Abstract
Purpose
Chloride, the predominant anion in extracellular fluid from humans, is essential to maintaining homeostasis. One important metric for thoroughly assessing kidney function is the estimated glomerular filtration rate (eGFR). However, the relationship between variations in serum chloride concentration and eGFR in general populations has been poorly studied. Therefore, the purpose of this study is to elucidate the correlation between serum chloride levels and eGFR within the United States’ adult population.
Methods
This cohort study was conducted using data from the National Health and Nutrition Examination Survey (NHANES), which covered the years 1999–2018. We employed multiple linear regression analysis and subgroup analysis to evaluate the correlation between serum chloride concentration and eGFR. To examine the nonlinear association between serum chloride levels and eGFR, restricted cubic spline analyses were employed.
Results
Data from 49,008 participants in this cohort study were used for the chloride analysis. In the comprehensively adjusted model, a noteworthy inverse relationship was discovered between chloride plasma concentration and eGFR. Restricted cubic spline analyses revealed a significant nonlinear relationship between chloride levels and eGFR (P for overall < 0.001 and P for nonlinear < 0.001). A significant interaction was observed between eGFR and plasma chloride concentration (all P < 0.001 for interaction) among the subgroups characterized by sex, household income to poverty ratio, BMI, hypertension, and diabetes.
Conclusion
Our findings suggest that higher levels of chloride plasma concentration were linked to decreased eGFR. These findings underscore the significance of monitoring chloride plasma concentration as a potential indicator for identifying individuals at risk of developing chronic kidney disease (CKD).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is acknowledged as a major worldwide public health issue [1, 2]. About 15–20% of the global population in the US suffer from CKD [1, 3], which is characterized by the presence of albuminuria (urine albumin to creatinine ratio [UACR] ≥ 30 mg/g) or an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2 persisting for at least 3 months [4, 5]. Increased incidences of kidney failure necessitating kidney replacement therapy, acute renal damage, overall mortality, and cardiovascular-related mortality have all been linked to reduced eGFR levels and intensified albuminuria. A study from the Global Burden of Disease group, published in 2020, identified CKD as a leading factor among the top ten in global poor prognosis [6]. The eGFR is commonly considered the most precise measure for assessing kidney function. In standard practice, serum creatinine serves as the predominant biochemical parameter for eGFR [7]. CKD is a prevalent disorder marked by the progressive decline in renal function, which is characterized by intimal fibrosis, glomerulosclerosis and tubule-interstitial alterations (including renal tubular atrophy and fibrosis), ultimately destroying kidney parenchyma and renal failure [8]. This progression may result in a gradual decrease in eGFR [9]. The eGFR plays a crucial role in categorizing stages and risks for CKD patients, given that a reduced eGFR is independently and progressively linked to a heightened risk of end-stage kidney disease (ESKD) and death [10,11,12]. eGFR can be calculated from routinely collected variables such as age, race, sex, and serum creatinine [13]. Studies have shown eGFR to be a strong indicator of various outcomes, including cardiovascular events, early mortality, increased hospitalizations, and kidney failure necessitating renal replacement therapy [14, 15]. A comprehensive understanding of eGFR allows clinicians to prescribe appropriate drugs, liquid remedies, and early interventions to avert end-stage renal failure [16].
Chloride, as the primary potent anion in human extracellular fluid, makes up about 33% of the plasma’s tonicity and 67% of its total negative charges. Their main restriction is to the fluid compartment outside the cell [17,18,19]. Chloride in the blood plays a vital role in a variety of physiological functions in the human body, including regulating osmotic pressure, maintaining the electrical balance of bodily fluids, facilitating muscle function, and controlling blood pressure [18, 20, 21]. The kidney and gastrointestinal system are primarily responsible for controlling serum chloride levels. Chloride, excreted in the form of hydrochloric acid within gastric juice, may be absorbed throughout the entirety of the intestinal tract during digestion. American adult male chloride consumption varies between 7.8 and 11.8 g daily, compared to 5.8–7.8 g for adult female intake. Most human body chloride comes from table salt (Na Cl) in dietary intake, as well as from foods that are rich in salt [20]. The kidney is the main organ that excretes chloride. Approximately, 19,440 millimoles (mmol) are filtered daily by the kidneys, with a significant 99.1% being reabsorbed, resulting in a mere 180 mmol being expelled daily. Most reabsorption is carried out in the proximal tubule by active coupled transport with other ions, passive reabsorption, or ion conductance [17, 20]. Serum chloride levels are often overlooked in laboratory testing and are usually only taken into account in cases of metabolic acidosis. Chloride, however, has emerged as a key player in a number of homeostatic processes, such as blood pressure response, renal sodium management, tubuloglomerular feedback, and renin secretion regulation [22,23,24,25,26]. A growing quantity of studies are recognizing chloride’s importance, leading to its escalated utility in therapy for forecasting adverse results. In those suffering from heart failure, lower chloride levels in their serum are associated with elevated plasma renin, reduced effectiveness of diuretics, and a slower rate of muscle clearance [27,28,29]. Previous studies indicate a connection between hypochloremia and an increased mortality risk in individuals with pulmonary arterial hypertension (PAH), independent of their serum sodium concentrations [30, 31]. In addition, among hypertension patients, a correlation between Cl and mortality risk was identified. After adjusting for initial confounders and the concentrations of Na+, K+, and HCO3− , a decrease of 1.5% in overall mortality correlated with a 1 mEq/L rise in serum Cl− [32]. Consequently, regardless of sodium presence, chloride might play a important role in the physiological pathology of heart-related disorders.
Cardiovascular disease and a heightened risk of mortality are directly linked to CKD. However, there are little data on the connection between Cl and the prognosis of CKD patients. The predictive significance of Cl in patients with CKD may differ from that of other patient populations due to the frequent acid–base imbalances that impact blood Cl levels. Some prior investigations have concentrated on the correlation between chloride plasma concentration and acute kidney injury (AKI). However, they were demonstrated to have the opposite connection. A multicenter observational study that included 4234 critically ill AKI patients uncovered a link between reduced serum chloride levels and an increase in mortality in ICU and during hospitalization [33]. In marked contrast, severe hyperchloremia poses a significant risk to patient outcomes. An elevated risk of AKI is linked to a greater fluctuation in chloride levels starting from the moment of hospitalization. Moreover, severe hyperchloremia serves as an independent indicator for in-hospital acute kidney injuries and death rates [34,35,36]. Among chronic kidney disease patients not undergoing dialysis, having low serum chloride levels correlated with increased death rates and an elevated risk of cardiovascular incidents [37, 38]. To our knowledge, not much research has been done on the general US population about the association between variations in eGFR and chloride plasma levels. Our hypothesis was tested by conducting a cross-sectional study with 49,008 participants from the National Health and Nutrition Examination Survey (NHANES: 1999–2018) to determine if changes in blood chloride levels correlate with a heightened CKD risk in adult US citizens.
Methods
Study population
The NHANES, developed by the National Center for Health Statistics, serves as a program for evaluating the frequency, disease risk elements, and the physical and nutritional state of adults and kids in the United States. NHANES serves as a cross-sectional survey crafted to depict the non-institutionalized civilian demographic of America. All data are accessible to the public and can be obtained from the NHANES at https: //www.cdc.gov/nchs/nhanes/index.htm. It was mandatory for NHANES participants to provide their informed consent in writing. The National Center for Health Statistics’ Research Ethics Review Board granted it authorization. Due to the deidentified and publicly available nature of NHANES data, this research did not require institutional review board permission, as per the Common Rule. Therefore, this study was not subject to any additional ethical approvals [39].
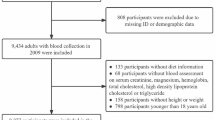
An analysis of ten 2-year cycles of NHANES data from 1999 to 2018 was conducted for this study. There were 101,316 individuals sampled in this study, with 86, 366 adults (age ≥ 20 years old) participants. Individuals were excluded if they lacked data on serum chloride concentration, or were missing data for the calculation of eGFR [13] (n = 37,358). Eventually, the present research incorporated a total of 49,008 individuals. Figure 1 displays the inclusion and exclusion criteria for the study population.
Assessments of serum chloride concentration
Specialists with certification collect and examine blood samples at a Mobile Examination Center (MEC), from which they are subsequently preserved in biological archives. The analysis of serum chloride content was performed using Beckman Synchron LX20, Beckman UniCel DxC800 Synchron (Beckman Coulter, Fullerton, CA, USA), or Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, IN 46250). Serum chloride concentration were assessed by the ion-selective electrode indirect (or diluted) method. Classification of the individuals was based on their serum chloride concentration quartiles. All the detection procedures about serum chloride measurements have been described in detail on the NHANES websites [39].
Measurement of estimated glomerular filtration rate
A Jaffe rate reaction was used to quantify creatinine concentration during NHANES mobile examination center screenings. Calculation of the eGFR value was based on age (in years), gender, and creatinine plasma levels (in mg/dL), utilizing the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation[13].eGFRCKD−EPI (mL/min/1.73 m2) = 141 × min (Scr/κ, 1) α × max (Scr/κ, 1) −1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where Scr represents serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max denotes the maximum of Scr/κ or 1. The eGFR-associated data of participants were obtained from NHANES [39].
Assessments of covariates
Following an extensive review of existing studies and our practical clinical experiences, our demographic covariates of interest included age, gender, racial and ethnicity, marital status (whether married, widowed, divorced, separated, unmarried, and cohabitating), education attainment (below 9th grade, 9 th to 11th grade, high school or GED level or something similar, college or AA education, and having a college degree or higher), and family income to poverty ratio. Based on self-report during the interview, race, and ethnicity were evaluated as fundamental demographic characteristics and categorized into five categories: Mexican American, non-Hispanic White, non-Hispanic Black, other Hispanic, and other (which includes non-Hispanic multiple races and other non-Hispanic non-Hispanic). These categories were gathered since their combined presence was thought to be confounding. We also incorporated various anthropometric measures and chronic health conditions in our study. These included body mass index (BMI), smoking habits (classified as every day, occasional, or never), alcohol consumption, hypertension (defined as being diagnosed by a physician, possessing a systolic and diastolic blood pressure levels of at least 130 mm Hg or 80 mm Hg, respectively), diabetes (self-reported diagnosis by a physician), congestive heart failure (self-reported diagnosis by a physician), stroke (self-reported diagnosis by a physician), and serum levels of total cholesterol, phosphorus, potassium, total calcium, sodium, and bicarbonate. Table 1 provides detailed information on the other categorical variables.
Statistical analysis
All the analyses in this paper take into account sample weights, strata, and primary sampling units because of the intricate sampling architecture of NHANES. According to the serum chloride concentration quartile, we separated the study population into four groups: 70 ≤ quartile 1 ≤ 101.3 mmol/L, 101.3 < quartile 2 ≤ 103.4 mmol/L, 103.4 < quartile 3 ≤ 105.0 mmol/L, and 105 < quartile 4 ≤ 120 mmol/L. Multiple linear regression analysis was employed to investigate the relationships between eGFR and blood chloride levels. To evaluate the study population’s initial characteristics, descriptive statistics were employed. The survey-weighted percentage with a 95% confidence range was computed for categorical variables, and the P value was derived via the survey-weighted Chi-square test. Regarding continuous variables, a survey-weighted average (with a 95% Confidence Interval) was computed, and in response, the P value derived through survey-weighted linear regression.
In addition to the above methods, we also employed weighted linear regression. When conducting regression analysis, we utilized the sample weight variables provided by the NHANES. This approach fully considered the complexity of the sample design, thereby ensuring the representativeness and accuracy of the research results. Three models were built. There was no covariate adjustment in Model 1. Age, gender, race, marital status, degree of education, and family income to poverty ratio were all taken into account in Model 2. In addition, Model 3 made adjustments for variables such as body mass index, smoking status, alcohol drinks, hypertension, diabetes, congestive heart failure, stroke, total cholesterol and serum bicarbonate. The multiple imputation using chained equations approach was used to impute covariates that had missing values. Multiple interpolation techniques were employed for continuous variables, while missing categorical variables were categorized separately. The nonlinear relationship between chloride plasm levels and eGFR was determined using multivariable restricted cubic spline (RCS) analysis. Age, gender, educational attainment, the ratio of family income to poverty, body mass index, hypertension, and diabetes were further stratified in our analyses. Age was categorized into three groups: those aged 39 or younger, those between 40 and 59, and individuals 60 years or older. Three categories were established for the family income to poverty ratio: lower than 1.0, between 1.0 and 3.0, and exceeding 3.0. BMI was determined by dividing weight in kilograms by the squared height in meters, assigning categories of less than 25.0, 25.0 to 29.9, and 30.0 or more.
The research utilized R software, version 4.3.1 (R Project for Statistical Computing), and EmpowerStats, version 6.0 for all statistical analyses conducted between October 1, 2023, and March 1, 2024. A significance criterion of P < 0.05 was applied to all studies.
Results
Baseline characteristics of the study participants
The final analysis included the 49,008 US adults qualified individuals who remained. The average age among the 49,008 individuals was 47.1 (95% CI, 46.8, 47.5) years, 51.8% of whom were female, and 68.6% of the participants were non-Hispanic White. Based on the quartiles, the participants’ serum chloride values were divided into four groups: 70 mmol/L ≤ quartile 1 ≤ 101.3 mmol/L, 101.3 mmol/L < quartile 2 ≤ 103.4 mmol/L, 103.4 mmol/L < quartile 3 ≤ 105.0 mmol/L, and 105 mmol/L < quartile 4 ≤ 120 mmol/L. After dividing participants by serum chlorideconcentration, there were 12,283 participants (mean [95% CI] age, 49.8 [49.2, 50.3] years; 6,522 [53.1%] men) in quartile 1, the lowest concentration quartile; 12,242 participants (mean [95% CI] age, 46.3 [45.8, 46.8] years; 6, 255 [51.1%] men) in quartile 2; 13,307 participants (mean [95% CI] age, 46.1 [45.7, 46.6] years; 6,121 [46.0%] men) in quartile 3; and 11,176 participants (mean [95% CI] age, 46.1 [45.6, 46.6] years; 4,682 [41.9%] men) in quartile 4, the highest concentration quartile. In summary, compared with individuals in the lowest quartile of serum chloride concentration, those with higher serum chloride were more likely to be younger, female, non-Hispanic White, married, and to drink more; they also had lower total cholesterol, lower serum total calcium, lower serum bicarbonate, and higher serum sodium levels; additionally, they were more unlikely to have hypertension, diabetes, congestive heart failure, and stroke (Table 1).
Association between serum chloride concentration and eGFR in NHANES (1999–2018)
The associations of chloride plasma concentration and eGFR were evaluated with multiple linear regression analysis. In Model 1, the β of eGFR in the fourth quartile was 2.3 (95% CI, 1.0, 3.5) compared with the reference group (P < 0.001 for trend). Compared with the lowest quartile of serum chloride, the highest quartile was associated with a lower eGFR in the partly adjusted Model 2 (β = −1.9 [95% CI −2.8, −1.1]; P < 0.001 for trend). Following adjustments for multiple variables in Model 3, compared with the reference group (the first quartile), the β for eGFR was −1.7 (95% CI −2.3, −1.1, P < 0.001) in the second quartile, − 1.8 (95% CI −2.4, −1.2, P < 0.001) in the third quartile, and − 2.3 (95% CI −3.2, −1.4, P < 0.001) in the fourth quartile (P < 0.001 for trend) (Table 2).
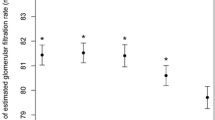
To investigate the association between serum chloride levels and eGFR, we analyzed restricted cubic spline (RCS) plots for the comprehensively adjusted model. The outcomes of multivariate linear regression using RCS are presented in Fig. 2. The findings indicated a significant nonlinear association between serum chloride concentration and eGFR (P < 0.001 for nonlinear) in the RCS (P < 0.001 for overall).
Restricted cubic spline (RCS) analysis with multivariate-adjusted associations between serum chloride levels and eGFR in adults. Models adjusted for age, gender, race, marital status, education level, family income to poverty ratio, body mass index, smoking status, alcohol intake, hypertension, diabetes, congestive heart failure, stroke, total cholesterol and serum bicarbonate. eGFR estimated glomerular filtration rate
Stratified analyses
Among the subgroups defined by sex, family income to poverty ratio, BMI, hypertension, and diabetes, we observed a significant interaction between chloride plasma concentration and them with eGFR (all P < 0.001 for interaction). In the subgroup exhibiting hypertension and diabetes versus the control group (the first quartile), the β values for eGFR in the fourth quartile stood at −2.2 (95% CI −3.4, −1.1, P < 0.001) and −2.2 (95% CI −4.2, −0.14, P = 0.036), correspondingly. When comparing the subgroup without hypertension and diabetes to the reference group, the β coefficients for eGFR in the fourth quartile were − 2.4 (95% CI −3.5, −1.3, P < 0.001) and −2.3 (95% CI −3.3, −1.3, P < 0.001), respectively. In the analysis that partitioned subjects based on BMI into categories of less than 25, between 25 and 29.9, and 30 or greater kg/m2, compared with the reference group (the first quartile), the β of eGFR in the fourth quartile was −2.6 (95% CI −3.9, −1.3, P < 0.001), −1.8 (95% CI −2.8, −0.72, P = 0.001) and −2.6 (95% CI −3.8, −1.4, P < 0.001), respectively. Nonetheless, the analysis failed to reveal any statistically significant interactions between serum chloride levels and other strata variables about eGFR (Table 3).
Discussion
This cross-sectional study, which incorporated 49,008 participants from the NHANES from 1999 to 2018, demonstrated an inverse correlation between serum chloride levels and eGFR. Elevated levels of blood chloride have been found to correlate with diminished eGFR, even after accounting for confounding factors that could influence either blood chloride levels and/or eGFR. RCS analysis disclosed a nonlinear relationship between serum chloride concentration and eGFR within the general population. This is, to our knowledge, the first study examining the relationship between eGFR and chloride plasma concentration in the broader US community. Our research contributes to the increasing amount of data suggesting that chloride may play a part in human illness.
Previous studies have primarily concentrated on patients afflicted with cardiovascular diseases, including heart failure, hypertension, and pulmonary arterial hypertension. Hypochloremia is associated with an increased likelihood of cardiovascular deaths among people, regardless of the presence of known cardiovascular hazards and other electrolyte levels, like serum sodium [40]. Recent studies have indicated that, in the context of chronic heart failure, serum chloride levels may be a more robust predictor of patient outcomes compared to serum sodium levels. Studies conducted by BEST trial indicated a strong correlation with increased death risk due to hypochloremia, exhibiting a hazard ratio (HR) of 1.3 (95% CI 1.18–1.42; P < 0.001) corresponding to each decrease in serum chloride levels by standard deviation. The link persisted irrespective of serum sodium concentration, a condition that did not affect death risks considering serum chloride amounts [41]. In a prospective cohort study with 1673 participants revealed a link between reduced chloride concentration, quantified per standard deviation, and a heightened adjusted mortality risk, evidenced by a hazard ratio of 1.29 and a 95% CI between 1.12 and 1.49, holding statistical importance (P < 0.001). When chloride levels were incorporated into a multivariable model, they resulted in a net reclassification improvement of 10.4%, which was statistically significant (P = 0.03). In contrast, sodium levels did not demonstrate prognostic significance within the same model (P = 0.30) [29]. Similar findings have been observed in the TOPCAT trial [42], associating reduced serum chloride with heightened risk of hospitalization for heart failure, cardiovascular fatalities, and overall mortality. The study clarified that reducing serum chloride levels by 4 mmol/L correlates with a heightened risk of death from various reasons, including cardiovascular incidents. Hazard ratios were determined to be 1.29 (95% confidence interval [CI] 1.02–1.62; P = 0.04) for all-cause mortality and 1.51 (95% CI 1.11–2.06; P < 0.008) for cardiovascular mortality, correspondingly. There is a connection between hypochloremia and negative results in individuals suffering from acute decompensated heart failure. A review of more than 1300 heart failure patients, sorted by admission serum chloride and sodium levels, showed a link between hypochloremia and declining survival rates over a 3-year monitoring span. Conversely, hyponatremia did not significantly affect survival rates if chloride levels were maintained within the normal range [28]. Over a 35-year period, McCallum and team, evaluating 12,968 hypertensive patients in their comprehensive study, found a 20% increased risk of death among those in the bottom quintile for serum chloride levels (less than 100 mEq/L), covering all causes including both cardiovascular and non-cardiovascular, compared to those treated with a higher serum chloride counterparts. Gradually, every 1 mEq/L increase in serum chloride correlated with a reduction of 1.5% in the risk of all-cause death (HR, 0.985; 95% CI 0.98–0.99), a relationship that remained even after accounting for initial variables such as serum sodium, potassium, and bicarbonate levels [32]. A research with 277 individuals suffering from idiopathic or inheritable PAH inheritable individuals with serum chloride concentration of 100 mM/L or less at 6 months showed a higher death rate. After accounting for factors such as age, gender, pulmonary vascular resistance, the use of prostacyclin or diuretics, and serum creatinine and sodium levels at 6 months, this link stayed statistically meaningful, resulting in a hazard ratio of 1.83 and a 95% confidence interval between 1.11 and 3.00 [30].
The profound interconnection between cardiac and renal physiology is paramount and warrants emphasis. The kidney is where chloride is mainly excreted. Historically, investigative efforts examining plasma chloride levels and renal function have predominantly centered on the context of AKI. Studies investigating the connection between blood chloride levels and eGFR are very rare. Yunos et al. [43] conducted a pre-post clinical experiment with more than 750 individuals to assess the effects of intravenous chloride levels on kidney health. During the period when a chloride-restricted intravenous fluid strategy was implemented, there was a notable decline of 50% in AKI occurrences as per the RIFLE criteria, along with a reduced use of renal replacement therapy (RRT), dropping from 10% in the control stage to 6.3% during the entire intervention period. In a retrospective study involving 250 adult participants within a multidisciplinary academic intensive care unit (ICU), 57% of participants developed hyperchloremia within the first 48 h post-ICU admission. Elevated chloride levels at 48 h were found to be a significant forecast of AKI, with an odds ratio (OR) of 6.44 (95% CI 2.95–14.10), and of increased mortality, OR of 2.46 (95% CI 1.22–4.94) on univariate studies. These correlations remained consistent upon multivariate analysis [44].
The study synthesized findings on the relationship between serum chloride concentration and eGFR. A negative association was delineated, with eGFR being discernibly reduced in subjects exhibiting elevated serum chloride levels. The determination of whether the correlation between chloride fluctuations and long-term eGFR alterations has a causal underpinning or significant clinical implications remains to be established through additional research and corroboration. Nonetheless, there exist potential physiological pathways that could underlie and elucidate the connections observed in these results. The chloride ion is essential to physiological processes, including the regulation of both extracellular and intracellular volume, as well as maintaining acid–base homeostasis. Research conducted on experimental animals has shown that it is the plasma chloride, not sodium, that results in denervated canine kidneys having less renal blood flow [26]. Studies have demonstrated that both Angiotensin II (ANG II) [45] and endothelin [46] contribute to the enhancement of chloride ion conductance, alongside mediating vasoconstriction in the afferent arteriole. Furthermore, the gradient between extracellular and intracellular chloride has been demonstrated to be critical in determining the responsiveness of the afferent arterioles. Low levels of chloride in the macula densa lead to an increase in the production of renin and local prostaglandins when there is a decrease in perfusion pressure. These mechanisms work through afferent vasodilation and efferent vasoconstriction to maintain GFR. An excess of chloride in the body, whether from normal saline or due to hyperchloremia, enhances the transport of chloride towards the macula densa in the distal region. This triggers a reduction in GFR through afferent vasoconstriction, a process facilitated by thromboxane and adenosine. Concurrently, there is a diminished response in efferent vasoconstriction attributed to decreased levels of renin and angiotensin II [47].
An alternative hypothesis accounting for the noted correlation could relate to metabolic acidosis, considering that elevated serum chloride concentration frequently occur alongside metabolic acidosis, a condition linked to an escalated risk of CKD advancement [48, 49]. This occurrence is explicable by the fact that metabolic acidosis elevates the process of ammoniagenesis, consequently amplifying the excretion of acid. Despite the overall decrease in ammonium excretion observed as CKD progresses, the generation of ammonia on a per-nephron basis experiences an upsurge, contributing to the sustenance of augmented acid secretion in the context of nephronic impairment [50, 51]. The adaptive response proves detrimental to the survival of nephrons, as it augments ammoniagenesis, leading to an elevated local ammonia concentration around the nephron. This, in turn, can initiate the activation of complement C3 and C5b-9, as well as the alternative complement cascade. Detrimental outcomes of this maladaptive activation of the complement system include the overproduction of inflammatory and fibrogenic factors. These mediators are implicated in exacerbating proteinuria, inflammatory responses, and fibrotic processes, thereby aggravating tubulointerstitial damage and the progression of CKD [52].
The present research possesses multiple noteworthy advantages that enhance its validity and reliability. This investigation encompassed a nationally representative sample, thereby enabling the extrapolation of the results to the broader United States populace. Furthermore, the study leveraged the comprehensive and robust data acquisition inherent in the NHANES to mitigate the influence of confounding variables encompassing demographic characteristics, socioeconomic status, dietary habits, lifestyle choices, health conditions, and familial chronic disease histories. Third, the employment of skilled personnel adhering to uniform procedures for the ascertainment of anthropometric measurements, such as height and weight, as well as the systematic gathering of laboratory and interview-based information, serves to augment the precision and veracity of the resultant dataset. Last but not least, analyses of subgroups were conducted to evaluate the strength of the association between serum chloride levels and eGFR across diverse cohorts.
Notwithstanding its merits, this investigation acknowledges multiple potential constraints. First, because exposures and outcomes are recorded simultaneously, cross-sectional studies, like the one we used, have some limitations. Therefore, the only use for our data is to evaluate associations. They cannot even be utilized to evaluate the temporal or directional nature of the observed relationships, let alone establish causality. Second, the prospect remains that certain confounding variables may not have been sufficiently addressed, potentially permitting the persistence of residual confounding elements and unidentified confounding factors that cannot be unequivocally ruled out. In addition, the evaluation of renal function was limited to a singular time point, disregarding anomalies that may occur under physiological conditions or those that emerge from AKI. Lastly, the restricted accessibility of extensive drug-related data in the NHANES database hindered our ability to assess the influence of medications, such as dialysis treatment and the utilization of diuretic medication, on our results. It is anticipated that future research initiatives will include additional covariates to enhance the validity of our findings.
Conclusion
The findings of this study, which focused on Americans adult, suggest that there may be a connection between higher plasma chloride levels and a higher likelihood of decreased eGFR. These findings underscore the critical role of monitoring chloride plasma concentration as a potential indicator for identifying individuals at risk of developing CKD. Nevertheless, to solidify these observations, further large-scale longitudinal investigations are desperately needed to validate the findings of this study.
Data availability
Researchers and data users from all over the world can access the survey data on the internet (www.cdc.gov/nchs/nhanes/).
References
Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS et al (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375(9731):2073–2081. https://doi.org/10.1016/S0140-6736(10)60674-5
Levin A, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK et al (2024) Executive summary of the KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease: known knowns and known unknowns. Kidney Int 105(4):684–701. https://doi.org/10.1016/j.kint.2023.10.016
Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R (2022) Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 18(11):696–707. https://doi.org/10.1038/s41581-022-00616-6
Writing Group for the CKDPC, Grams ME, Coresh J, Matsushita K, Ballew SH, Sang Y et al (2023) Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA 330(13):1266–1277. https://doi.org/10.1001/jama.2023.17002
Collaboration GBDCKD (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Collaborators GBDRF (2020) Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396(10258):1223–1249. https://doi.org/10.1016/S0140-6736(20)30752-2
Villa P, Jimenez M, Soriano MC, Manzanares J, Casasnovas P (2005) Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care 9(2):R139-143. https://doi.org/10.1186/cc3044
Yuan Q, Ren Q, Li L, Tan H, Lu M, Tian Y et al (2022) A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-beta signaling. Nat Commun 13(1):438. https://doi.org/10.1038/s41467-022-28096-z
Abdelhafiz AH, Brown SH, Bello A, El Nahas M (2010) Chronic kidney disease in older people: physiology, pathology or both? Nephron Clin Pract 116(1):c19-24. https://doi.org/10.1159/000314545
Chen DC, Shlipak MG, Scherzer R, Bauer SR, Potok OA, Rifkin DE et al (2022) Association of intraindividual difference in estimated glomerular filtration rate by creatinine vs cystatin c and end-stage kidney disease and mortality. JAMA Netw Open 5(2):e2148940. https://doi.org/10.1001/jamanetworkopen.2021.48940
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354(23):2473–2483. https://doi.org/10.1056/NEJMra054415
Ferguson TW, Komenda P, Tangri N (2016) Change in estimated glomerular filtration rate and outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 25(3):240–244. https://doi.org/10.1097/MNH.0000000000000210
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305. https://doi.org/10.1056/NEJMoa041031
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K et al (2011) The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int 80(1):17–28. https://doi.org/10.1038/ki.2010.483
Work DF, Schwartz GJ (2008) Estimating and measuring glomerular filtration rate in children. Curr Opin Nephrol Hypertens 17(3):320–325. https://doi.org/10.1097/MNH.0b013e3282fb77f2
NaM Y, Bellomo R, Story D, Kellum J (2010) Bench-to-bedside review: chloride in critical illness. Critical Care (London, England) 14(4):226. https://doi.org/10.1186/cc9052
Hou X, Xu W, Zhang C, Song Z, Zhu M, Guo Q et al (2023) L-shaped association of serum chloride level with all-cause and cause-specific mortality in american adults: population-based prospective cohort study. JMIR Public Health Surveill 9:e49291. https://doi.org/10.2196/49291
Koch SM, Taylor RW (1992) Chloride ion in intensive care medicine. Crit Care Med 20(2):227–240. https://doi.org/10.1097/00003246-199202000-00012
Berend K, van Hulsteijn LH, Gans RO (2012) Chloride: the queen of electrolytes? Eur J Intern Med 23(3):203–211. https://doi.org/10.1016/j.ejim.2011.11.013
Kazory A, Costanzo MR (2020) The dynamic relationship between serum chloride and cardiorenal syndrome. Rev Cardiovasc Med 21(1):25–29. https://doi.org/10.31083/j.rcm.2020.01.6
Kotchen TA, Luke RG, Ott CE, Galla JH, Whitescarver S (1983) Effect of chloride on renin and blood pressure responses to sodium chloride. Ann Intern Med 98(5 Pt 2):817–822. https://doi.org/10.7326/0003-4819-98-5-817
Kurtz TW, Morris RC (1983) Dietary chloride as a determinant of “sodium-dependent” hypertension. Science 222(4628):1139–1141. https://doi.org/10.1126/science.6648527
Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP (1991) Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol 260(4 Pt 2):F486–F493. https://doi.org/10.1152/ajprenal.1991.260.4.F486
Schnermann J, Ploth DW, Hermle M (1976) Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch 362(3):229–240. https://doi.org/10.1007/BF00581175
Wilcox CS (1983) Regulation of renal blood flow by plasma chloride. J Clin Invest 71(3):726–735. https://doi.org/10.1172/jci110820
ter Maaten JM, Damman K, Hanberg JS, Givertz MM, Metra M, O’Connor CM et al (2016) Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circulat: Heart Failure. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003109
Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M et al (2015) Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol 66(6):659–666. https://doi.org/10.1016/j.jacc.2015.06.007
Grodin JL, Verbrugge FH, Ellis SG, Mullens W, Testani JM, Tang WH (2016) Importance of abnormal chloride homeostasis in stable chronic heart failure. Circ Heart Fail 9(1):e002453. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002453
Naal T, Abuhalimeh B, Khirfan G, Dweik RA, Tang WHW, Tonelli AR (2018) Serum chloride levels track with survival in patients with pulmonary arterial hypertension. Chest 154(3):541–549. https://doi.org/10.1016/j.chest.2018.04.022
Prins KW, Kalra R, Rose L, Assad TR, Archer SL, Bajaj NS et al (2020) Hypochloremia is a noninvasive predictor of mortality in pulmonary arterial hypertension. J Am Heart Assoc 9(5):e015221. https://doi.org/10.1161/JAHA.119.015221
McCallum L, Jeemon P, Hastie CE, Patel RK, Williamson C, Redzuan AM et al (2013) Serum chloride is an independent predictor of mortality in hypertensive patients. Hypertension 62(5):836–843. https://doi.org/10.1161/HYPERTENSIONAHA.113.01793
Zhu X, Xue J, Liu Z, Dai W, Xiang J, Xu H et al (2022) Association between serum chloride levels with mortality in critically ill patients with acute kidney injury: an observational multicenter study employing the eICU database. PLoS ONE 17(8):e0273283. https://doi.org/10.1371/journal.pone.0273283
Lombardi G, Ferraro PM, Bargagli M, Naticchia A, D’Alonzo S, Gambaro G (2020) Hyperchloremia and acute kidney injury: a retrospective observational cohort study on a general mixed medical-surgical not ICU-hospitalized population. Intern Emerg Med 15(2):273–280. https://doi.org/10.1007/s11739-019-02165-6
Kee YK, Jeon HJ, Oh J, Shin DH (2020) Dyschloremia is associated with failure to restore renal function in survivors with acute kidney injury: an observation retrospective study. Sci Rep 10(1):19623. https://doi.org/10.1038/s41598-020-76798-5
Barhight MF, Brinton JT, Soranno DE, Faubel S, Mourani PM, Gist KM (2020) Effects of hyperchloremia on renal recovery in critically ill children with acute kidney injury. Pediatr Nephrol 35(7):1331–1339. https://doi.org/10.1007/s00467-020-04513-7
Mandai S, Kanda E, Iimori S, Naito S, Noda Y, Kikuchi H et al (2017) Association of serum chloride level with mortality and cardiovascular events in chronic kidney disease: the CKD-ROUTE study. Clin Exp Nephrol 21(1):104–111. https://doi.org/10.1007/s10157-016-1261-0
Kubota K, Sakaguchi Y, Hamano T, Oka T, Yamaguchi S, Shimada K et al (2020) Prognostic value of hypochloremia versus hyponatremia among patients with chronic kidney disease-a retrospective cohort study. Nephrol Dial Transplant 35(6):987–994. https://doi.org/10.1093/ndt/gfy299
National Health and Nutrition Examination Survey data [https://wwwn.cdc.gov/nchs/nhanes/Default.aspx]
De Bacquer D, De Backer G, De Buyzere M, Kornitzer M (1998) Is low serum chloride level a risk factor for cardiovascular mortality? J Cardiovasc Risk 5(3):177–184
Testani JM, Hanberg JS, Arroyo JP, Brisco MA, Ter Maaten JM, Wilson FP et al (2016) Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail 18(6):660–668. https://doi.org/10.1002/ejhf.477
Grodin JL, Testani JM, Pandey A, Sambandam K, Drazner MH, Fang JC et al (2018) Perturbations in serum chloride homeostasis in heart failure with preserved ejection fraction: insights from TOPCAT. Eur J Heart Fail 20(10):1436–1443. https://doi.org/10.1002/ejhf.1229
NaM Y, Bellomo R, Hegarty C, Story D, Ho L, Bailey M (2012) Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308(15):1566–1572. https://doi.org/10.1001/jama.2012.13356
de Vasconcellos K, Skinner DL (2018) Hyperchloraemia is associated with acute kidney injury and mortality in the critically ill: a retrospective observational study in a multidisciplinary intensive care unit. J Crit Care 45:45–51. https://doi.org/10.1016/j.jcrc.2018.01.019
Takenaka T, Kanno Y, Kitamura Y, Hayashi K, Suzuki H, Saruta T (1996) Role of chloride channels in afferent arteriolar constriction. Kidney Int 50(3):864–872. https://doi.org/10.1038/ki.1996.386
Takenaka T, Epstein M, Forster H, Landry DW, Iijima K, Goligorsky MS (1992) Attenuation of endothelin effects by a chloride channel inhibitor, indanyloxyacetic acid. Am J Physiol 262(5 Pt 2):F799–F806. https://doi.org/10.1152/ajprenal.1992.262.5.F799
Rein JL, Coca SG (2019) “I don’t get no respect”: the role of chloride in acute kidney injury. Am J Phy Renal Phy 316(3):F587–F605. https://doi.org/10.1152/ajprenal.00130.2018
Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ et al (2010) Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis 56(5):907–914. https://doi.org/10.1053/j.ajkd.2010.03.023
Shah SN, Abramowitz M, Hostetter TH, Melamed ML (2009) Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 54(2):270–277. https://doi.org/10.1053/j.ajkd.2009.02.014
Simpson DP (1971) Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 50(6):503–541. https://doi.org/10.1097/00005792-197111000-00002
Halperin ML, Ethier JH, Kamel KS (1989) Ammonium excretion in chronic metabolic acidosis: benefits and risks. Am J Kidney Dis Off J Nat Kidney Found 14(4):267–271. https://doi.org/10.1016/s0272-6386(89)80200-8
Wesson DE, Buysse JM, Bushinsky DA (2020) Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol 31(3):469–482. https://doi.org/10.1681/ASN.2019070677
Acknowledgements
We extend our gratitude to all the participants who contributed to this study.
Funding
This work was supported by the High-level Local Universities (Cultivation) Construction Program (22MC2022001).
Author information
Authors and Affiliations
Contributions
PZ and HD designed the research and wrote the original draft. PZ and YL collected and analyzed the data. PY, ZF, LG, BH revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors certify that they have no conflicting financial interests.
Ethical approval
The NCHS Ethics Review Board approved all techniques used in this study that involved using materials, data, or human subjects in accordance with the Declaration of Helsinki. With written informed consent, the patients/participants gave their approval to be included in this research.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, P., Li, Y., Fei, Z. et al. Association between serum chloride levels and estimated glomerular filtration rate among US adults: evidence from NHANES 1999–2018. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04119-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04119-0