Abstract
Kidney transplantation represents the gold standard treatment option for patients with end-stage renal disease. Improvements in surgical technique and pharmacologic treatment have continuously prolonged allograft survival in recent years. However, urological complications are frequently observed, leading to both postoperative morbidity and putative deterioration of allograft function. While open redo surgery in these patients is often accompanied by elevated surgical risk, endoscopic management of urological complications is an alternative, minimal-invasive option. In the present article, we reviewed the literature on relevant urological postoperative complications after kidney transplantation and describe preventive approaches during the pre-transplantation assessment and their management using minimal-invasive approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplantation (KT) is a therapeutic option for patients with end-stage renal disease, deliberating the recipients from the burden of regular dialysis. In 2018, over 90,000 KT were performed globally [1]. KT improves the survival of patients compared to peritoneal or hemo-dialysis [2]. Improvements in operative technique, donor and recipient selection, and immunosuppression have improved patients’ quality of life and also allograft survival in the last decades [3, 4]. From 2010 to 2014, the 1 and 5 years survival rate of deceased donor kidney allograft in the United States of America were 93.4 and 72.4%, respectively [5]. However, early and late-onset of surgical complications are frequently observed: Koçak et al. reported an overall complication rate of 15.7% in 362 cases of living donor KT, of which 8% were of urological nature (urinoma, ureteral stenosis, renal calculi, vesicoureteral reflux, lymphocele [6], ureteral necrosis [7]), while other surgical complications included vascular and wound healing problems, hematoma formation, and graft rupture [6]. Other authors reported on urological complication rates from 2.9 to 12.5% [7, 8].
The management of complications after KT may be challenging, due to an elevated risk after previous surgery and the need for ongoing immunosuppression in frequently comorbid patients [9]. Given recent advances in endoscopic urology, a minimal-invasive approach for urological complications might be a reasonable alternative. Moreover, meticulous preoperative urologic work-up prior to KT is demanded to identify risk profiles prior to surgery.
In the present article, we summarize available evidence for endoscopic and minimal-invasive treatment of post-KT urologic complications and provide putative approaches for clinical practice in adult patient. Given the paucity of high level evidence in the field, we conducted a targeted review of high-quality PubMed- indexed articles on the topic of urological complications in conjunction with KT. Moreover, we integrated additional aspects on the management of urological complications in the non-transplanted patient to further discuss the role of minimal-invasive approaches.
Special features of pre-operative preparation and surgical techniques
Pre-emptive urological assessment of the kidney transplant recipient and management of abnormal urinary tract
Pre-operative urological evaluation is an important prerequisite for preparation for KT and emphasis should be taken on the assessment of all functional and anatomical aspects of the urinary tract, as well as the exclusion of malignancy or chronic infection. Thorough patient history with voiding diary in presence of residual diuresis, physical examination, and ultrasound represent basic investigations which may be followed by uroflowmetry, cystoscopy, micturating cystourethrogram [10] and urodynamics [11], if indicated [12, 13]. Patients with a history of vesicoureteral reflux (VUR), pre-existing bladder emptying disorder, recurrent urinary tract infections (UTI), stone disease, urinary tract anomaly or after prior urologic surgery are at elevated risk to develop postoperative urologic complications [14] and may need extensive work-up. When the evaluation indicates bladder outlet obstruction (BOO) due to benign prostate hyperplasia (BPH) in male patients, the same stratification for either medical or surgical treatment according to non-transplanted patients may be performed [15]. Noteworthy, BPH was shown to be independently associated with urinary retention, urinary tract infection, and graft loss in patients after KT [16]. If surgical deobstruction e.g. with transurethral resection of the prostate (TUR-P) is indicated, it may either be performed prior [17] or after KT, although low or missing urine output before transplantation seems to promote bladder neck and urethral scarring [18]. In analogy, urethral strictures can be safely treated endoscopically with urethrotomy or open reconstruction surgery in dependence of the extent and the localization of the stricture [19, 20]. Using the endoscopic technique, it should be kept in mind that the risk of disease recurrence is elevated, compared to open urethral reconstruction. Regular urological follow-up in these patients is mandatory to screen for recurrent urinary retention with subsequent allograft damage.
Smaller surgical series have shown that transplantation in a dysfunctional bladder can be successful depending on the precautious selection of cases: high intravesical pressure (> 100 cmH2O peak pressure) and low bladder capacity (< 100 ml of volume) were identified to predispose to complications after KT [21]. In these cases, bladder augmentation may be discussed. In patients with a history of urethral valves [22], urinary diversion [23], or bladder augmentation [24] successful KT has been described but should be reserved for specialized centers.
In specific indications prior to transplantation, native nephrectomy may become necessary: unilateral or bilateral nephrectomy can improve pre-transplant clinical conditions of the patient in case of large proteinuria, UTI on the basis of VUR [25], and hypertension [26]. In cases of large polycystic kidneys, nephrectomy creates intraabdominal space and may remit possible symptoms like abdominal or flank pain, recurrent cyst infections or recurrent bleeding due to rupture of cysts [17].
Technique of ureteral re-implantation and association to urological complications
The standard procedure for KT is an extraperitoneal approach after pelvic access, usually in the right, less common in the left iliac fossa (first described in 1951 from Kuss et al. [27]). Another surgical approach is a midline incision and placement of the graft intra-abdominally. This approach is chosen for example when the KT is combined with pancreas transplantation, in a complex vascular situation or after previous bilateral KT. The vessels are connected to the external or internal iliac artery on the one hand and to the external iliac vein on the other hand [28].
Subsequently, the ureteroneocystostomy (UCN) is conducted. Implantation techniques aim to achieve reflux protection while preserving optimal urine outlet and avoiding scar formation or inadequate perfusion of the transplant ureter. First anti-refluxive ureter implantation techniques have been developed in the 1950s by Politano imitating natural urinary tract conditions based on the assumption, that VUR can impair allograft function through increasing upper urinary tract pressure and the risk of pyelonephritis. Since then, the technique itself has been modified and other surgical techniques have been developed. The Politano–Leadbetter operating technique (PL) foresees an anterior cystostomy, the creation of a submucosal tunnel of 2–3 cm from inside the bladder and the feeding of the distal ureter through a new opening close to the trigonum [29, 30]. The Lich–Gregoir (LG) technique uses a 4 cm extravesical incision through the seromuscularis, and a 1 cm incision into the mucosa at the distal edge of the primary incision. The distal ureter is then sutured to the mucosa and the seromuscularis is closed over the ureter course to provide reflux protection [30]. Another extravesical approach (Woodruff) incises the seromuscularis from the outside of the bladder, conducts a smaller incision in the mucosa, implants the spatulated ureter into the mucosa but does not close the submucosal incision over the ureter course [31].
Thrasher et al. compared two subgroups of each 160 patients receiving ureter re-implantation by either the PL- technique or the LG- technique. The total number of urological complications was 9.4% in the PL- technique group and 3.7% in the LG technique group (p = 0.04). With 3.7%, the rate of uretero-vesical junction obstruction was significantly higher in the PL- technique group compared to the LG technique group (0.6%; p* = 0.05) [30]. A meta-analysis conducted by Alberts et al. in 2014 comparing the two main techniques arrives at the same result: the prevalence of both urinoma and hematuria were significantly reduced when using the LG- technique [32]. Today, anti-refluxive ureter implantation techniques are the gold standard in KT, and, based on the data mentioned above, guidelines strongly favor the use of the LG- approach. As an alternative, pyeloureteral or uretero-ureteral anastomosis can be discussed [33]. The perioperative placement of ureteral stents is a strongly recommended measure to avoid ureteral complications in the early postoperative course after KT [33]. In a study by Kumar et al., evaluating 670 living donor KTs, the application of ureteral stents reduced ureteral complications from 8.5 to 0.22% [34]. Nevertheless, stent-associated complications have also been reported: the incidence of UTI seems to be elevated in kidney graft recipients with ureteral stents [35], with the incidence increasing with the indwelling time of the stent [36].
Endoscopic management of postoperative urologic complications in kidney allograft recipients
An overview of the data on endoscopic treatment of urological complications after KT can be found in Table 1.
Ureteral stenosis
Ureteral stenosis (US) after KT may appear in form of ureterovesical junction obstruction (UVJO) and more proximal US. US is a complication that occurs in 2–10% of cases and typically becomes evident within the first 3 months after transplantation. Mostly, it is caused by ureteral ischemia due to loss of distal ureteral perfusion through graft explantation, less common causes are pre-existing anatomic anomaly, hematoma, lymphocele, calculi, tumor manifestation or scarring following UCN [37]. The diagnosis of obstruction may be challenging due to the possible absence of hydronephrosis. However, renal scintigraphy can help to reveal urinary obstruction. The European Association of Urology (EAU) guidelines recommend the placement of a nephrostomy as the primary approach and simultaneous diagnostic measure for UVJO [33, 34], followed by definite therapy where appropriate. Data on endoscopic treatment of US and UVJO after KT is available from a limited number of single-center series. Here, the principles of endoscopic treatment are adapted from clinical experience with the benign US in non-transplanted patients.
The permanent supply with standard polymer ureteral stent is a reasonable option in patients unfit or unwilling to undergo surgery: the literature reports of success rates of up to 100% in the intrinsic US [38, 39]. Stent exchange via the ureteral implantation site at the bladder dome can be challenging, so the use of assisting devices, like a 14/16 French ureteral access sheath, have been described [40].
To avoid the inconvenience and costs of regular stent exchanges, two different permanent metal stent approaches have been introduced. The all-metal Resonance® stent has shown success rates of 80% in the benign US [41] while reducing material and operation costs over 50% per year per patient [42]. The self-expanding thermolabile nitinol stent Memokath™ has also been applied for the supply US after KT: In 2013, Bach et al. demonstrated a success rate of 87% with a medium indwelling time of the stent of 4 years and no perioperative complications [43].
Apart from the application of permanent stents, balloon dilation and US-incision are the two main operative principles described for endoscopic treatment of US. Here, the incision of the stricture may be performed by laser, cold knife or electrosurgical instrument. Usually, the incision is conducted in dorsolateral direction for the proximal US and in ventromedial direction for distal US [44]. The report success rates for the incision of distal US/UVJO varies between 62 and 88% [45,46,47,48,49]. In 2013, Arrabal-Martín published data of 18 patients with obstructive megaureter, obstruction secondary to bladder surgery, and orthotopic ureterocele with lithiasis with ureteral ostium incision. The incision was performed after placement of a guidewire or ureteral catheter via endoscopic scissors and cold cutting in medial and dorsal direction (5 o’clock position on the right side and 7 o’clock position on the left side). The procedure turned out to be a safe and effective treatment: all but one patient were treated on an outpatient basis, the grade of hydronephrosis decreased or disappeared after 3 months and the preoperative pain resolved completely in 17 out of 18 cases after 3 and 24–36 months [50].
In more proximal benign US, the endoscopic treatment also appeared to be a feasible therapeutic option with low complication rates: Lojanapiwat et al. report success rates of 75% after transurethral incision [49].
Comparing laser and cold knife incision in endoscopic US treatment, Dutkiewicz et al. in 2012 observed no differences in the success or complication rates [51]. Even though heterogeneous data exists as to which technique should be preferred [52], the current guidelines favor the antegrade approach and laser incision in short strictures [53].
Another endoscopic approach is the balloon dilation of the US: Byun et al. performed a 5–10 min balloon dilation of 21 benign US with a subsequent ureteral stent supply of 3 weeks releasing success rates of 57% [54].
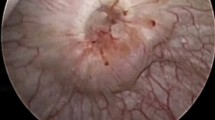
Balloon dilation seems efficient in benign, non-ischemic strictures shorter than 2 cm [54,55,56]. Figure 1 illustrates balloon dilatation in a proximal ureteral stenosis after KT. In the literature, balloon dilation of US in KT patients was reported with a success rate of only 12.5% after a median follow up of 11.4 months [57]. Gil-Sousa et al. described a US recurrence rate of 47% with a mean time to recurrence of 6.9 months after balloon dilation [58]. One reason for the unsatisfactory data might be the mostly ischemic etiology after KT, which warrants consideration in planning the procedure. The combination of both incision and dilation of the US seems to improve the success rate of balloon dilation alone. In 2012, Christman et al. described the endoscopic treatment in 17 pediatric patients with a median age of 7 years suffering from primary obstructive megaureter (POM) using combined laser and balloon dilatation: if shorter than 2 cm, the stricture was dilated with balloon only, if longer, the stricture was incised with laser and then dilated with a balloon. All patients experienced resolution of obstruction during the median follow-up time of 3.2 years [59]. In KT patients, Gdor et al. described a success rate of 67% in 6 KT patients with UVJO treated with holmium laser incision and balloon dilation at the same time, with the success rate being 100% in strictures with a maximum expansion of 1 cm in a mean follow up of 52 months [60].
a–d Interventional balloon dilatation in secondary proximal ureteral stricture caused by superinfected lymphocele formation. a Radiographic illustration of short proximal ureter stricture (indicated by arrow). b Insertion of endoscopic balloon inflation device (arrow: radiopaque proximal and the distal end of the inflation balloon). c Activation of the dilation balloon and stricture dilation. d Final result with regular contrast passage after dilation
While endoscopic techniques appear helpful in the treatment of US in KT patients and these interventions are reflected by contemporary guidelines, length of the stricture and etiology seem to be the most relevant predictive and selection parameters for the minimally invasive approach. Therefore, for US shorter than 3 cm, the panel recommends the endoscopic approach (balloon dilation or antegrade laser incision) or primary surgical reconstruction, for longer or recurrent US, surgical reconstruction is being favored [33, 61].
Endoscopic management of vesicoureteral reflux (VUR) after KT
The incidence of vesicoureteral reflux (VUR) after KT varies from 10.5 [62] up to 86% [63] in literature, with data suggesting that deceased donor organs have a greater risk of developing VUR compared to living donor allografts [64]. Comparing the different UCN techniques, the incidence of VUR using the LG technique was reported to be higher than using the PL technique [62]. Even though older data indicates that VUR may increase the risk of long term graft failure [65], more recent data could not confirm the results: in a study cohort of 1008 adult kidney transplant recipients examined by Molenaar et al. in 2017, VUR did not have an impact on bacteriuria, allograft function after 3 and 12 months, as well as 1- and 5-year-graft survival [62]. Favi et al. showed similar results in 2017 [66]. Still, asymptomatic VUR needs to be distinguished from VUR with recurrent UTI. UTI is the most common type of infection following kidney transplantation (up to 47%) [67]. Especially in combination with VUR, UTI may manifest as graft pyelonephritis. A series of dimercaptosuccinic acid (DMSA) scans described that 69% of the pediatric patients suffering from VUR into the native kidneys and recurrent urinary tract infection showed renal scarring (p = 0.001) [68], a finding confirmed by pathological examinations [69]. In analogy, in high-grade VUR with recurrent pyelonephritis, therapeutic intervention may be indicated after KT.
While in pediatric patients with low-grade VUR, the disease tends to resolve spontaneously (13% annual rate for the first 5 years [70]), this can by nature not be expected after KT. However, conservative treatment with prophylactic antibiotics like trimethoprim (± sulfamethoxazole) and nitrofurantoin [71] can be administered in KT patients bearing in mind limitations of these compounds in patients with reduced GFR [72]. The injection of a bulking agent like dextranomer/hyaluronic acid copolymer (DHAC, Zuidex™) below the ureteral orifice or in the ureteral tunnel resolves the VUR by a single injection and only 3% of pediatric patients experience febrile UTI in the follow-up period of several years [73]. The injection of bulking agents in refluxive orifices of adult KT has also been described as a convenient therapeutic option: in 2011, Pichler et al. showed a significantly reduced incidence of UTI in 19 kidney transplant recipients with VUR after DHAC application in a median follow up time of 6.5 months [74]. Despite an overall low level of evidence in the KT population, current guidelines recommend the endoscopic approach as first-line treatment [33].
Apart from VUR in the kidney allograft, the presence of VUR in the native kidneys in case of reflux nephropathy increases the risk of UTI, with increasing numbers in higher grade reflux [75]. Techniques like native nephrectomy in high-grade reflux or distal ureter ligation in low-grade reflux during transplantation were shown to have a protective effect [76].
Endoscopic management of urolithiasis in the transplanted patient
The formation of renal calculi is a rare complication after KT. The incidence of urolithiasis after KT is 1% after a median time of 28 months. The stone composition is merely calcium-based in 67%, struvite in 20% and uric acid in 13% [77]. Co-existing conditions of end-stage renal disease like hyperparathyroidism, hypercalciuria, hypophosphatemia, and UTI may increase the risk of calculi formation [78, 79]. Due to renal and ureteral denervation in kidney grafts, ureterolithiasis may occur without pain in transplanted patients causing other complications like hydronephrosis and acute kidney injury or hematuria. In the acute situation, ureteral stent placement resolves hydronephrosis. Like in native kidneys, the definite treatment depends on calculi genesis: in uric acid stones, the urine alkalisation with, for example, potassium sodium hydrogen citrate (Uralyt-U®) seems to be safe under strict potassium monitoring in patients with normal renal function [80]. In any other stone composition, extracorporeal shock-wave lithotripsy (SWL) is an option [81, 82]. The endoscopic treatment via ante- or retrograde flexible ureteroscopy has proven to be a safe and effective option although retrograde access can be challenging [83, 84]. For larger calculi, percutaneous nephrolithotomy (PCNL) can be performed via easy access because of the position of the allograft in the iliac fossa and the short puncture distance between skin and renal pelvis [85, 86]. Using mini- PCNL, the tissue damage to the graft can be reduced [87]. In 2018, Emiliani et al. reported that in 51 cases of urolithiasis therapies in transplanted kidneys including active surveillance, ante- and retrograde ureteroscopy, ESWL, PCNL and the open approach they found no loss of renal function through intervention and no case of allograft loss [88]. In the case of urolithiasis being present in a specimen at transplantation, an ex vivo ureteroscopy or pyelotomy should be performed to minimize the risk of post-transplant complication through spontaneous stone passing [89].
Urinoma formation after KT
The formation of urinoma occurs in less than 10% of cases after KT mostly due to the insufficiency of the UCN, but also due to rupture of the bladder in case of long-term anuria before KT. It usually becomes evident in the early postoperative course of the first 90 days after surgery [90, 91]. The symptoms of urinoma vary from pain and infection to creeping creatinine and acute kidney injury. The placement of nephrostomy and antegrade ureteral stenting represent the first-line therapy in the acute phase as the retrograde approach is challenging and often challenging due to the implantation site of the ureter. In case of large fluid collections, additional drainage can be placed using ultrasound- or CT- guidance [92]. Moreover, the supply with transurethral catheter lowers pressure and minimizes reflux via the ureteral stent and thus supports recovery. The conservative regimen is often successful and the ureteral stent can usually be removed after 6–12 weeks [93, 94].
In case of a non-spontaneously resolving leak, extended fluid collection or ischemic ureter, a surgical revision is required. Distal ureteral injuries can be treated with open ureteral re-implantation. If the damaged ureteral part is extended, psoas-hitch technique may be combined with Boari tubularized bladder flap. An atrophic bladder in the recipient can make the formation of a bladder flap impossible, so uretero-ureterostomy to the native ureter or uretero-ileal interposition may be options in selected cases [95].
Lymphatic fistula
The formation of lymphatic fistula after RT is a common complication occurring in up to 20% of the cases [96,97,98], caused by dissection of lymphatic vessels of the donor kidney during preparation and recipient pelvis during allograft implantation. Data indicates that the use of an absorbable polysaccharide hemostatic powder (HaemoCer™) can reduce the incidence of lymphatic fistula [99]. Only a minimum of patients requires interventional therapy in case of symptoms like pain or allograft dysfunction due to lymphocele infection or vascular compression. However, patients with lymphatic fistula in need of an intervention are at a higher overall risk of allograft rejection or delayed graft function [100]. Risk factors for lymphatic fistula formation include surgical preparation technique, implantation site, choice of immunosuppression (e.g. sirolimus/MMF/prednisolone), and recipient properties [101]. Available therapeutic options are puncture and aspiration, percutaneous drainage placement with or without sclerotherapy, and open or laparoscopic fenestration [102]. Endoscopic treatment of lymphatic fistula via percutaneous puncture, dilatation of the channel, insertion of flexible ureteroscope, and fulguration of lymphocele wall has been described [103]. Given the success rates of the more conventional techniques, however, this approach should be restricted to specific indications.
Recurrent UTI after kidney transplantation
UTI is the most common type of infection following KT (up to 47%) [67]. Additionally, many patients develop asymptomatic bacteriuria (ASB) early after KT. While most centers treat ASB within 1–3 months ager KT, there is increasing data that this may be unnecessary [104].
Particularly in the first month after transplantation, the risk of UTI seems to be elevated through ureteral stents [105]. Early stent removal reduced the risk of UTI without increasing the risk of urinary leakage and should be performed around 3 weeks after KT [36]. However, even after stent removal for UTI the risk for repeat UTIs remains higher in patients who already suffered from stent-associated UTI [35].
All patients with persistent or recurrent UTI after KT should undergo a thorough assessment for anatomic or functional abnormalities of the urinary tract as already delineated earlier.
Risk factors for UTI after KT are female gender, immunosuppression, history of acute rejection, cytomegalovirus infection, UVJO [106], re-transplantation [67], polycystic kidney disease [107], diabetes mellitus [108] VUR in the native kidneys. While in the acute situation and for primary recurrence, prophylactic antibiotics should be administered, care should be taken to screen for functional or anatomical allograft pathologies.
Despite missing high evidence evaluation of behavioral and non-antibiotic prophylaxis for recurrent UTI in KT, patients should be advised to apply conservative measures in accordance with general recommendations. An excretion minimum of > 2 l/day, measures of genital hygiene, urine pH 5.8–6.5 (e.g. with an intake of vitamin C or methionine), application of vaginal estrogen/lactobacillus, and intake of cranberry products (juice/tablets) should be considered. In cases of residual urine, intermittent self-catheterization may be recommended. In addition, vaccination with inactivated species of E. coli, Morganella morganii, Proteus, Klebsiella, Enterococcus faecalis is an option [109].
The Pneumocystis jirovecii prophylaxis advised by many centers (3–6 months trimethoprim-sulfamethoxazole) seems to decrease the incidence of recurrent UTIs likewise [110].
Conclusions
Urologic complications after kidney transplantation can lead to severe consequences up to chronic allograft dysfunction and eventually allograft loss. Successful transplantation is therefore heavily reliant on both thorough urologic work-up prior to transplantation, and early recognition of complications after surgery. Despite the lack of major systematic prospective evaluation, endoscopic treatment options offer minimally invasive options for patients with a variety of urological complications after kidney transplantation.
References
https://www.lamoncloa.gob.es/lang/en/gobierno/news/Paginas/2019/20180828transplant.aspx. Accessed 26 Jul 2020
European Commission (2014) Journalist workshop on organ donation and transplantation recent facts & figures. European Commission
Coemans M, Susal C, Dohler B et al (2018) Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int 94(5):964–973
Gondos A, Dohler B, Brenner H, Opelz G (2013) Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation 95(2):267–274
Wang JH, Skeans MA, Israni AK (2016) Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis 23(5):281–286
Kocak T, Nane I, Ander H, Ziylan O, Oktar T, Ozsoy C (2004) Urological and surgical complications in 362 consecutive living related donor kidney transplantations. Urol Int 72(3):252–256
Gogus C, Yaman O, Soygur T, Beduk Y, Gogus O (2002) Urological complications in renal transplantation: long-term follow-up of the Woodruff ureteroneocystostomy procedure in 433 patients. Urol Int 69(2):99–101
Mundy AR, Podesta ML, Bewick M, Rudge CJ, Ellis FG (1981) The urological complications of 1000 renal transplants. Br J Urol 53(5):397–402
Wu C, Evans I, Joseph R et al (2005) Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol 16(11):3437–3444
Shandera KC, Rozanski TA, Jaffers G (1996) The necessity of voiding cystourethrogram in the pretransplant urologic evaluation. Urology 47(2):198–200
Errando C, Batista JE, Caparros J, Vicente J, Arano P (2000) Urodynamic evaluation and management prior to renal transplantation. Eur Urol 38(4):415–418
Power RE, Hickey DP, Little DM (2004) Urological evaluation prior to renal transplantation. Transplant Proc 36(10):2962–2967
Antoniewicz AA, Zapala L, Bogucki A, Malecki R (2015) The standard of urological consultation of patients qualified for renal transplant—a review. Cent European J Urol 68(3):376–382
Cairns HS, Leaker B, Woodhouse CR, Rudge CJ, Neild GH (1991) Renal transplantation into abnormal lower urinary tract. Lancet 338(8779):1376–1379
Emberton M, Fitzpatrick JM, Rees J (2011) Risk stratification for benign prostatic hyperplasia (BPH) treatment. BJU Int 107(6):876–880
Hurst FP, Neff RT, Falta EM et al (2009) Incidence, predictors, and associated outcomes of prostatism after kidney transplantation. Clin J Am Soc Nephrol 4(2):329–336
Bae KS, Huh JS, Kim YJ, Chang SG (2005) Clinical characteristics of renal transplant recipients that underwent urologic surgery for de novo disease before and after transplantation. J Korean Med Sci 20(1):75–78
Shenasky JH 2nd (1976) Renal transplantation in patients with urologic abnormalities. J Urol 115(5):490–493
Krajewski W, Dembowski J, Kolodziej A et al (2016) Urological complications after renal transplantation—a single centre experience. Cent European J Urol 69(3):306–311
Xie L, Lin T, Wazir R, Wang K, Lu Y (2014) The management of urethral stricture after kidney transplantation. Int Urol Nephrol 46(11):2143–2145
Serrano DP, Flechner SM, Modlin CS, Wyner LM, Novick AC (1996) Transplantation into the long-term defunctionalized bladder. J Urol 156(3):885–888
Salomon L, Fontaine E, Gagnadoux MF, Broyer M, Beurton D (1997) Posterior urethral valves: long-term renal function consequences after transplantation. J Urol 157(3):992–995
MacGregor P, Novick AC, Cunningham R et al (1986) Renal transplantation in end stage renal disease patients with existing urinary diversion. J Urol 135(4):686–688
Fontaine E, Gagnadoux MF, Niaudet P, Broyer M, Beurton D (1998) Renal transplantation in children with augmentation cystoplasty: long-term results. J Urol 159(6):2110–2113
Darby CR, Cranston D, Raine AE, Morris PJ (1991) Bilateral nephrectomy before transplantation: indications, surgical approach, morbidity and mortality. Br J Surg 78(3):305–307
Sharbaf FG, Bitzan M, Szymanski KM et al (2012) Native nephrectomy prior to pediatric kidney transplantation: biological and clinical aspects. Pediatr Nephrol 27(7):1179–1188
Kuss R (1951) Some attempts at kidney transplantation in man. Mem Acad Chir (Paris) 77(22–24):755–764
Humar A, Matas AJ (2005) Surgical complications after kidney transplantation. Semin Dial 18(6):505–510
Politano VA, Leadbetter WF (1958) An operative technique for the correction of vesicoureteral reflux. J Urol 79(6):932–941
Thrasher JB, Temple DR, Spees EK (1990) Extravesical versus Leadbetter–Politano ureteroneocystostomy: a comparison of urological complications in 320 renal transplants. J Urol 144(5):1105–1109
Starzl TE, Shapiro R, Tzakis A, Hakala TR (1989) A new technique of extravesical ureteroneocystostomy for renal transplantation. Transplant Proc 21(5):3856–3858
Alberts VP, Idu MM, Legemate DA, Laguna Pes MP, Minnee RC (2014) Ureterovesical anastomotic techniques for kidney transplantation: a systematic review and meta-analysis. Transpl Int 27(6):593–605
Breda ABK, Figueiredo A, Lledó García L, Olsburgh J, Regele H et al (2020) European association of urology guidelines on renal transplantation. EAU Guidelines Office, Arnhem, The Netherland. ISBN 978-94-92671-0
Kumar A, Verma BS, Srivastava A, Bhandari M, Gupta A, Sharma R (2000) Evaluation of the urological complications of living related renal transplantation at a single center during the last 10 years: impact of the double-J* stent. J Urol 164(3 Pt 1):657–660
Ranganathan M, Akbar M, Ilham MA, Chavez R, Kumar N, Asderakis A (2009) Infective complications associated with ureteral stents in renal transplant recipients. Transplant Proc 41(1):162–164
Visser IJ, van der Staaij JPT, Muthusamy A, Willicombe M, Lafranca JA, Dor F (2019) Timing of ureteric stent removal and occurrence of urological complications after kidney transplantation: a systematic review and meta-analysis. J Clin Med 8(5):689
Sandhu C (2002) Renal transplantation dysfunction: the role of interventional radiology. Clin Radiol 57:772–783
Rosevear HM, Kim SP, Wenzler DL, Faerber GJ, Roberts WW, Wolf JS Jr (2007) Retrograde ureteral stents for extrinsic ureteral obstruction: nine years’ experience at University of Michigan. Urology 70(5):846–850
Yossepowitch O, Lifshitz DA, Dekel Y et al (2001) Predicting the success of retrograde stenting for managing ureteral obstruction. J Urol 166(5):1746–1749
Halstuch D, Ehrlich Y, Shenhar C et al (2018) Transplant kidney retrograde ureteral stent placement and exchange: overcoming the challenge. Urology 111:220–224
Kadlec AO, Ellimoottil CS, Greco KA, Turk TM (2013) Five-year experience with metallic stents for chronic ureteral obstruction. J Urol 190(3):937–941
Baumgarten AS, Hakky TS, Carrion RE, Lockhart JL, Spiess PE (2014) A single-institution experience with metallic ureteral stents: a cost-effective method of managing deficiencies in ureteral drainage. Int Braz J Urol 40(2):225–231
Bach C, Kabir MN, Goyal A et al (2013) A self-expanding thermolabile nitinol stent as a minimally invasive treatment alternative for ureteral strictures in renal transplant patients. J Endourol 27(12):1543–1545
Goldfischer ER, Gerber GS (1997) Endoscopic management of ureteral strictures. J Urol 157(3):770–775
Hafez KS, Wolf JS Jr (2003) Update on minimally invasive management of ureteral strictures. J Endourol 17(7):453–464
Gdor Y, Gabr AH, Faerber GJ, Roberts WW, Wolf JS Jr (2008) Success of laser endoureterotomy of ureteral strictures associated with ureteral stones is related to stone impaction. J Endourol 22(11):2507–2511
Lane BR, Desai MM, Hegarty NJ, Streem SB (2006) Long-term efficacy of holmium laser endoureterotomy for benign ureteral strictures. Urology 67(5):894–897
Tran H, Arsovska O, Paterson RF, Chew BH (2015) Evaluation of risk factors and treatment options in patients with ureteral stricture disease at a single institution. Can Urol Assoc J 9(11–12):E921–E924
Lojanapiwat B, Soonthonpun S, Wudhikarn S (2002) Endoscopic treatment of benign ureteral strictures. Asian J Surg 25(2):130–133
Arrabal-Martin M, Zuluaga-Gomez A, Merino-Salas S, Nogueras-Ocana M, Arrabal-Polo MA (2013) Endoscopic treatment of ureterovesical junction obstructive pathology: a description of the oblique meatotomy technique and results. Can Urol Assoc J 7(11–12):E728–E731
Dutkiewicz SA, Wroblewski M (2012) Comparison of treatment results between holmium laser endourethrotomy and optical internal urethrotomy for urethral stricture. Int Urol Nephrol 44(3):717–724
Kristo B, Phelan MW, Gritsch HA, Schulam PG (2003) Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium: YAG laser endoureterotomy. Urology 62(5):831–834
Faba OR, Boissier R, Budde K et al (2018) European association of urology guidelines on renal transplantation: update 2018. Eur Urol Focus 4(2):208–215
Byun SS, Kim JH, Oh SJ, Kim HH (2003) Simple retrograde balloon dilation for treatment of ureteral strictures: etiology-based analysis. Yonsei Med J 44(2):273–278
Lang EK, Glorioso LW 3rd (1988) Antegrade transluminal dilatation of benign ureteral strictures: long-term results. AJR Am J Roentgenol 150(1):131–134
Junior NRN, Ferreira U, Lemos GC, Claro JF (1990) Endourological management of ureteral strictures. J Urol 144(3):631–634
Rabenalt R, Winter C, Potthoff SA et al (2011) Retrograde balloon dilation > 10 weeks after renal transplantation for transplant ureter stenosis—our experience and review of the literature. Arab J Urol 9(2):93–99
Gil-Sousa D, Oliveira-Reis D, Teves F et al (2017) Ureteral stenosis after renal transplantation-a single-center 10-year experience. Transplant Proc 49(4):777–782
Christman MS, Kasturi S, Lambert SM, Kovell RC, Casale P (2012) Endoscopic management and the role of double stenting for primary obstructive megaureters. J Urol 187(3):1018–1022
Gdor Y, Gabr AH, Faerber GJ, Wolf JS Jr (2008) Holmium: yttrium-aluminum-garnet laser endoureterotomy for the treatment of transplant kidney ureteral strictures. Transplantation 85(9):1318–1321
Bromwich E, Coles S, Atchley J, Fairley I, Brown JL, Keoghane SR (2006) A 4-year review of balloon dilation of ureteral strictures in renal allografts. J Endourol 20(12):1060–1061
Molenaar NM, Minnee RC, Bemelman FJ, Idu MM (2017) Vesicoureteral reflux in kidney transplantation. Prog Transplant 27(2):196–199
Jung GO, Chun JM, Park JB et al (2008) Clinical significance of posttransplantation vesicoureteral reflux during short-term period after kidney transplantation. Transplant Proc 40(7):2339–2341
Margreiter M, Gyori GP, Bohmig GA, Trubel S, Muhlbacher F, Steininger R (2013) Value of routine voiding cystourethrography after renal transplantation. Am J Transplant 13(1):130–135
Grunberger T, Gnant M, Sautner T et al (1993) Impact of vesicoureteral reflux on graft survival in renal transplantation. Transplant Proc 25(1 Pt 2):1058–1059
Favi E, Spagnoletti G, Valentini AL et al (2009) Long-term clinical impact of vesicoureteral reflux in kidney transplantation. Transplant Proc 41(4):1218–1220
Golebiewska J, Debska-Slizien A, Komarnicka J, Samet A, Rutkowski B (2011) Urinary tract infections in renal transplant recipients. Transplant Proc 43(8):2985–2990
Coulthard MG, Keir MJ (2006) Reflux nephropathy in kidney transplants, demonstrated by dimercaptosuccinic acid scanning. Transplantation 82(2):205–210
Howie AJ, Buist LJ, Coulthard MG (2002) Reflux nephropathy in transplants. Pediatr Nephrol 17(7):485–490
Schwab CW Jr, Wu HY, Selman H, Smith GH, Snyder HM 3rd, Canning DA (2002) Spontaneous resolution of vesicoureteral reflux: a 15-year perspective. J Urol 168(6):2594–2599
Bollgren I (1999) Antibacterial prophylaxis in children with urinary tract infection. Acta Paediatr Suppl 88(431):48–52
Samaan F, Requiao-Moura LR, Pinheiro HS, Ozaki KS, Camara NOS, Pacheco-Silva A (2011) Prevalence and progression of chronic kidney disease after renal transplantation. Transplant Proc 43(7):2587–2591
Stenberg A, Lackgren G (2007) Treatment of vesicoureteral reflux in children using stabilized non-animal hyaluronic acid/dextranomer gel (NASHA/DX): a long-term observational study. J Pediatr Urol 3(2):80–85
Pichler R, Buttazzoni A, Rehder P, Bartsch G, Steiner H, Oswald J (2011) Endoscopic application of dextranomer/hyaluronic acid copolymer in the treatment of vesico-ureteric reflux after renal transplantation. BJU Int 107(12):1967–1972
Bouchot O, Guillonneau B, Cantarovich D et al (1991) Vesicoureteral reflux in the renal transplantation candidate. Eur Urol 20(1):26–28
Dinckan A, Aliosmanoglu I, Kocak H et al (2013) Surgical correction of vesico-ureteric reflux for recurrent febrile urinary tract infections after kidney transplantation. BJU Int 112(4):E366–E371
Cheungpasitporn W, Thongprayoon C, Mao MA et al (2016) Incidence of kidney stones in kidney transplant recipients: a systematic review and meta-analysis. World J Transplant 6(4):790–797
Mamarelis G, Vernadakis S, Moris D et al (2014) Lithiasis of the renal allograft, a rare urological complication following renal transplantation: a single-center experience of 2045 renal transplantations. Transplant Proc 46(9):3203–3205
Harper JM, Samuell CT, Hallson PC, Wood SM, Mansell MA (1994) Risk factors for calculus formation in patients with renal transplants. Br J Urol 74(2):147–150
Wang L, Cui Y, Zhang J, Zhang Q (2017) Safety of potassium-bearing citrate in patients with renal transplantation: a case report. Medicine (Baltimore) 96(42):e6933
Li SD, Wang QT, Chen WG (2011) Treatment of urinary lithiasis following kidney transplantation with extracorporeal shock-wave lithotripsy. Chin Med J (Engl) 124(9):1431–1434
Rhoderick TM, Yang HC, Escobar FS, Belis JA, Gifford RR (1992) Extracorporeal shock wave lithotripsy in the renal transplant patient: a case report and review of literature. Clin Transplant 6(5):375–378
Hyams E, Marien T, Bruhn A et al (2012) Ureteroscopy for transplant lithiasis. J Endourol 26(7):819–822
Swearingen R, Roberts WW, Wolf JS Jr (2015) Ureteroscopy for nephrolithiasis in transplanted kidneys. Can J Urol 22(2):7727–7731
McAlpine K, Leveridge MJ, Beiko D (2015) Outpatient percutaneous nephrolithotomy in a renal transplant patient: world’s first case. Can Urol Assoc J 9(5–6):E324–E328
Francesca F, Felipetto R, Mosca F, Boggi U, Rizzo G, Puccini R (2002) Percutaneous nephrolithotomy of transplanted kidney. J Endourol 16(4):225–227
Markic D, Krpina K, Ahel J et al (2016) Treatment of kidney stone in a kidney-transplanted patient with mini-percutaneous laser lithotripsy: a case report. Case Rep Nephrol Dial 6(1):26–31
Emiliani E, Subiela JD, Regis F, Angerri O, Palou J (2018) Over 30-yr experience on the management of graft stones after renal transplantation. Eur Urol Focus 4(2):169–174
Harraz AM, Kamal AI, Shokeir AA (2016) Urolithiasis in renal transplant donors and recipients: an update. Int J Surg 36(Pt D):693–697
Isa WA, Robles JE, Rosell D et al (1991) Urologic complications in 237 recipients of cadaveric kidney transplantation. Actas Urol Esp 15(4):351–356
Buresley S, Samhan M, Moniri S, Codaj J, Al-Mousawi M (2008) Postrenal transplantation urologic complications. Transplant Proc 40(7):2345–2346
Titton RL, Gervais DA, Hahn PF, Harisinghani MG, Arellano RS, Mueller PR (2003) Urine leaks and urinomas: diagnosis and imaging-guided intervention. Radiographics 23(5):1133–1147
Streeter EH, Little DM, Cranston DW, Morris PJ (2002) The urological complications of renal transplantation: a series of 1535 patients. BJU Int 90(7):627–634
Shoskes DA, Hanbury D, Cranston D, Morris PJ (1995) Urological complications in 1000 consecutive renal transplant recipients. J Urol 153(1):18–21
Shokeir AA, Shamaa MA, Bakr MA, el-Diasty TA, Ghoneim MA (1993) Salvage of difficult transplant urinary fistulae by ileal substitution of the ureter. Scand J Urol Nephrol 27(4):537–540
Dubeaux VT, Oliveira RM, Moura VJ, Pereira JM, Henriques FP (2004) Assessment of lymphocele incidence following 450 renal transplantations. Int Braz J Urol 30(1):18–21
Ulrich F, Niedzwiecki S, Fikatas P et al (2010) Symptomatic lymphoceles after kidney transplantation—multivariate analysis of risk factors and outcome after laparoscopic fenestration. Clin Transplant 24(2):273–280
Mehrabi A, Kulu Y, Sabagh M et al (2020) Consensus on definition and severity grading of lymphatic complications after kidney transplantation. Br J Surg 107(7):801–811
Burghuber CK, Kandioler D, Strobl S et al (2019) Standardized intraoperative application of an absorbable polysaccharide hemostatic powder to reduce the incidence of lymphocele after kidney transplantation—a prospective trial. Transpl Int 32(1):59–65
Bzoma B, Kostro J, Debska-Slizien A et al (2016) Treatment of the lymphocele after kidney transplantation: a single-center experience. Transplant Proc 48(5):1637–1640
Ranghino A, Segoloni GP, Lasaponara F, Biancone L (2015) Lymphatic disorders after renal transplantation: new insights for an old complication. Clin Kidney J 8(5):615–622
Iwan-Zietek I, Zietek Z, Sulikowski T et al (2009) Minimally invasive methods for the treatment of lymphocele after kidney transplantation. Transplant Proc 41(8):3073–3076
McDougall EM, Clayman RV (1994) Endoscopic management of persistent lymphocele following laparoscopic pelvic lymphadenectomy. Urology 43(3):404–407
Coussement J, Maggiore U, Manuel O et al (2018) Diagnosis and management of asymptomatic bacteriuria in kidney transplant recipients: a survey of current practice in Europe. Nephrol Dial Transplant 33(9):1661–1668
Gomez-Ochoa SA, Vega-Vera A (2020) Systematic review and meta-analysis of asymptomatic bacteriuria after renal transplantation: incidence, risk of complications, and treatment outcomes. Transpl Infect Dis 22(1):e13221
Alangaden GJ, Thyagarajan R, Gruber SA et al (2006) Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant 20(4):401–409
Illesy L, Kovacs DA, Szabo RP, Asztalos L, Nemes B (2017) Autosomal dominant polycystic kidney disease transplant recipients after kidney transplantation: a single-center experience. Transplant Proc 49(7):1522–1525
Almond PS, Matas A, Gillingham K et al (1993) Risk factors for chronic rejection in renal allograft recipients. Transplantation 55(4):752–756 (discussion 6–7)
Giessing M (2012) Urinary tract infection in renal transplantation. Arab J Urol 10(2):162–168
Green H, Rahamimov R, Gafter U, Leibovitci L, Paul M (2011) Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis 13(5):441–447
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deininger, S., Nadalin, S., Amend, B. et al. Minimal-invasive management of urological complications after kidney transplantation. Int Urol Nephrol 53, 1267–1277 (2021). https://doi.org/10.1007/s11255-021-02825-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02825-7