E, Pyšek P, Saul W-C, Yannelli FA, Jeschke JM (2020) A conceptual map of invasion biology: integrating hypotheses into a consensus network. Glob Ecol Biogeogr 29:978–991. https://doi.org/10.1111/geb.13082
Flora of Australia 11B (2001) Mimosaceae, Acacia part 2. ABRS/CSIRO Publishing, Melbourne
Fox J, Weisberg S, Price B, Adler D, Bates D, Baud-Bovy G, Bolker B, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S, Heiberger R, Krivitsky P, Laboissiere R, Maechler M, Monette G, Murdoch D, Nilsson H, Ogle D, Ripley B, Short T, Venables W, Walker S, Winsemius D, Zeileis A, R-Core (2023) car: Companion to applied regression. https://CRAN.R-project.org/package=lme4 Accessed 09 June 2023
Gibson MR, Richardson DM, Marchante E, Marchante H, Rodger JG, Stone GN, Byrne M, Fuentes-Ramírez A, George N, Harris C, Johnson SD, Roux JJL, Miller JT, Murphy DJ, Pauw A, Prescott MN, Wandrag EM, Wilson JRU (2011) Reproductive biology of Australian acacias: important mediator of invasiveness? Divers Distrib 17:911–933. https://doi.org/10.1111/j.1472-4642.2011.00808.x
Gioria M, Hulme PE, Richardson DM, Pyšek P (2023) Why are invasive plants successful? Annu Rev Plant Biol 74:635–670. https://doi.org/10.1146/annurev-arplant-070522-071021
Guo K, Pyšek P, Chytrý M, Divíšek J, Lososová Z, van Kleunen M, Pierce S, Guo W-Y (2022) Ruderals naturalize, competitors invade: varying roles of plant adaptive strategies along the invasion continuum. Funct Ecol 36:2469–2479. https://doi.org/10.1111/1365-2435.14145
Hamad-Sheip Y, Abdul-Hamid H, Abiri R, Saleh M-N, Mohamed J, Jalil A-M, Naji HR (2021) Effect of Acacia mangium canopy on physicochemical characteristics and nutrient concentrations of the soil at Ayer Hitam forest reserve. Malays For 12:1259. https://doi.org/10.3390/f12091259
Harrell Jr. FE, Dupont C (2023) Hmisc: Harrell miscellaneous. https://CRAN.R-project.org/package=Hmisc Accessed 09 June 2023
Harris CJ, Dormontt EE, Le Roux JJ, Lowe A, Leishman MR (2012) No consistent association between changes in genetic diversity and adaptive responses of Australian acacias in novel ranges. Evol Ecol 26:1345–1360. https://doi.org/10.1007/s10682-012-9570-6
Hoagland DR (1932) Mineral nutrition of plants. Annu Rev Biochem 1:618–636. https://doi.org/10.1146/annurev.bi.01.070132.003154
Husson F, Josse J, Le S, Mazet J (2023) FactoMineR: multivariate exploratory data analysis and data mining. https://CRAN.R-project.org/package=FactoMineR Accessed 09 June 2023
IPBES (2023) Summary for policymakers of the thematic assessment report on invasive alien species and their control of the intergovernmental science-policy platform on biodiversity and ecosystem services. Roy HE, Pauchard A, Stoett P, Renard Truong T, Bacher S, Galil BS, Hulme PE, Ikeda T, Sankaran KV, McGeoch MA, Meyerson LA, Nuñez MA, Ordonez A, Rahlao SJ, Schwindt E, Seebens H, Sheppard AW, Vandvik V (eds) IPBES secretariat, Bonn, Germany. https://doi.org/10.5281/zenodo.7430692
Kassambara A, Mundt F (2020) factoextra: Extract and visualize the results of multivariate data analyses. https://CRAN.R-project.org/package=factoextra Accessed 09 June 2023
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World Map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Kull CA, Shackleton CM, Cunningham PJ, Ducatillon C, Dufour-Dror J-M, Esler KJ, Friday JB, Gouveia AC, Griffin AR, Marchante E, Midgley SJ, Pauchard A, Rangan H, Richardson DM, Rinaudo T, Tassin J, Urgenson LS, von Maltitz GP, Zenni RD, Zylstra MJ (2011) Adoption, use and perception of Australian acacias around the world. Divers Distrib 17:822–836. https://doi.org/10.1111/j.1472-4642.2011.00783.x
Le Maitre DC, Gaertner M, Marchante E, Ens E-J, Holmes PM, Pauchard A, O’Farrell PJ, Rogers AM, Blanchard R, Blignaut J, Richardson DM (2011) Impacts of invasive Australian acacias: implications for management and restoration. Divers Distrib 17:1015–1029. https://doi.org/10.1111/j.1472-4642.2011.00816.x
Le Roux JJ, Crous PW, Kamutando CN, Richardson DM, Strasberg D, Wingfield MJ, Wright MG, Valverde A (2021) A core of rhizosphere bacterial taxa associates with two of the world’s most isolated plant congeners. Plant Soil 468:277–294. https://doi.org/10.1007/s11104-021-05049-x
Lenth RV, Bolker B, Buerkner P, Giné-Vázquez I, Herve M, Jung M, Love J, Miguez F, Riebl H, Singmann H (2023) emmeans: Estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans Accessed 09 June 2023
Lorenzo P, Rodríguez-Echeverría S (2015) Soil changes mediated by invasive Australian acacias. Ecosistemas 24:59–66. https://doi.org/10.7818/ECOS.2015.24-1.10
Lozano-Isla F, Benites-Alfaro OE, Pompelli MF (2019) GerminaR: an R package for germination analysis with the interactive web application “GerminaQuant for R.” Ecol Res 34:339–346. https://doi.org/10.1111/1440-1703.1275
Magona N, Richardson DM, Le Roux JJ, Kritzinger-Klopper S, Wilson JRU (2018) Even well-studied groups of alien species might be poorly inventoried: Australian Acacia species in South Africa as a case study. NeoBiota 39:1–29. https://doi.org/10.3897/neobiota.39.23135
Marchante H, Freitas H, Hoffmann JH (2010) Seed ecology of an invasive alien species, Acacia longifolia (Fabaceae), in Portuguese dune ecosystems. Am J Bot 97:1780–1790. https://doi.org/10.3732/ajb.1000091
Matzek V (2011) Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol Invasions 13:3005. https://doi.org/10.1007/s10530-011-9985-y
Matzek V (2012) Trait values, not trait plasticity, best explain invasive species’ performance in a changing environment. PLoS ONE 7:e48821. https://doi.org/10.1371/journal.pone.0048821
Montesinos D (2022) Fast invasives fastly become faster: invasive plants align largely with the fast side of the plant economics spectrum. J Ecol 110:1010–1014. https://doi.org/10.1111/1365-2745.13616
Morais MC, Freitas H (2012) The acclimation potential of Acacia longifolia to water stress: implications for invasiveness. Plant Sci 196:77–84. https://doi.org/10.1016/j.plantsci.2012.08.007
Morais MC, Panuccio MR, Muscolo A, Freitas H (2012) Salt tolerance traits increase the invasive success of Acacia longifolia in Portuguese coastal dunes. Plant Physiol Biochem 55:60–65. https://doi.org/10.1016/j.plaphy.2012.03.013
Morris TL, Esler KJ, Barger NN, Jacobs SM, Cramer MD (2011) Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Divers Distrib 17:898–910. https://doi.org/10.1111/j.1472-4642.2011.00802.x
Mounger J, Ainouche ML, Bossdorf O, Cavé-Radet A, Li B, Parepa M, Salmon A, Yang J, Richards CL (2021) Epigenetics and the success of invasive plants. Phil Trans R Soc B 376:20200117. https://doi.org/10.1098/rstb.2020.0117
Ni M, Liu Y, Chu C, Xu H, Fang S (2018) Fast seedling root growth leads to competitive superiority of invasive plants. Biol Invasions 20:1821–1832. https://doi.org/10.1007/s10530-018-1664-9
Ortmans W, Mahy G, Monty A (2016) Effects of seed traits variation on seedling performance of the invasive weed, Ambrosia artemisiifolia L. Acta Oecol 71:39–46. https://doi.org/10.1016/j.actao.2016.01.008
Peperkorn R, Werner C, Beyschlag W, Peperkorn R, Werner C, Beyschlag W (2005) Phenotypic plasticity of an invasive acacia versus two native Mediterranean species. Funct Plant Biol 32:933–944. https://doi.org/10.1071/FP04197
Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM (2020) Scientists’ warning on invasive alien species. Biol Rev 95:1511–1534. https://doi.org/10.1111/brv.12627
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/ Accessed 09 June 2023
Rascher KG, Große-Stoltenberg A, Máguas C, Meira-Neto JAA, Werner C (2011) Acacia longifolia invasion impacts vegetation structure and regeneration dynamics in open dunes and pine forests. Biol Invasions 13:1099–1113. https://doi.org/10.1007/s10530-011-9949-2
Richards CL, Pennings SC, Donovan LA (2005) Habitat range and phenotypic variation in salt marsh plants. Plant Ecol 176:263–273. https://doi.org/10.1007/s11258-004-0841-3
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993
Richardson DM, Kluge RL (2008) Seed banks of invasive Australian Acacia species in South Africa: role in invasiveness and options for management. Perspect Plant Ecol Evolut Syst 10:161–177. https://doi.org/10.1016/j.ppees.2008.03.001
Richardson DM, Carruthers J, Hui C, Impson FAC, Miller JT, Robertson MP, Rouget M, Le Roux JJ, Wilson JRU (2011) Human-mediated introductions of Australian acacias: a global experiment in biogeography. Divers Distrib 17:771–787. https://doi.org/10.1111/j.1472-4642.2011.00824.x
Richardson DM, Le Roux JJ, Wilson JR (2015) Australian acacias as invasive species: lessons to be learnt from regions with long planting histories. South For 77:31–39. https://doi.org/10.2989/20702620.2014.999305
Richardson DM, Marchante E, Le Roux JJ (2023) Acacia species around the world: historical, social, evolutionary and ecological insights into one of the planet’s most widespread plant genera. In: Richardson DM, Le Roux JJ, Marchante E (eds) Wattles: Australian Acacia species around the world. CABI, UK, pp 1–26
Rodríguez-Echeverría S (2010) Rhizobial hitchhikers from down under: invasional meltdown in a plant–bacteria mutualism? J Biogeogr 37:1611–1622. https://doi.org/10.1111/j.1365-2699.2010.02284.x
Rodríguez-Echeverría S, Crisóstomo JA, Nabais C, Freitas H (2009) Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol Invasions 11:651–661. https://doi.org/10.1007/s10530-008-9280-8
Sampaio C, Vicente S, Antunes M, Máguas C, Trindade H, Sampaio C, Vicente S, Antunes M, Máguas C, Trindade H (2023) Soil origin impacts Acacia longifolia above and belowground development: water and nutrition as players. Soil Res 61:510–522. https://doi.org/10.1071/SR22109
Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Skálová H, Havlíčková V, Pyšek P (2012) Seedling traits, plasticity and local differentiation as strategies of invasive species of Impatiens in central Europe. Ann Bot 110:1429–1438. https://doi.org/10.1093/aob/mcr316
Souza-Alonso P, Rodríguez J, González L, Lorenzo P (2017) Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann For Sci 74:55. https://doi.org/10.1007/s13595-017-0651-0
Ulm F, Jacinto J, Cruz C, Máguas C (2017a) How to outgrow your native neighbour? Belowground changes under native shrubs at an early stage of invasion. Land Degrad Dev 28:2380–2388. https://doi.org/10.1002/ldr.2768
Ulm F, Hellmann C, Cruz C, Máguas C (2017b) N/P imbalance as a key driver for the invasion of oligotrophic dune systems by a woody legume. Oikos 126:231–240. https://doi.org/10.1111/oik.03810
Vicente S, Meira-Neto J, Trindade H, Máguas C (2020) The distribution of the invasive Acacia longifolia shows an expansion towards southern latitudes in South America. BioInvasions Records 9:723–729. https://doi.org/10.3391/bir.2020.9.4.06
Vicente S, Máguas C, Richardson DM, Trindade H, Wilson JRU, Le Roux JJ (2021) Highly diverse and highly successful: invasive Australian acacias have not experienced genetic bottlenecks globally. Ann Bot 128:149–157. https://doi.org/10.1093/aob/mcab053
Vicente S, Trindade H, Máguas C, Dickson CR, Pascoe PP, Le Roux JJ (2023a) Intraspecific genetic and ecological differentiation in Australian Acacia Species: insights from Acacia longifolia. In: Richardson DM, Le Roux JJ, Marchante E (eds) Wattles: Australian Acacia species around the world. CABI, UK, pp 103–117
Vicente S, Trindade H, Máguas C, Le Roux JJ (2023b) Genetic analyses reveal a complex introduction history of the globally invasive tree Acacia longifolia. NeoBiota 82:89–117. https://doi.org/10.3897/neobiota.82.87455
Warrington S, Ellis A, Novoa A, Wandrag EM, Hulme PE, Duncan RP, Valentine A, Le Roux JJ (2019) Cointroductions of Australian acacias and their rhizobial mutualists in the Southern Hemisphere. J Biogeogr 46:1519–1531. https://doi.org/10.1111/jbi.13602
Welgama A, Florentine S, Marchante H, Javaid MM, Turville C (2019) The germination success of Acacia longifolia subsp. longifolia (Fabaceae): a comparison between its native and exotic ranges. Aust J Bot 67:414–424. https://doi.org/10.1071/BT19018
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159. https://doi.org/10.1146/annurev.ecolsys.33.010802.150452
Whibley DJE, Symon DE (1992) Acacias of South Australia, 2nd edn. The Flora and Fauna of South Australia Handbooks Committee, Adelaide, South Australia, Australia
Xue B, Leibler S (2018) Benefits of phenotypic plasticity for population growth in varying environments. Proc Natl Acad Sci 115:12745–12750. https://doi.org/10.1073/pnas.1813447115
Yelenik SG, Stock WD, Richardson DM (2007) Functional group identity does not predict invader impacts: differential effects of nitrogen-fixing exotic plants on ecosystem function. Biol Invasions 9:117–125. https://doi.org/10.1007/s10530-006-0008-3
Young ARM, Young RW (2001) Soils in the Australian landscape. Oxford University Press, UK
Zhang H, Jiang Y, Song M, He J, Guan D (2020) Improving understanding of carbon stock characteristics of Eucalyptus and Acacia trees in southern China through litter layer and woody debris. Sci Rep 10:4735. https://doi.org/10.1038/s41598-020-61476-3
Acknowledgements
The authors thank Alexandra Machado, Cláudia Tavares, Joana Jesus, and Daniela Carvalho for their assistance in harvesting the seedlings in the glasshouse. We also thank Daniel Montesinos, Margaret Byrne and Luke Flory for their insightful comments and suggestions on an earlier draft of our manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on). This research was funded by Fundação para a Ciência e a Tecnologia (FCT, Portugal), FCT/MCTES, through the financial support to CESAM (UIDP/50017/2020, UIDB/50017/2020 and LA/P/0094/2020) and the financial support to cE3c (UIDB/00329/2020, https://doi.org/10.54499/UIDB/00329/2020). SV worked under the following scholarships: PD/BD/135536/2018 and COVID/BD/152524/2022 awarded by FCT, Portugal, and International Cotutelle Macquarie University Research Excellence Scholarship (iMQRES Tuition – Cotutelle & MQRES Stipend – Cotutelle). JLR received an Outside Studies Program (OSP) Fellowship from Macquarie University’s Faculty of Science and Engineering that supported part of his contribution to this work.
Author information
Authors and Affiliations
Contributions
SV, HT, JLR and CM were involved in the research conceptualisation, data interpretation, and in writing, reviewing, and editing the manuscript. SV and MC performed seed germination and glasshouse work. SV performed the laboratory work and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Additional information
Communicated by Stephen Bonser.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vicente, S., Condessa, M., Trindade, H. et al. Early development of Acacia longifolia is more severely impacted by water and nutrient stress in invasive than native seedlings. Plant Ecol 225, 629–640 (2024). https://doi.org/10.1007/s11258-024-01420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-024-01420-x