Abstract
Hedera helix L. is an evergreen vine native to Europe. Nowadays, it is commonly recognized as an expansive species, posing danger to biodiversity of natural ecosystems. Obtaining the relationships between morphological variability, productive parameters of vegetative individuals, and variations in environmental factors (on the example of the Kórnik Arboretum) is important for understanding the successful adaptation and dispersion of H. helix. Thus, the issue of our studies was to discover the link between environmental factors and morphometric measurements of H. helix. We collected data on leaf length, leaf width, leaf petiole length, plant stem length, and the number of leaves per stem. We assessed values of ecological plasticity and correlation between the studied parameters. The results indicated the tolerance of ivy to wide variation in edaphic conditions. The limiting environmental factors for growth of the studied subpopulations were soil moisture and light intensity. We found large variability of leaf morphometric parameters and shoot length. Close correlation links were detected between leaf length/leaf width, leaf length/leaf petiole length, and leaf width/leaf petiole length. The length of the average leaf and the length of leaf petiole were the most variable parameters under different values of light intensity. Weak relationships were found between light intensity at the soil surface and plant stem length/the number of leaves per shoot. A high level of index of morphological integration of H. helix individuals and a close relationship between the quality index/subpopulation compositions were established in this study. A direct relationship between light intensity and specific leaf area values was established. The value of specific leaf area decreased linearly with increasing light intensity at the soil surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hedera helix L. (English ivy, common ivy, European ivy) is affiliated with the family Araliaceae (Wen et al. 2001; Ackerfield and Wen 2003; Metcalfe 2005). Ivy can be found in different life forms: perennial evergreen woody vine or clambering plant, a green forage, a woody subshrub, or seldom a tree (Putz 1984; Grime et al. 1988; Metcalfe 2005). Ivy is a native European species in the Mediterranean and Atlantic regions. The distribution area of ivy in Europe also comprises the southern Scandinavia Peninsula, Balkan Peninsula, Estonia, Latvia, Lithuania, Belarus, and Ukraine. Hedera helix is nowadays extensively introduced into areas of the world with moderate climates (Green et al. 2011), having become naturalized in Australia, Brazil, Canada, Hawaii, New Zealand, India, South Africa and the continental USA (Rose 1996; Larocque 1999; Swearingen and Diedrich 2006; Strelau et al. 2018). Ivy covers many aboveground natural ecosystems. Competitive displacement of native species is increasingly detected in natural ecosystems (Clements and Ditommaso 2011; Strelau et al. 2018). Moreover, its invasion of woodlands and forests is associated with natural or anthropogenic disturbance, as well as forest plantations (Schnitzler and Heuzé 2006). It is particularly expansive in temperate deciduous forests, where it can take advantage of the extra light in the cold season (Okerman 2000).
The eastern part of Poland lies at the European eastern border of the H. helix range. The density of ivy’s habitats within Poland decreases from the western part to the eastern and northeastern parts (Boratyńska 1987; Zając and Zając 2001; Koziarz 2015). European ivy is an ecotone species, occurring in fringe communities, in deciduous and mixed deciduous forests in fresh and moist habitats, occupied by oak-hornbeam and riparian alder-ash forests in Central Poland (Koziarz 2015). In Poland, the specimens of ivy were subjected to legal protection in 1946–2014 (Romuald 1979; Regulation of the Minister of the Environment 2014). Since the mid-twentieth century on the territory of Poland, the common ivy has become an expansive species with an increasing number of locations where reproductive individuals are commonly encountered (Kucharski et al. 2019). Owing to its clear range limits (both altitudinal and northeastern) and population characteristics, ivy has long been considered a species particularly suited to monitoring the shift of forest types as a result of climate change (Walther 2002).
Juvenile and adult individuals of ivy have differentiating phenotypic properties (Bunk et al. 2019). The juvenile period lasts 15 years or more (Reichard and Hamilton 1997). Phenotypically, juvenile leaves are shade leaves and adult leaves are sun leaves. The most commonly recognized form of H. helix is the juvenile, with its palmately lobed leaves (3–5 lobes) that are dark green and glossy with whitish veins (Okerman 2000; Metcalfe 2005). The juvenile form cannot produce seeds, but it can reproduce vegetatively and it is typically this growth phase that produces adventitious roots (Okerman 2000). During the juvenile or non-reproductive stage, H. helix is typically an aboveground cover growing to a height of 50 cm (Metcalfe 2005). The leaves of the adult or generative individual are mainly a lighter green, thick, ovate, or rhombic in shape and have imperceptible whitish veins. Reproductive individuals of H. helix produce terminal fascicles of greenish-white flowers in the fall, which are pollinated by insects. The following spring common ivy produces a dark purple, fleshy drupe (fruit) (Metcalfe 2005). Generative organs of the ivy appear on the shoots climbing the trunks of trees only when the diameter of the ivy shoot exceeds 1 cm at the height of 1.3 m aboveground (Green et al. 2011). The differential replication of DNA is a natural switching phase in morphogenesis from non-reproductive to reproductive stages. Reversal may also occur biologically in non-intensive light and high temperatures and adult individuals may revert to the juvenile stage of ivy development (Ackerfield and Wen 2003; Rogler and Hackett 2006).
Ivy has a wide potential ecological niche under edaphic factors. H. helix is a plant associated with warm growing conditions (temperature environmental parameter) (Ellenberg 1998). Ivy is favored by fertile soils ranging from rather dry to slightly damp. Common ivy is quite abundant on clay-rich soils and occurs less often on poor and well-drained sandy soils (Thomas 1998; Metcalfe 2005).
The limiting environmental factors for ivy are moisture of soil and soil shade (Ellenberg 1998; Koziarz 2015). Ivy is semi-shade plant (Ellenberg 1998; Sack et al. 2003). Several studies (Hoflacher and Bauer 2006; Sack and Grubb 2002; Schnitzler and Heuze 2006; Stafne et al. 2005) show that English ivy develops over a wide light range, from full shade to full sunlight. Ivy seedling density was closely correlated with soil moisture in shaded conditions (Kollmann and Grubb 1999). Shade plays a protective role. The physiological adaptation is manifest in reducing exhaling demand and photosynthetic activity (Sack and Grubb 2001; Sack 2004). Ivy has a relatively low light compensation point in comparison with other scramblers (Carter and Teramura 1988). Vegetative leaves have lower photosynthetic capacity and have limited acclimation to high light fluxes (Bauer and Thöni 1988; Hoflacher and Bauer 2006). A differential characteristic of adult leaves and juvenile leaves from the same individual is different photosynthetic capacity (Bauer and Bauer 1980). Leaves show limited acclimation to moderately intensive light of soil during leaf development or even on exposure to higher light after full development (Bauer and Thöni 1988). Sun leaves which are exposed to a significant increase in ambient light in autumn show reduced net photosynthesis (Oberhuber and Bauer 1991). Ivy exhibits moderate morphological plasticity of leaves in deep shade with higher specific leaf areas (SLAs) (Sack and Grubb 2002). Leaves absorb some energy by sharp increases in both reflectance and transmittance. Light quality has impacts on vitality and growth of H. helix (Sack and Grubb 2002). Although the above-mentioned studies deal with the ecological properties of vegetative ivy, there are no studies which examine the variation of leaf and shoot traits in different soil, light, and moisture conditions. Therefore, the issue of the link between environmental factors and the vegetative growth phase of H. helix in connection with its expansion and wide ecological amplitude is relevant. Considering the results of the previous studies we hypothesized that (1) moisture and soil shade are limiting environmental factors for growth of vegetative subpopulations of ivy, (2) soil shade has the biggest impact on variability and plasticity of morphometric leaf traits, and (3) vitality of H. helix subpopulations are the best in semi-shaded conditions. The current study is aimed at assessing morphology-, vitality-, and productivity-related spectrums of the vegetative stage of H. helix in subpopulation loci and their dependence on changes in environmental factors.
Material and methods
Study site
The research was conducted during July–August 2022 on the territory of the Kórnik Arboretum (western Poland; 52.2448°N, 17.09698°E, 75 m a.s.l.; Fig. Sl). The study was carried out at the same hours of the day from 10 a.m. to 12 p.m. Eleven experimental plots 50 × 50 m (EP) in different environmental conditions and dominant soil types of the Arboretum were laid out to examine phytocenotic characteristics (Table 1). We identified the species in the field, and all identifications were verified in the laboratory. Analysis was carried out at the level of loci (clusters) of subpopulations of H. helix (Table S1).
The number of ivy individuals in the vegetative stage of growth has recently increased in the Arboretum. Individuals of the initial stages of ontogeny, as well as individuals of immature and virginal stages of the generative phase were not found in the experimental plots at the time of the study. Studied loci of subpopulations of H. helix were incomplete. A predominance of juvenile individuals of this vine species was established. H. helix was a sub-edificator of the herbaceous layer and formed fixed synusia on EPs. The studied subpopulations were not inferior in their position in the artificial phytocenoses (by projected coverage) regardless of the inferiority spectrum of the ontogenic development. The horizontal composition of subpopulations was accidental. Vegetative reproduction was detected.
Data analysis
Phytoindicative analysis
The phytoindication method was used to determine the rates of the main ecological factors (Didukh 2012). The parameters of edaphic factors were determined using unified scales of environmental amplitudes. The effects of edaphic factors were analyzed: Hd—soil moisture, fH—variability of soil moisture, Rc—soil acidity, Tr—soil salinity, Sl—general salt regime, Nt—available soil nitrogen, Ae—soil aeration, Lc—soil shade (light at the soil surface). The average score of the environmental gradient was determined for all species on EPs. The means of indicator values were calculated as weighted means based on species cover within six subplots on each EP. To collect the geobotanical data, six subplots 5 × 5 m (25 m2) were arranged within each EP (1–11).
Vitality composition and morphometric parameters
We measured only a representative subset of vegetative individuals. Seedlings were excluded from our study. For our analysis, the density and vitality composition of subpopulations were established. Subpopulation density was calculated on 1 m2 plots (fourfold replication on EP). A sample size of 20–25 vegetative individuals was selected from each subpopulation. The harvested ivy from EP was separated into dry and fresh and only the fresh vegetative shoots were applied for biometric measurements in this study.
Vitality analysis was performed to assess the state of the individuals using a one-dimensional approach according to standard methods (Zlobin 2009). Vitality state of individuals of H. helix was determined by morphometric parameters: stem height (Ls), leaf petiole length (Lp), leaf length (Ll) and width (Wl), and number of lobes per leaf (Nl) of average formation. Stem height was measured from the soil surface to the top leaf with a measuring tape. Green leaves of each individual of H. helix were counted. Morphometric measurements of the leaves (Lp, Ll, Wl, Nl) in 20 replicates for each shoot were carried out. The ecological plasticity of a leaf morphological parameter was calculated for each individual as the difference between the maximal value and the minimal value of that parameter divided by the minimum of that parameter ([max–min]/min). The coefficient of variance was used to estimate the variability of the characteristics (CV, %). The following limits were established to identify the degree of variability of characteristics according to Pelabon et al. (2020).
The quality index of subpopulation places or sides or niches (Q) was determined as follows:
where a is the number of individuals in the first vitality class and b is the number of individuals in the middle vitality class. The type is considered prosperous when Q > c, equilibrium when Q ≈ c, and depressed when Q < c.
To assess the morphological integrity of plants, we used the morphological integrity index according to Zlobin (2009):
where IMI is the index of morphological integrity; B is the number of statistically significant correlation coefficients (p-value 0.95) in the matrix; and n is the total number of estimated morphometric parameters.
Productivity parameters
Photosynthetic Active Radiation (PAR) was measured by Quantitherm PAR (Hansatech). Light intensity measurement was recorded at a height of 10 cm above climbing individuals of H. helix on each subplot (2.2.1). DLI (daily light integral) refers to the total amount of PAR expressed in units of moles per square meter per day (mol × m−2 × d−1). Individuals of H. helix were divided into components (leaves, stems) and then dried in an oven with forced air circulation at 65 °C to a constant biomass. All the components were weighed.
Specific leaf area (SLA) is an important morphological parameter with great ecological significance as it strongly correlates with other morphological plant traits. SLA also responds to environmental changes. Specific leaf area (SLA) was calculated as follows:
where LA is the leaf area (cm2); W is the leaf dry weight (g).
LA was calculated by non-destructive method, based on the dimensions of the leaves (Ll, Wl) according to Rosu and Sala (2022):
where Ll is the leaf length (cm); Wl is the leaf width (cm); CF is the specific correction factor for ivy leaves. The optimal value of the correction factor (CF) specific to ivy leaves was used according to the proposed model by Sala et al. (2015).
Statistical analysis
Descriptive statistical analysis was used for the general analysis of the obtained field data and the phytoindicative parameter values (min, max, average, standard deviation, standard error). Interdependence relationships between morphological leaf parameters, ecological plasticity of measured traces, and DLI were quantified by correlation analysis (Pearson), for which statistical certainty was also determined (p < 0.05; p < 0.01, p < 0.001). The variability within the data series was quantified for each morphometric parameter (coefficient of variation, CV). Linear regression analysis was used to describe the fit relationships between Ll, Wl, and DLI, as well as other interdependence relationships between determined parameters and for safety, p and R2 parameters, and the F test, were considered. The interdependence relationships among phytoindicative parameter values of edaphic factors, SLA, Q, and DLI were considered by multivariate analysis (Principal Component Analysis). For analysis and statistical processing of experimental data, mathematical calculations were based on OriginPro 2022 software.
Results
Phytoindicative analysis
Phytoindicative assessment of the soil conditions on Eps was revealed by low/high ranges in the parameter values. Mesophytic conditions (by evaluation of Hd, fH) were predominant in the investigated area (Table 1). Water productivity of soil at the level of 100–145 mm was detected according to phytoindicative data. Index values of variability of soil moisture (fH) were 4.9–5.7. The documented edaphic conditions, which were favorable for plants of fresh forest-meadow ecotopes, had moderately uneven moisture of the soil root layer due to complete wetting by precipitation and melt water (coefficient of unevenness of moisture ω = 0.17–0.23 mm). The level of soil acidity (RC) on Eps was within the range of 7.1–7.9 (estimated as equivalent to pH 6.5–7.1), indicating that soil conditions were favorable for neutrophytes and sub-acidophilous plants. This was confirmed by the analysis of the species composition of the herbaceous layer on the Eps. Analysis of the salt regime (SL) ranged from 5.8 to 7.5. Such salt conditions were suitable for development of eutrophic plants, which grew on soils rich in salt (150–200 mg × l−1). The assessment of the nitrate soil regime was confirmed by dominance of nitrophilous plants on all Eps (soils with relatively high supplies of mineral nitrogen). The value of NT ranged from 5.9 to 7.0. The higher values of NT were characteristic for Eps located on soils of anthropogenic origin. The evaluation of soil aeration (AE) showed that the conditions were suitable for growth of plants on dry loamy or wet sandy soils on all Eps. The values of soil shade (Lc) had more variation in comparison with other environmental factors (3.5–7.8 score). The obtained values were confirmed by the species which dominated the herbaceous layers on the EPs. Soils of anthropogenic genesis were characterized by more variation in the numerical score of phytoindicative scales (Table 1). Principally, the obtained phytoindicative data on the Arboretum territory coincided with the main characteristics of the indicated soil types.
Morphological variability of diagnostic traces (parameters) of vegetative individuals of H. helix
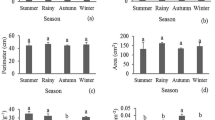
Large ranges of the morphometric parameters of vegetative individuals of ivy were found. The width of the leaf of H. helix was varied from min 0.97 ± 0.43 cm (EP4) to max 12.34 ± 0.95 cm (EP1) among the studied EPs (Fig. 1). At the same time, the average value of the leaf width varied from 3.31 to 6.39 cm. The maximum value of leaf length was recorded on EP3, EP6, and EP9. The average value of leaf length varied from 2.86 to 5.31 cm. The maximum values of length of the leaf petiole were recorded on EP1 and EP2, and the minimum values were found on EP10 and EP4. It should be noted that the average value of this parameter varied from 6.41 to 9.08 cm. The morphometric parameters with the highest variability among EPs were maximum length of the petiole and maximum leaf width. The most stable of parameters measured of vegetative individuals of H. helix was leaf length.
The ecological plasticity of the measured morphological leaf parameters significantly varied. The ecological plasticity of the leaf petiole (ANOVA, F = 30.02, SS = 35.41, MS = 3.54, p < 0.0001) had the highest variability (Fig. 2a), corresponding to a high class of ecological plasticity, while the width (ANOVA, SS = 13.06, MS = 1.31, F = 12.82, p < 0.0001, Fig. 2c) and length (ANOVA, SS = 10.54, MS = 1.05, F = 11.17, p < 0.0001, Fig. 2b) of the leaf had an average value of ecological plasticity. The ecological plasticity of length of leaf petiole of H. helix were largest for Lp5 and Lp10. The length of leaf per plant reached its maximum for Ll2, Ll3, Ll6, Ll7, and Ll10 and minimum for Ll5 (Fig. 2b). The width of the leaf tended to be higher for Wl4–Wl6 than for the other treatments (Fig. 2c). The coefficient of variation for Wl ranged from 19.6% (EP9) to 36.3% (EP3), for Ll from 23.6% (EP4) to 29.1% (EP3), and for Lp 28.3% (EP7) to 42.3% (EP2). All investigated morphometric leaf parameters (according to the values of the variation coefficients) were assigned to the high class of variability.
The average length of juvenile shoots and the number of leaves located on them were also analyzed. The specified parameters had a wide range of variability among EPs. In particular, the maximum value of shoot length occurred on EP2 (281.30 ± 14.07 cm) and the minimum value (130.38 ± 6.51 cm) on EP4. The coefficient of variation of shoot length was 14.7–31.1% (high class). The average length of the juvenile shoot was 213.15 ± 10.66 cm (SD = 42.40). The number of leaves on a juvenile shoot ranged from 28 (EP10) to 74 (EP2). The greatest number of leaves (74) was identified on juvenile shoots with the longest length on EP2. The minimum number of leaves was detected on a juvenile shoot with a length of 248.24 cm (EP10). The average number of leaves on a juvenile shoot of H. helix was 50 (SD = 15.61). The number of lobes per leaf of individual H. helix plants was 3–5 on all EPs.
A close correlation link was observed betwixt the traits of leaves: leaf length and leaf width (0.91), leaf length and leaf petiole length (0.87), and leaf width and leaf petiole length (0.80). The weaker correlation link was detected between the shoot height and the leaf petiole length (0.76). The link between the number of leaves and their lobes was only 0.18. No more correlations were recorded between the specified quantitative traces at this level of reliability.
Subpopulation composition of H. helix as a function of vitality analysis
At the subpopulation level, most of the loci studied (according to vitality analysis) belong to the prosperous type, while two loci were found to be equilibrial (EP2 and EP10) and one locus was of the depressive subpopulation type (EP 4) (Table 2). The highest values of the quality index (Q) had the loci EP3 and EP6 subpopulations and they were characterized by high abundance rates of individuals compared to other EPs of the Arboretum. It is worth mentioning that the share of c-class individuals was only 3% on EP3. Individuals of EP4 were characterized by the lowest values of the quality index (0.265) and the highest share of c-class individuals. IMI values ranged from 0.61 for the locus on EP4 to 0.78 for the locus on EP3, indicating a generally high level of morphological integration of H. helix individuals.
DLI, SLA, and subpopulation composition
The conducted phytoindicative analysis indicated that the main limiting environmental factor of growth and dispersion of vegetative individuals of H. helix was soil shade. It was important to identify the dependences between light intensity and SLA. SLA is one of the objective components of the analysis of the environmental condition and plant growth, and it is mathematically scaled positively and linearly relative to the growth rate of the plant (Fig. 3). The values of DLI from 8.0 mol × m−2 × d−1 to 13.0 mol × m−2 × d−1 corresponded to the average values of SLA on EP1, EP6–EP9, and EP11. The values of SLA at the corresponding light intensity at the soil surface ranged from 182.04 ± 9.13 cm2 × g−1 to 186.02 ± 9.34 cm2 × g−1. The value of SLA decreased linearly to 109–112 cm2 × g−1 with a further increase of DLI to 22 mol × m−2 × d−1 and 25 mol × m−2 × d−1, respectively. The maximum values of SLA were found on EP3 and EP5—190.09 ± 9.57 cm2 × g−1 and 194.16 ± 9.75 cm2 × g−1 corresponding to the lowest values of DLI. The value of SLA was half as much (84.03 ± 4.26 cm2 × g−1) at the maximum level of light intensity (DLI, 63 mol × m−2 × d−1) on EP4.
The evaluation of the subpopulation composition showed that within the DLI range of 3–13 mol × m−2 × d−1, the loci of H. helix retained a prosperous type (Fig. 3). The subpopulation locus changed to the depressive type on EP4 with increasing light intensity of soil. The equilibrial type was characteristic of the average value of DLI. In general, the obtained data indicated that the reduction of SLA and the negative deviation of the vitality composition (from equilibrial or prosperous condition) were associated with the stressful effect of the limiting factor of soil shade.
Link between morphological variability, SLA of H. helix, and edaphic factors
The evaluation of phytoindicative score values of 8 edaphic factors/productive parameter SLA/vitality was carried out. The closest link between the value of SLA, the type of subpopulation (Q), and the phytoindicative value of soil moisture Hd (in one quadrant) was detected among the analyzed edaphic factors (Fig. 4). At the same time, inverse relationships were shown between SLA, Q, and the Lc (opposite quadrants). The relationships between SLA, Q, and Lc appeared to be closer (0.85 and 0.88) compared to Hd (0.76 and 0.77), respectively. The relationships between SLA, Q, and other edaphic factors were not confirmed or were weak. The obtained results showed that the limiting edaphic factors which affected the vitality and dispersion of vegetative individuals of H. helix were to a greater degree soil shade and to a lesser degree, moisture of soil.
The obtained data proved the necessity of analyzing the relationships between the variation of values of DLI and morphometric parameters. The difference of soil shade among the EPs of the Arboretum was closely related to the average values of the width and length of the leaves of vegetative individuals of H. helix (Fig. 5). A significant variability of the specified parameters was detected at DLI of 63 mol × m−2 × d−1. Ranges in the variability of the specified parameters were insignificant at DLI up to 20–25 mol × m−2 × d−1. We ascertained that the length of the average leaf was a more variable parameter than the width of the leaf under differences of soil shade.
Analysis of the relationships between DLI and the average values of shoot length, leaf petioles, and the number of leaves per shoot of H. helix showed only weak relationships (Fig. 6). In particular, the relationship between DLI and plant shoot length was the closest, but rather weak (r = -0.58). Weak relationships were detected between DLI and the length of leaf petioles/the number of leaves on the shoot (r = 0.26 and r = -0.30, respectively). The established relationship betwixt the length of the shoot and the number of leaves was quite expected (r = 0.75). Analysis of the relationships between DLI and SLA showed a close relationship (r = 0.87) (Fig. 6). Weak relationships were detected between SLA and length of the shoot/the length of leaf petioles/ (r = 0.06 and r = 0.07, respectively). The relationship between SLA and the number of leaves on the shoot was closer than length of the shoot/the length of leaf petioles, but rather weak (r = 0.25).
Discussion
Hedera helix is often viewed as having a high potential impact on the composition of forest ecosystems and more specifically in completely covering the forest floor. This species is a versatile plant growing in a variety of natural and anthropogenic biotopes. It is worth mentioning that the non-reproductive phase typically forms the ground cover. A literature review indicated that English ivy seedlings establish well on anthropogenies disturbed or open locations, although it may not establish well under intensive man-made disturbance (Metcalfe 2005). The factors for changes in the ecological adaptation and dispersion of H. helix are the increases in the average air temperatures, precipitation and solar radiation intensity, which have been observed in temperate forests (Kucharski et al. 2019). In Europe, at its northern, eastern, and high-altitude distribution limits, H. helix will remain at its vegetative phase (Andergassen and Bauer 2002). The increase in the number of individuals of H. helix in the juvenile stage of development and the formation of stable synusia on the Arboretum territory were confirmed by the existing data regarding the expansive characteristics of dispersion of this species in secondary or artificial parklands (Rackham 1990; Swearingen and Diedrich 2006; Kucharski et al. 2019). Juvenile leaves of the species formed a thick canopy just above the ground and prevented sunlight from reaching other plants (Swearingen and Diedrich 2006). Other authors noted that foliage cover of individuals of English ivy grown in full sun was more than 20% (Stafne et al. 2005). The species with the most similar habitat preferences include A. podagraria, B. ramosa, G. urbanum, F. vesca, M. uniflora, and M. perennis (Grime et al. 1988; Metcalfe 2005) (Table S1). This study established that the listed species are typically interspersed with H. helix in the herbaceous cover.
Generally, H. helix appears to be a species tolerant of a rather broad spectrum of environmental conditions in the temperate zone. Ivy is classified as a soil moisture indicator, being well developed chiefly on soils of average dampness and absent from waterlogged land and ground which may dry out (Ellenberg 1998; Kollmann and Grubb 1999). Other authors indicated that H. helix tolerates a wide range of soil moisture regimes (Reichard 2000; Koziarz 2015). English ivy plantlet density was positively correlated with soil moisture (r2 = 0.65) and associated with relatively high soil moisture content (Kollmann and Grubb 1999). The phytoindicative study conducted here confirms results obtained by other authors, who demonstrated that the limiting environmental factors for the juvenile growth stage in H. helix were soil moisture and soil shade. It has been shown that frequent short-term flooding has a positive effect on the growth of ivy, e.g., by increasing the availability of certain nutrients (Heuzé et al. 2009). Our data have proved the ecological dominance of the species in the fresh forest-meadow ecotypes of the Arboretum with moderately uneven moisture in the root layer of the soil. Researchers predicted that English ivy may be favored over other forest species in Europe if climates become drier and average temperatures increase (Karl et al. 1995; Sack and Grubb 2002; Sack et al. 2003). A detailed understanding of the mechanism of water storage by ivy requires further research (Heuzé et al. 2009).
Ivy is found in all but the most acid (< 4 pH), waterlogged, or very dry soils. It has been noted to grow well on acidic and basic soils (Reichard 2000) and to tolerate a wide range of soil pH, but is most frequent and abundant above pH 6 and seldom found below pH 4 (Grime et al. 1988). The results of this research indicated the tolerance of ivy for pH of 6.5–7.1.
Data on nitrate and total soil salinity as substrate conditions for H. helix were also shown in the literature. Ivy was often considered as a nitrogen indicator species which is also tolerant of salt deposition (Lameire et al. 2000; Binggeli 2005). Vidra et al. (2006) also found a positive correlation in an urban forest betwixt introduced ivy’s richness and soil fertility. Our data provide additional information about this issue. In particular, it was found that H. helix grows on soils which are rich in salts and have relatively high supplies of mineral nitrogen. The high cover degree of ivy often produced in temperate forests suppresses natural regeneration of understory and herbaceous species, possibly as a result of increased nitrogen levels produced by its leaf litter (Thomas 1998; Akerson and Gounaris 2000; Reichard 2000). Nitrophile conditions of the edaphotope, with increase in phytoindicative point values up to 7.0 on Arboretum soils of anthropogenic origin, were obtained in this study.
The non-reproductive form of ivy most often grows in shade conditions (Hoflacher and Bauer 2006; Metcalfe 2005; Schnitzler and Heuze 2006), while the reproductive form occurs in light conditions (Wallerstein and Hackett 1989). The sun and shade leaf differentiation were confirmed by functional mechanisms of frond structure (Terashima et al. 2005). The adaptation of individuals of H. helix to different intensities of soil light may be based on the ability of the plant to regulate efficiency of photosynthetic capacity and photosynthetic nutrient (Hoflacher and Bauer 2006). Developing leaves have less photosynthetic capacity and photosynthetic nutrient than adult leaves (Hoflacher and Bauer 2006; Metcalfe 2005). Juvenile leaves did not have morphological and physiological damages in intensive light fluxes. Generally, both life phases of H. helix are capable of acclimating to sun, but to different extents. However, several authors have claimed that juvenile leaves have limited ability for acclimation to high light conditions (Hoflacher and Bauer 1982, 2006; Bauer and Thöni 1988).
Juvenile individuals of H. helix were actively developed in the understory with low photosynthetically active radiation (PAR, 30 μmol × m−1 × s−1) and light intensity at the soil surface (DLI, 3 mol × m−2 × d−1) (Carter and Teramura 1988). Our study confirmed the tolerance of vegetative individuals to changes in light intensity at the soil surface (DLI, 3–63 mol × m−2 × d−1) without visible damage to the leaf surface. The changes in the leaf structure of ivy at the tissue level are the results of functional adaptation to the impact of a limiting environmental factor (Oberhuber and Bauer 1991). This opinion was confirmed by the morpho-histological characteristics of leaves of H. helix (water content and chlorophyll) at the different levels of light intensity (Baldini et al. 1997; Ganthaler et al. 2019). Ivy demonstrates moderate morphological plasticity of leaves with increased SLAs in shade conditions (Wright et al. 2001; Sack and Grubb 2002). Notably, SLA in high irradiance declined with increasing drought tolerance.
SLA ranged between 100 and 200 cm2 × g−1 in the shade/sun (Baldini et al. 1997; Sack et al. 2003). Ivy leaves demonstrated a definite peak of absorbance at 400 and 700 nm, with a slight reduction in assimilation at around 550 nm (Baldini et al. 1997). Our study completely coincides with the data of other authors indicating a substantial alteration of SLA of juvenile H. helix leaves, depending on the light intensity at the soil surface.
We concur with the consequence that a significant fluctuation in SLA of H. helix is an ontogenetic strategy and a habitat-specific feature of resource allocation (Wyka et al. 2019).
How was the dual tolerance (moisture and light) of H. helix possible? Many traits can contribute to a reduced demand for irradiance and water simultaneously, such as small size (Grime 1966), long-lived leaves of low SLA (Cornelissen 1999; Wright et al. 2001), and a high below-ground allocation (Sack et al. 2003). Strong relationships were shown between soil water and SLA, too (Doğan et al. 2015).
English ivy may reach its greatest projective cover and vitality in shady conditions in forests. In particular, H. helix reached its highest frequency in a parkland on dark plots with less than 2.5% light transmission (Siebel and Bouwma 1998). Our study confirmed this phenomenon. The number of individuals of the highest development classes and, accordingly, the quality index of the subpopulation increased significantly at the lowest values of DLI. The type of subpopulation was deteriorated to the depressed type under intensive lighting conditions (DLI more than 60 mol × m−2 × d−1). English ivy had the greatest abundance and vitality in the shade. The relationship between the intensity of light at the soil surface and the vitality of H. helix was also given in other works (Sack and Grubb 2002). Some authors indicated a close relationship between the integrated variation in lighting/temperature and the fluctuation of stem length, SLA, and other morphometric parameters (Pollet et al. 2009).
Morphometric parameters of leaves of vegetative individuals of H. helix are not only an indicative parameter that responds to modified environmental factors, but also a key feature for species identification (Ackerfield and Wen 2002). The authors recorded that the most indicative parameters of a leaf were number of lobes per leaf, length of the middle lobe, ratio of trichome center diameter to overall size, and overall width of the leaf. The lengths of the leaf and the petiole of the leaf were revealed as indicative properties in this study. The maximum length of the leaf petiole for H. helix was previously set at 10.00 cm for the temperate zone of Europe. However, the maximum value of this parameter nevertheless reached 17.54 cm in our research. The weighted average, minimum and maximum values of width and length of leaf, and number of lobes per leaf of H. helix were consistent with the reference data (Miller 2003).
Details on ecological plasticity of the indicative leaf parameters of H. helix were recorded for the first time. The ecological plasticity of the leaf petiole had the highest variability. It was confirmed that expansive species demonstrated significantly higher ecological plasticity than native plant species (Davidson et al. 2011). The identified ranges of the morphometric parameters of leaves of vegetative individuals due to diversity of light intensity coincided with the data of other authors (Baldini et al. 1997). Regardless, the link between variability of soil moisture and leaf morphometric parameters was not detected. This may be explained by the small amplitude of range of the humidity factor within the Arboretum.
Wide potential ecological niche, variability and ecological plasticity of morphometric parameters of leaves, and impacts of limiting environmental factors on the vitality of loci can explain the peculiarities of expansion and adaptation of juvenile individuals in artificial parkland. Determination of the dependence between the variability of limiting edaphic factors of high amplitude of oscillation in different forest types and the adaptive strategy of H. helix are future perspectives needed to clarify the reasons for the expansion of the species.
Conclusions
Wide potential ecological niche (phytoindicative analysis of edaphic soil factors), variability of morphometric parameters of diagnostic vegetative traces, ecological plasticity of morphometric parameters of leaves, and impact of the limiting environmental factor of soil shade on the deviation of the vitality composition (from equilibrial to prosperous condition) were detected at the level of loci of subpopulations of H. helix. The length of the average leaf and the length of leaf petiole were the most variable parameters under different values of light intensity. Weak links were found between light intensity at the soil surface and plant stem length/the number of leaves per shoot. A high level of index of morphological integration of H. helix individuals and a close relationship between the quality index/subpopulation compositions were established. A direct relationship between light intensity and specific leaf area values was established.
Data availability
The data that support the findings of this study are available on request from the corresponding author [Blinkova Olena].
References
Ackerfield J, Wen J (2002) A morphometric analysis of Hedera L. (the Ivy Genus, Araliaceae) and its taxonomic implications. Adansonia 24(2):197–212
Ackerfield J, Wen J (2003) Evolution of Hedera (the Ivy genus, Araliaceae): insights from chloroplast DNA data. Int J Plant Sci 164:593–602. https://doi.org/10.1086/375423
Akerson J, Gounaris K (2000) Strategic plan for managing alien invasive vegetation. Colonial National Historical Park Yorktown, Virginia. http://data2.itc.nps.gov/nature/documents/ACF34.pdf.
Andergassen S, Bauer H (2002) Frost hardiness in the juvenile and adult life phase of ivy (Hedera helix L.). Plant Ecol 161:207–213. https://doi.org/10.1023/A:1020365422879
Baldini E, Facini O, Nerozzi F, Rossi F, Rotondi F (1997) Leaf characteristics and optical properties of different woody species. Trees 12:73–81. https://doi.org/10.1007/s004680050124
Bauer H, Bauer U (1980) Photosynthesis in leaves of the juvenile and adult phase of ivy (Hedera helix). Physiol Plant 49(4):366–372. https://doi.org/10.1111/j.1399-3054.1980.tb03318.x
Bauer H, Thöni W (1988) Photosynthetic light acclimation in fully developed leaves of the juvenile and adult life phases of Hedera helix. Physiologia Plantarium 73(1):31–37. https://doi.org/10.1111/j.1399-3054.1988.tb09189.x
Binggeli P (2005) Crop Protection compendium-Hedera helix L. CAB International. http://members.multimania.co.uk/woodyplantecology/docs/CPC-Hedera_helix.pdf. Accessed October 2012.
Boratyńska K (1987) Flowering and fructifying specimens of Hedera helix L. in Poland. Arboretum Kórnickie 32:19–36
Bunk K, Krassovitski S, Speck T, Masselter T (2019) Branching morphology and biomechanics of ivy (Hedera helix) stem-branch attachments. Am J Bot 106(9):1143–1155. https://doi.org/10.1002/ajb2.1341
Carter GA, Teramura AH (1988) Vine photosynthesis and relationships to climbing mechanics in a forest understory. Am J Bot 75(7):1011–1018
Clements DR, Ditommaso A (2011) Climate change and weed adaptation: can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res 51:227–240
Cornelissen JHC (1999) A triangular relationship between leaf size and seed size among woody species: allometry, ontogeny, ecology and taxonomy. Oecologia 118(2):248–255
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14(4):419–431. https://doi.org/10.1111/j.1461-0248.2011.01596.x
Didukh YP (2012) Fundamentals of bioindication. Naukova Dumka, Kyiv
Doğan A, Yalçin E, Sürmen B, Kutbay HG (2015) Seasonal and altitudinal changes in leaf nutrient concentrations of Hedera helix L. (Araliaceae). Revue d’Ecologie (Terre et Vie) 70(2):166–181. https://doi.org/10.3406/revec.2015.1779
Ellenberg H (1998) Vegetation ecology of central Europe, 4th edn. Cambridge University Press, Cambridge
Ganthaler A, Marx K, Beikircher B, Mayr S (2019) Are hydraulic patterns of lianas different from trees? New insights from Hedera helix. J Exp Bot 70(10):2811–2822. https://doi.org/10.1093/jxb/erz071
Green AF, Ramsey TS, Ramsey J (2011) Phylogeny and biogeography of ivies (Hedera Spp., Araliaceae), a polyploid complex of woody vines. System Bot 36(4):1114–1127
Grime JP (1966) Shade avoidance and shade tolerance in flowering plants. In: Evans GC, Bainbridge R, Rackham O (eds) Light as an ecological factor. Blackwell Press, London, pp 187–207
Grime JP, Hodgson JG, Hunt R (1988) Comparative plant ecology: A functional approach to common British species. Unwin-Hyman, London
Heuzé P, Dupouey JL, Schnitzler A (2009) Radial growth response of Hedera helix to hydrological changes and climatic variability in the Rhine floodplain. River Res Appl 25:393–404. https://doi.org/10.1002/rra.1165
Hoflacher H, Bauer H (2006) Light acclimation in leaves of the juvenile and adult life phases of ivy (Hedera helix L.). Physiologia Plantarum 56(2):177–182. https://doi.org/10.1111/j.1399-3054.1982.tb00321.x
Karl T, Knight RW, Plummer N (1995) Trends in high-frequency climate variability in the twentieth century. Nature 377:217–220. https://doi.org/10.1038/377217a0
Kollmann J, Grubb PJ (1999) Recruitment of fleshy-fruited species under different shrub species: control by under-canopy environment. Ecol Res 14:9–21
Koziarz M (2015) Ekologiczne uwarunkowania występowania bluszczu pospolitego (Hedera helix L.). Sylwan 159(2):168–176
Kucharski L, Kloss M, Sienkiewicz J, Liszewska M, Kieltyk P (2019) Impact of climate change on ivy (Hedera helix L) expansion in forests of Central Poland. Folia Forestalia Polonica, Series A 61(3):211–221. https://doi.org/10.2478/ffp-2019-0020
Lameire S, Hermy M, Honnay O (2000) Two decades of change in the ground vegetation of a mixed deciduous forest in an agricultural landscape. J Veg Sci 11:695–704. https://doi.org/10.2307/3236576
Larocque KL (1999) Blurred park boundaries and the spread of English Ivy (Hedera helix L.): case studies from Greater Victoria, British Columbia. M.Sc. thesis, University of Victoria, Victoria
Metcalfe DL (2005) Biological flora of the British Isles: Hedera helix L. J Ecol 93(3):632–648
Miller JH (2003) Nonnative invasive plants of southern forests: a field guide for identification and control. Gen. Tech. Rep. SRS-62. Asheville: U.S. Department of Agriculture, Forest Service, Southern Research Station. http://www.srs.fs.usda.gov/pubs/gtr/gtr_srs062/
Oberhuber W, Bauer H (1991) Photoinhibition of photosynthesis under natural conditions in ivy (Hedera helix L.) growing in an understory of deciduous species. Planta 185:545–553
Okerman A (2000) Combating the “Ivy Desert”: the invasion of Hedera helix (English ivy) in the Pacific Northwest United States. Restor Reclam Rev 6(4):1–10
Pelabon C, Hilde CH, Einum S, Gamelon M (2020) On the use of the coefficient of variation to quantify and compare trait variation. Evolution Letters 4(3):180–188. https://doi.org/10.1002/evl3.171
Pollet B, Steppe K, Dambre P, Van Labeke MC, Lemeur R (2009) Temperature integration of Hedera helix L: quality aspects and growth response. Sci Hortic 120(1):89–95
Putz FE (1984) The natural history of lianas on Barro-Colorado island. Panama Ecology 65(6):1713–1724. https://doi.org/10.2307/1937767
Rackham O (1990) Trees and woodland in the British landscape, 2nd edn. Dent, London
Regulation of the Minister of the Environment (2014) Rozporządzenie Ministra Środowiska z dnia 9 października 2014 r. w sprawie ochrony gatunkowej roślin (Dz. U. 2014 poz. 1409)
Reichard S (2000) Hedera helix. In: Bossard CC, Randall JM, Hoshovsky MC (eds) Invasive plants of California’s wildlands. University of California Press, Berkeley, pp 212–216
Reichard SH, Hamilton CW (1997) Predicting invasions of woody plants introduced into North America. Conserv Biol 11(1):193–203. https://doi.org/10.1046/j.1523-1739.1997.95473.x
Rogler CE, Hackett WP (2006) Phase change in Hedera helix: induction of the mature to juvenile phase change by Gibberellin A3. Physiol Plant 34(2):141–147. https://doi.org/10.1111/j.1399-3054.1975.tb03809.x
Romuald O (1979) Owocujący bluszcz pospolity Hedera helix w Polsce środkowej. Chrońmy Przyrodę Ojczystą 35(5):5–17
Rose PQ (1996) The gardener’s guide to growing ivies. Timber Press, Portland
Rosu S, Sala F (2022) Characterization of the Hedera helix L. leaves geometry based on some dimensional parameters and calculated ratios. Life Sci Sustain Dev 3(2):7–16. https://doi.org/10.58509/lssd.v3i2.201
Sack L (2004) Response of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation? Oikos 107(1):110–127. https://doi.org/10.1111/j.0030-1299.2004.13184.x
Sack L, Grubb PJ (2001) Why do species of woody seedlings change rank in relative growth rate between low and high irradiance? Funct Ecol 15:145–154. https://doi.org/10.1046/j.1365-2435.2001.00507.x
Sack L, Grubb PJ (2002) The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 131(2):175–185. https://doi.org/10.1007/s00442-002-0873-0
Sack L, Grubb PJ, Marañón T (2003) The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecol 168:139–163. https://doi.org/10.1023/A:1024423820136
Sala F, Arsene GG, Iordanescu O, Boldea MV (2015) Leaf area constant model in optimizing foliar area measurement in plants: a case study in apple tree. Sci Hortic 193:218–224. https://doi.org/10.1016/j.scienta.2015.07.008
Schnitzler A, Heuzé P (2006) Ivy (Hedera helix L.) dynamics in riverine forests: effects of river regulation and forest disturbance. For Ecol Manage 236:12–17. https://doi.org/10.1016/j.foreco.2006.05.060
Siebel HN, Bouwma IM (1998) The occurrence of herbs and woody juveniles in a hardwood floodplain forest in relation to flooding and light. J Veg Sci 9(5):623–630. https://doi.org/10.2307/3237280
Stafne RA, Einert AE, Klingaman GL (2005) Fertilizer applications on establishment and growth of three groundcover species in sun and shade. J Environ Hortic 23(3):157–161. https://doi.org/10.24266/0738-2898-23.3.157
Strelau M, Clements DR, Benner J, Prasad R (2018) The biology of Canadian weeds: 157. Hedera helix L. and Hedera hibernica (G. Kirchn.) Bean. Can J Plant Sci 98(5):1005–1022. https://doi.org/10.1139/cjps-2018-0009
Swearingen JM, Diedrich S (2006) Fact sheet: English ivy. Plant conservation alliance’s alien plant working group. http://www.nps.gov/plants/alien; https://www.invasive.org/weedcd/pdfs/wgw/englishivy.pdf
Terashima I, Araya T, Miyazawa SI, Sone K, Yano S (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Ann Bot 95:507–519. https://doi.org/10.1093/aob/mci049
Thomas LK Jr (1998) Topographic alterations, forest structure, and invasion by English ivy (Hedera helix L.) in the rock creek floodplain, Washington. DC. Natural Areas Journal 18(2):164–168
Vidra RL, Shear TH, Wentworth TR (2006) Testing the paradigms of exotic species invasion in urban riparian forests. Nat Areas J 26(4):339–350. https://doi.org/10.3375/0885-8608(2006)26[339:ttpoes]2.0.co;2
Wallerstein I, Hackett WP (1989) Inflorescence induction and initiation in Hedera helix. Isr J Bot 38(2–3):71–83. https://doi.org/10.1080/0021213X.1989.10677114
Walther GR (2002) Weakening of climatic constraints with global warming and its consequences for evergreen broad-leaved species. Folia Geobot 37(1):129–139. https://doi.org/10.1007/BF02803195
Wen J, Plunkett GM, Mitchell AD, Wagstaff SJ (2001) The evolution of Araliaceae: a phylogenetic analysis based on ITS sequences of nuclear ribosomal DNA. Syst Bot 26:144–167. https://doi.org/10.1043/0363-6445-26.1.144
Wright IJ, Reich PB, Westoby M (2001) Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol 15(4):423–434. https://doi.org/10.1046/j.0269-8463.2001.00542.x
Wyka TP, Zadworny M, Mucha J, Zytkowiak R, Nowak K, Oleksyn Y (2019) Biomass and nitrogen distribution ratios reveal a reduced root investment in temperate lianas vs self-supporting plants. Ann Bot 124(5):777–790. https://doi.org/10.1093/aob/mcz061
Zając A, Zając M (2001) Atlas rozmieszczenia roślin naczyniowych w Polsce. Pracownia Chorologii Komputerowej Instytutu Botaniki Uniwersytetu Jagiellońskiego, Kraków
Zlobin YA (2009) Population plant ecology: current state, points of growth. University, Sumy
Funding
This work was supported by the Institute of Dendrology, Polish Academy of Sciences, Kórnik.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by OB, KR, and AMJ. The first draft of the manuscript was written by OB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no financial or non-financial conflict of interest.
Additional information
Communicated by Ling Zhang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blinkova, O., Rawlik, K. & Jagodziński, A.M. The impact of environmental factors on traits of Hedera helix L. vegetative shoots. Plant Ecol 224, 973–986 (2023). https://doi.org/10.1007/s11258-023-01354-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-023-01354-w