Abstract
Background
Ferric citrate hydrate (FC), an oral iron product is approved as iron preparation for iron deficiency anemia and phosphate binder for chronic kidney disease (CKD). We investigated whether gastric acid secretion inhibitors (GASI) influenced on iron absorption and phosphate-lowering effects of FC.
Methods
Two phase 3 studies of FC for treatment of hyperphosphatemia in CKD patients (non-dialysis-dependent, 12 weeks, and hemodialysis, 52 weeks), were retrospectively analyzed. Patients were divided into with or without concomitant GASI and levels of iron- and phosphate-related parameters were analyzed.
Results
In non-dialysis study (FC, 60 patients; placebo, 30 patients), 14 FC patients and 14 placebo patients used GASI. No significant differences were found between the FC and placebo groups for adjusted mean differences (95% CI) of changes from baseline to end of treatment (EOT) in serum ferritin [104.84 ng/mL (35.97, 173.71) with GASI vs 145.30 ng/mL (96.34, 194.25) without GASI, P = 0.34], and transferrin saturation (TSAT) [12.56% (− 0.83, 25.95) with GASI vs 18.56% (8.15, 28.98) without GASI, P = 0.49]. In hemodialysis study, 95/180 patients used GASI. Mean changes (SD) from baseline to EOT in serum ferritin were 166.32 ng/mL (153.70) with GASI and 155.16 ng/mL (139.47) without GASI, and for TSAT were 16.60% (19.44) with GASI and 16.02% (18.81) without GASI. In both studies, there were no differences in the changes from baseline to EOT in serum phosphate between with and without GASI cohorts.
Conclusion
GASI did not influence on the changes in serum ferritin, TSAT and serum phosphate by FC administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is important for various biological processes and, therefore, its homeostasis is tightly regulated. Ingested iron is dissolved at a low pH by gastric acid secreted in the stomach, which is an important process for its effective absorption in the small intestine [1]. Patients taking gastric acid secretion inhibitors (GASI), such as proton pump inhibitors and histamine-2 receptor antagonists, showed decreased iron absorption and increased risk of iron deficiency, which were dose- and treatment duration-dependent [2]. The incidence rate of iron deficiency was high in patients who underwent gastrectomy or gastric bypass surgery [3, 4]. These findings demonstrate that the low pH of gastric acid is essential for dietary iron absorption.
Ferric citrate hydrate (FC, Riona®, Torii Pharmaceutical Co., Ltd., Tokyo, Japan) is an oral iron-based phosphate binder, and the ferric iron of FC binds to dietary phosphorus to form an insoluble complex that promotes the fecal excretion of phosphorus [5], thereby effectively lowering serum phosphate in patients with chronic kidney disease (CKD) who are non-dialysis-dependent [6] or undergoing dialysis [7,8,9]. In addition, ferric iron from FC was enzymatically reduced to ferrous iron and absorbed in the small intestine [10, 11], which improved anemia in patients with iron deficiency anemia [12]. Therefore, FC is also indicated for use as an iron preparation for patients with iron deficiency anemia in Japan. In the USA, ferric citrate (Auryxia®; Akebia Therapeutics Inc., Cambridge, MA, USA) is indicated for use as a phosphate binder in patients with CKD undergoing dialysis and as an iron preparation to treat iron deficiency anemia in patients with CKD not undergoing dialysis. In Taiwan, ferric citrate (Nephoxil®, Panion & BF Biotech Inc., Taipei, Taiwan) is indicated for use as a phosphate binder in patients with CKD undergoing hemodialysis.
FC needs to be dissolved in the stomach to exert its phosphate-lowering and iron absorption effects. In a previous study, FC showed a similar phosphate-lowering effect in CKD patients with hyperphosphatemia undergoing hemodialysis when treated with or without a concomitant histamine-2 receptor antagonist [13]. These results suggest FC can be dissolved, even when gastric acid secretion has been inhibited, and has a consistent phosphate-lowering effect regardless of the pH level of the stomach.
The suppression of gastric acid by omeprazole (proton pump inhibitor) was reported to impair the absorption of an oral ferrous iron preparation (ferrous sulfate) in a rat model and in patients with iron deficiency anemia [14, 15]; however, no study has investigated the absorption of oral ferric iron preparations. Therefore, it is unclear whether iron absorption from FC is affected by GASI use.
In this study, we retrospectively investigated data from two phase 3 clinical studies to determine the influence of GASI on the iron absorption and phosphate-lowering effects of FC in CKD patients with hyperphosphatemia who were non-dialysis-dependent [6] or who were undergoing hemodialysis [7].
Materials and methods
Study design
This was a retrospective study using data from two previous clinical studies to investigate FC in CKD patients with hyperphosphatemia. The GBA4-4 study was a 12-week, phase 3, multicenter, randomized, double-blind, placebo-controlled, dose-titration study in non-dialysis-dependent CKD patients [6], and the GBA4-6 study was a 52 week, phase 3, multicenter, open-label, dose-titration study in CKD patients undergoing hemodialysis [7].
Patients
Detailed inclusion and exclusion criteria and interventions for these studies were described previously [6, 7]. Briefly, CKD patients with hyperphosphatemia, who were ≥ 20 years old when informed consent was provided, were recruited. Patients included in GBA4-4 were at CKD stages 3 − 5 and received standard conservation therapy and had a serum phosphate level ≥ 5.0 mg/dL and < 8.0 mg/dL at 2 weeks before, 1 week before, or at FC treatment initiation. Patients included in GBA4-6 were undergoing hemodialysis three times a week for ≥ 3 months before treatment initiation and had a serum phosphate level ≥ 3.5 mg/dL and < 10.0 mg/dL with hyperphosphatemia therapy or ≥ 6.1 mg/dL and < 10.0 mg/dL without hyperphosphatemia therapy at FC treatment initiation. Patients who had gastrointestinal disease and previous gastrectomy or duodenectomy were excluded. FC (Riona® 250 mg, containing approximately 60 mg of ferric iron) was taken orally three times a day immediately after meals. The starting dose was 1500 mg/day and the dose was adjusted up to 6000 mg/day to achieve a serum phosphate level ≥ 2.5 mg/dL and ≤ 4.5 mg/dL (GBA4-4) or ≥ 3.5 mg/dL and ≤ 6.0 mg/dL (GBA4-6). The FC treatment periods were 12 weeks (GBA4-4) and 52 weeks (GBA4-6).
The use of GASI [proton pump inhibitors (Omeprazole, Lansoprazole, and Rabeprazole Sodium) or histamine-2 receptor antagonists (Cimetidine, Ranitidine Hydrochloride, Famotidine, Nizatidine, Roxatidine Acetate Hydrochloride, and Lafutidine)] from 4 weeks before treatment initiation until the end of treatment (EOT, end of the study or treatment discontinuation) was determined for all included patients. Patients were divided into two cohorts: patients treated with or without GASI. For the GBA4-4 study, patients were divided into two cohorts (with or without GASI) for each treatment group (FC and placebo groups).
Analysis of iron-related and mineral and bone disorder-related parameters
From each study, iron-related parameters, including the levels of serum iron, serum ferritin, total iron-binding capacity (TIBC), transferrin saturation (TSAT), and hemoglobin (Hb), were collected from the safety analysis set, and mineral and bone disorder (MBD)-related parameters, including serum phosphate, serum corrected calcium (cCa), intact parathyroid hormone (iPTH), and calcium–phosphate calculated variable (Ca*P), were collected from the efficacy analysis set (see “Statistics”). These evaluation items were measured at screening, baseline, and at pre-determined intervals throughout the study period.
Statistics
Iron-related parameters and Hb were analyzed from data of patients who received FC at least once and data for safety evaluation items were collected (safety analysis set). MBD-related parameters were analyzed from the data of patients who received FC and data for efficacy evaluation items were collected at least once (efficacy analysis set). In each study, changes from baseline to the EOT (at 12 weeks for GBA4-4, 52 weeks for GBA4-6, or at discontinuation) were calculated for all safety and efficacy evaluation items. For the GBA4-4 study, adjusted mean differences [(least square mean of FC) − (least square mean of placebo)] and 95% confidence intervals (CI) were calculated for differences between FC and placebo groups in changes from baseline to EOT and compared using analysis of covariance. For the GBA4-6 study, changes from baseline were summarized descriptively. SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Use of GASI
Data were collected from 90 non-dialysis-dependent CKD patients from GBA4-4. All patients were randomized 2:1 to FC (n = 60) or placebo (n = 30) groups and were included in the safety analysis. Patients, whose administration was wrong or effective evaluation was missing, were excluded from the efficacy analysis (n = 3 from the FC group and n = 1 from the placebo group). Approximately 25% of patients in the FC group (13/60 patients) and 50% in the placebo group (13/30 patients) were treated with GASI (Fig. 1). Data were collected from 180 patients in GBA4-6 undergoing hemodialysis. All of these patients were treated with FC and all efficacy evaluation items were present; therefore, all data were analyzed for safety and efficacy. Approximately half of these (95/180 patients) were treated with GASI (Fig. 2).
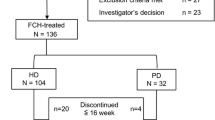
Patient flow in the GBA4-4 study. Flow of non-dialysis-dependent patients from the GBA4-4 study. a A patient may have multiple reasons to withdraw. b Serum phosphate level was < 2.5 mg/dL in two consecutive investigations after FC treatment initiation. BL baseline, EOT end of treatment, FC ferric citrate hydrate, GASI gastric acid secretion inhibitor
Patient flow in the GBA4-6 study. Flow of patients undergoing hemodialysis from the GBA4-6 study. a Ferritin level ≥ 800 ng/mL. b Serum phosphate level was < 3.0 mg/dL in two consecutive investigations. c Investigation was not possible for patient’s reason. d Serum corrected calcium level was < 7.5 mg/dL in two consecutive investigations. e Serum phosphate level was ≥ 10.0 mg/dL in two consecutive investigations. BL baseline, EOT end of treatment, FC ferric citrate hydrate, GASI gastric acid secretion inhibitor
Influence of GASI on non-dialysis-dependent patients (GBA4-4)
Time-course changes in serum ferritin and TSAT levels are shown in Fig. 3. The levels of serum ferritin gradually increased in the FC group after FC treatment initiation and this increase was also observed in with and without GASI cohorts (Fig. 3a). TSAT levels were also increased with time in the FC group, and the increase was similar in with and without GASI cohorts (Fig. 3b). The changes from baseline to EOT in these iron-related parameters and Hb are summarized in Table 1. Serum ferritin increased from baseline to EOT in the FC group regardless of GASI use and the adjusted mean differences (95% CI) compared with the placebo group were 104.84 ng/mL (35.97, 173.71) with GASI and 145.30 ng/mL (96.34, 194.25) without GASI use. No significant interaction with GASI use was detected (P = 0.34). The adjusted mean differences (95% CI) in changes in TSAT between the FC and placebo groups were 12.56% (− 0.83, 25.95) with GASI and 18.56% (8.15, 28.98) without GASI use. There were no significant interactions for GASI use (P = 0.49). Similarly, an analysis of covariance did not detect any significant influence of GASI use on serum iron, TIBC, and Hb.
Time-course changes in serum ferritin and TSAT in non-dialysis-dependent patients from the GBA4-4 study (safety analysis set). Time-course changes in serum ferritin (a) and TSAT (b). Blue lines, FC group; gray lines, placebo group; solid lines, with GASI cohort; broken lines, without GASI cohorts. Data are the mean ± standard deviation. BL baseline, EOT end of treatment, FC ferric citrate hydrate, GASI gastric acid secretion inhibitor, Scr screening, TSAT transferrin saturation
Time-course changes in serum phosphate are shown in Fig. 4. Serum phosphate levels were similar in the FC and placebo groups at baseline, and they were lower in the FC group compared with the placebo group when the treatment advanced. There was no notable difference in these parameters between with and without GASI cohorts. The changes in MBD-related parameters from baseline to EOT in the FC and placebo groups with and without GASI use are summarized in Table 2. The adjusted mean differences (95% CI) in the reduction of serum phosphate in the FC group compared with the placebo group were − 0.85 mg/dL (− 1.70, − 0.01) with GASI and − 1.61 mg/dL (− 2.23, − 0.98) without GASI, indicating no significant interaction with GASI use (P = 0.16). Similarly, there was no interaction with GASI use in Ca*P change (adjusted mean differences (95% CI) − 6.37 (mg/dL)2 (− 13.17, 0.43) with GASI and − 12.66 (mg/dL)2 (− 17.68, − 7.64) without GASI, P = 0.14). GASI use did not influence changes in serum cCa by FC treatment, but did influence changes in iPTH [adjusted mean differences (95% CI): 0.4 pg/mL (− 79.5, 80.3) with GASI and − 116.3 pg/mL (− 175.5, − 57.2) without GASI, P = 0.02].
Time-course changes in serum phosphate in non-dialysis-dependent patients from the GBA4-4 study (efficacy analysis set). Time-course changes in serum phosphate. Blue lines, FC group; gray lines, placebo group; solid lines, with GASI cohort; broken lines, without GASI cohorts. Data are the mean ± standard deviation. BL baseline, EOT end of treatment, FC ferric citrate hydrate, GASI gastric acid secretion inhibitor, Scr screening
The mean (standard deviation; SD) doses of FC in the safety analysis set were 3,240 mg/day (725) with GASI (n = 14) and 3,445 mg/day (831) without GASI (n = 46), respectively.
Influence of GASI on patients under hemodialysis (GBA4-6)
In GBA4-6, patients undergoing hemodialysis were treated with FC for 52 weeks. Time-course changes in iron-related parameters for with and without GASI cohorts are shown in Fig. 5. Serum ferritin (Fig. 5a) and TSAT (Fig. 5b) gradually increased during the treatment period and the levels were similar in with and without GASI cohorts. Changes from baseline to EOT in iron-related parameters and Hb are summarized in Table 3. Mean changes (SD) from baseline to EOT in serum ferritin were 166.32 ng/mL (153.70) in patients with GASI and 155.16 ng/mL (139.47) in patients without GASI, and those in TSAT were 16.60% (19.44) with GASI and 16.02% (18.81) without GASI. Changes in other parameters were similar in both cohorts.
Time-course changes in serum ferritin and TSAT in patients undergoing hemodialysis from the GBA4-6 study (safety analysis set) Time-course changes in serum ferritin (a) and TSAT (b). Solid line, with GASI cohort; broken line, without GASI cohorts. Data are the mean ± standard deviation. BL baseline, EOT end of treatment, FC ferric citrate hydrate, GASI gastric acid secretion inhibitor, Scr screening, TSAT transferrin saturation
Time-course changes in serum phosphate are shown in Fig. 6. The levels of serum phosphate were similar in with and without GASI cohorts throughout the observation period. The mean changes (SD) from baseline to EOT in serum phosphate were − 0.23 mg/mL (1.41) in patients with GASI and 0.02 mg/mL (1.69) in patients without GASI. Similarly, the changes from baseline to EOT in serum cCa, iPTH, and Ca*P were comparable between with and without GASI cohorts (Table 3).
Time-course change in serum phosphate in patients undergoing hemodialysis from the GBA4-6 study (efficacy analysis set) Time-course changes in serum phosphate. Solid line, with GASI cohort; broken lines, without GASI cohorts. Data are the mean ± standard deviation. BL baseline, EOT end of treatment, FC ferric citrate hydrate, GASI gastric acid secretion inhibitor, Scr screening
The mean (SD) dose of FC in the safety analysis population was 2619 mg/day (1113) in the with GASI cohort (n = 95) and 2854 mg/day (1164) in the without GASI cohort (n = 85).
Discussion
It is thought that the low pH of gastric acid is essential for the absorption of dietary iron and oral iron preparations, whether ferrous iron or ferric iron [1], and that the increase in intragastric pH by concomitant use of GASI might affect iron absorption from FC. Therefore, this study retrospectively investigated whether the iron absorption and phosphate-lowering effects of FC were influenced by GASI use. In the 12 week, randomized control study of non-dialysis-dependent CKD patients (GBA4-4), the FC group had a tendency towards increased serum ferritin and TSAT compared with placebo. This tendency was observed regardless of GASI use. Serum phosphate tended to be decreased in the FC group compared with placebo, regardless of GASI use. In the 52 week study of CKD patients undergoing hemodialysis (GBA4-6), the effect of FC to increase serum ferritin and TSAT was intact regardless of GASI use. Furthermore, FC maintained lower levels of serum phosphate regardless of GASI use. The dose of FC might strongly influence iron-related parameters, however, the mean doses of FC in both studies were similar between with or without GASI. This study demonstrated that GASI use considered not to influence on the iron absorption and phosphate-lowering effects of FC in patients with CKD.
The phosphate-lowering effect of other phosphate binders used to treat hyperphosphatemia in CKD patients undergoing hemodialysis, such as calcium carbonate and lanthanum carbonate, was hindered in patients with concomitant GASI use [16,17,18]. In the present study, we confirmed that the use of GASI did not interfere with the serum phosphate-lowering effect of FC in non-dialysis-dependent CKD patients and CKD patients undergoing hemodialysis, as predicted by a previous clinical study in patients with CKD undergoing hemodialysis treated with or without a concomitant histamine-2 receptor antagonist [13].
A deficiency or overload of iron can cause serious health problems; therefore, to maintain homeostasis, iron absorption is strictly regulated. FC is a trivalent ferric iron, which must be reduced to divalent ferrous iron to be absorbed in the small intestine. The absorption of dietary ferric iron or iron preparations is reported to be lower than that of dietary ferrous iron or iron preparations because of their lower solubility and bioavailability [10, 20]. FC is formulated to have a large surface area for high solubility (32.4–39.9 m2/g for FC vs 0.62 m2/g for general ferric citrate products that are approved as a dietary supplement) [19], which may have resulted in the solubility at pH 6.8 being as rapid as that at pH 1.2 and the comparable elution behavior of iron from FC at pH 1.2, 4.0, and 6.8 (Fig. 7) [19]. Ferric Citrate (Auryxia®) which is a similar FC product and approved in the USA, the rate of dissolution at pH 8 was reported to be 3.08 times the rate of commercial-grade ferric citrate [11]. The properties of FC that allow it to be soluble at a high pH might enhance ferric iron absorption in the duodenum [10] and contribute to its effects on iron absorption and phosphate-lowering, as shown in this study.
Elution behavior of iron from ferric citrate hydrate (Riona®) under different pH conditions. The elution solution was filtered through a 0.45 µm membrane filter, followed by reduction using L-ascorbic acid. The iron concentration was determined by the 1,10-phenanthroline spectrometric method and the elution rate was calculated using an iron standard solution (1000 mg/L Japan Calibration Service System), which was used as a 100% elution solution. The mean elution ratio of solutions adjusted for pH 1.2 (a), pH 4.0 (b), pH 6.8 (c), and a water control (d) (n = 6 for each). Modified from Koyama et al. [19]
FC achieves its iron absorption and phosphate-lowering effects via two contrasting mechanisms: ferric iron from FC, enzymatically reduced to ferrous iron, is absorbed in the small intestine [10, 20], and concurrently, ferric iron from FC binds to dietary phosphorus to form an insoluble complex that promotes the fecal excretion of phosphorus [5]. How this absorption–excretion balance is regulated is not known. Iron and phosphate are essential for the human body; therefore, they are thought to be regulated individually by such as hepcidin [21, 22] and fibroblast growth factor-23 [23, 24]. Gastrointestinal complications are known to occur in approximately 70% of patients with renal failure [25]. Accordingly, FC is considered to be effective in patients with CKD who are taking GASI not only as a phosphate binder but also as an iron preparation.
This was a retrospective study and the number of patients included was insufficient to make firm conclusions. Further prospective studies are needed and should include patients with iron deficiency anemia without CKD.
Conclusions
This retrospective study using data from FC-treated CKD patients who were non-dialysis-dependent or were undergoing hemodialysis demonstrated that GASI use did not influence on the changes in iron-related parameters, such as serum ferritin and TSAT and serum phosphate by FC administration.
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.
References
Jacobs A, Miles PM (1969) Role of gastric secretion in iron absorption. Gut 10:226–229
Lam JR, Schneider JL, Quesenberry CP, Corley DA (2017) Proton pump inhibitor and histamine-2 receptor antagonist use and iron deficiency. Gastroenterology 152:821–9.e1
Geokas MC, McKenna RD (1967) Iron-deficiency anemia after partial gastrectomy. Can Med Assoc J 96:411–417
Sandvik J, Bjerkan KK, Græslie H, Hoff DAL, Johnsen G, Klöckner C et al (2021) Iron deficiency and anemia 10 years after Roux-en-Y gastric bypass for severe obesity. Front Endocrinol (Lausanne) 12:679066
Iida A, Kemmochi Y, Kakimoto K, Tanimoto M, Mimura T, Shinozaki Y et al (2013) Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am J Nephrol 37:346–358
Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K et al (2014) Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9:543–552
Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y et al (2014) Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. J Ren Nutr 24:261–267
Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Hirakata H (2014) JTT-751 for treatment of patients with hyperphosphatemia on peritoneal dialysis. Nephron Clin Pract 128:135–140
Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y et al (2014) A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant 29:1053–1060
Pergola PE, Fishbane S, Ganz T (2019) Novel oral iron therapies for iron deficiency anemia in chronic kidney disease. Adv Chronic Kidney Dis 26:272–291
Ganz T, Bino A, Salusky IB (2019) Mechanism of action and clinical attributes of Auryxia® (Ferric Citrate). Drugs 79:957–968
Komatsu N, Arita K, Mitsui H, Nemoto T, Hanaki K (2021) Efficacy and safety of ferric citrate hydrate compared with sodium ferrous citrate in Japanese patients with iron deficiency anemia: a randomized, double-blind, phase 3 non-inferiority study. Int J Hematol 114:8–17
Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y (2012) Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol 36:478–487
Golubov J, Flanagan P, Adams P (1991) Inhibition of iron absorption by omeprazole in rat model. Dig Dis Sci 36:405–408
Sharma VR, Brannon MA, Carloss EA (2004) Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J 97:887–889
Matsunaga C, Izumi S, Furukubo T, Satoh M, Yamakawa T, Uchida T et al (2007) Effect of famotidine and lansoprazole on serum phosphorus levels in hemodialysis patients on calcium carbonate therapy. Clin Nephrol 68:93–98
Takahashi N, Shoji T, Matsubara K, Hitomi H, Hashimoto M, Kiyomoto H et al (1999) Effect of histamine H2-receptor antagonist on the phosphorus-binding abilities of calcium carbonate and calcium lactate in hemodialysis patients. J Am Soc Nephrol 10:1090–1094
Minakuchi H, Yoshida T, Kaburagi N, Fujino T, Endo S, Takemitsu TY et al (2020) Proton pump inhibitors may hinder hypophosphatemic effect of lanthanum carbonate, but not of ferric citrate hydrate or sucroferric oxyhydroxide, in hemodialysis patients. Ren Fail 42:799–806
Komiyama J, Yamane S, Shiota Y, Miyake S, Uehara H, Serigaya T et al (2015) Formulation characteristics of the new phosphate-binder, Riona® 250 mg. Jpn J Clin Dial 31:1543–1549 (ISSN:0910-5808 Japanese)
Wheby MS, Suttle GE, Ford KT 3rd (1970) Intestinal absorption of hemoglobin iron. Gastroenterology 58:647–654
Ganz T, Nemeth E (2012) Hepcidin and iron homeostasis. Biochim Biophys Acta 1823:1434–1443
Yokoyama K, Fukagawa M, Akiba T, Nakayama M, Ito K, Hanaki K et al (2019) Randomised clinical trial of ferric citrate hydrate on anaemia management in haemodialysis patients with hyperphosphataemia: ASTRIO study. Sci Rep 9:8877
Nakano C, Hamano T, Fujii N, Matsui I, Tomida K, Mikami S et al (2012) Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol 7:810–819
Ito K, Yokoyama K, Nakayama M, Fukagawa M, Hirakata H (2021) Association of fibroblast growth factor 23 and α-klotho in hemodialysis patients during administration of ferric citrate hydrate: post hoc analysis of ASTRIO study. BMC Nephrol 22:374
Shirazian S, Radhakrishnan J (2010) Gastrointestinal disorders and renal failure: exploring the connection. Nature Rev Nephrol 6:480–492
Acknowledgements
The original statistical analysis plan was made and conducted by Japan Tobacco Inc. We thank Mr. Minoru Kudo and Mr. Yoji Matsunaga of Torii Pharmaceutical Co., Ltd. for supporting this work. Medical writing support was provided by ASCA Corporation (Osaka, Japan). We received a permission to use (Fig. 7) from Nihon Medical Center Ltd.
Author information
Authors and Affiliations
Contributions
KI and KY: conceived and designed the study, and interpreted the data. KI: drafted the manuscript. All authors reviewed the manuscript and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
Kyoko Ito is an employee of Torii Pharmaceutical Co., Ltd. and Keitaro Yokoyama has received honoraria from Japan Tobacco Inc., Torii Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Kyowa Kirin Co., Ltd.
Ethical approval
The two studies that provided data for this study were conducted by Japan Tobacco Inc., in accordance with the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice and were registered at the Japan Pharmaceutical Information Center as CTI-111435 (GBA4-4) and CTI-111437 (GBA4-6). Informed consent was obtained from all individual participants before study enrollment [6, 7].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ito, K., Yokoyama, K. Iron absorption and phosphate-lowering effects of ferric citrate hydrate are not influenced by gastric acid secretion inhibitors in patients with chronic kidney disease: a retrospective post hoc analysis. Int Urol Nephrol 55, 141–150 (2023). https://doi.org/10.1007/s11255-022-03287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03287-1