Abstract
Ureteral stents are one of the most frequently used tools in urology. These medical devices have a multitude of both prophylactic and therapeutic indications. Among the first ones, the use of stents related to the treatment of ureteral or renal stones, in renal transplant surgery and in reconstructive surgery of the upper urinary tract by endourological, laparoscopic, robotic or conventional surgical approach. Therapeutic indications are related to urinary tract decompression in obstructive uropathy and as in the conservative treatment of upper urinary tract trauma.
Although it has clear benefits, unfortunately it is not free of side effects. Complications associated with ureteral stents can be classified as intraoperative, early complications if they appear between 2 and 4 weeks after stenting, and late complications, depending on the time of onset of side effects. The most common side effects are the development of vesicoureteral reflux, LUTS and stent discomfort. The complications with the highest rate of incidence are asymptomatic bacteriuria and urinary tract infection. There are also common events related to ureteral stent dislodgement and crystallization of the stent surface resulting in encrustation.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Indications of Polymeric Double J Stents
Double J stents are used in a wide variety of scenarios, which we will divide into two groups of indications for didactic purposes: prophylactic and therapeutic.
1.1 Prophylactic Indications
The insertion of a double J stent can prevent the advent of perioperative complications in specific procedures involving the upper urinary tract. These interventions are mainly focused on urinary stone management, followed by reconstructive procedures.
1.1.1 Stone Interventional Treatment
Stents can be placed either before or after stone treatment interventions, for different reasons. Overall, they aim at minimizing the risk of obstruction due to fragments, blood clots or edema after ureteral manipulation [1].
Prior to shock wave lithotripsy (SWL), ureteral stents try to prevent ureteral obstruction secondary to the passage of stone fragments or the formation of a steinstrasse after the treatment. Although very common in the past, it has been demonstrated that this practice doesn’t increase the stone free and auxiliary treatment rates. Stenting is generally recommended for stones larger than 1.5–2 cm in diameter, since SWL in these situations will generate more fragments possibly leading to ureteral obstruction. Currently, these stone burdens are more efficiently treated by flexible ureteroscopy or miniaturized percutaneous surgery, in which a preoperative stent is not usually required. However, whenever SWL is the treatment of choice in these cases, double J stenting and its morbidity should be discussed with the patients, as well as the probable need for further lithotripsy sessions [2,3,4,5,6].
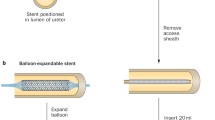
Prior to ureteroscopy or retrograde intrarenal surgery, the use of a double J stent aims at creating a passive dilation of the ureter that eases the insertion of the ureteroscope or the ureteral access sheath [7].
This maneuver was very common in the past due to the size of the ureteroscopes available, since not all the ureters admitted such large calibers of endoscopes or ureteral access sheaths. There are data in the literature that show that pre-stenting should lead to better stone-free rates and lessen the incidence of complications, but this finding is mainly based on retrospective studies and is therefore controversial [8,9,10,11].
Besides these data, primarily from old series, our opinion and that of the urological guidelines is that with the current armamentarium preoperative stenting should not be systematically recommended. However, placing a double J is advised when the access sheath or the ureteroscope does not go up smoothly into the ureter, in order to create a passive dilation which should allow the passage of these instruments in 1–2 weeks [12, 13].
Post ureteroscopy, be it semirigid or flexible, the use of double J is not routinely recommended, and the stenting decision must be analyzed individually. Clinicians must weigh up the risk of readmission when not leaving a stent against the morbidity of bearing it. Overall, stenting should be mandatory when there is ureteral damage, high risk of obstruction due to edema, fragments or blood clots, when an infective complication occurs or is likely to happen in the postoperative period, as well as in all doubtful cases [14,15,16,17,18,19].
Besides these recommendations, many groups place double J stents following ureteroscopy in the majority of cases, with considerable differences across countries [20]. In general, when a ureteral access sheath is used, many authors recommend leaving a double J stent at the end of the procedure, due to the considerable incidence of ureteral wall lesions found as a result of the insertion of these sheaths [21]. Therefore, it is advisable to endoscopically review the ureter after these procedures to have more information regarding the urothelium status before the decision to stent [22]. Nevertheless, there is a randomized trial showing that omitting the stent in these cases should be safe and feasible, mainly if the patient has been pre-stented [22, 23].
There are no solid data on the ideal indwelling time, but the vast majority of groups advocate for 1–2 weeks. In some situations, leaving a ureteral stent overnight or a double J on strings for 2–3 days are reasonable alternatives that can lessen the morbidity of bearing a stent for 2 weeks or longer [24,25,26].
Post percutaneous surgery, the use of double J has been increased in the last years due to the more frequent practice of tubeless surgeries. The decision of leaving a double J after these procedures instead of performing a totally tubeless surgery is mainly based on the surgeon’s experience, the characteristics of the case and patient preferences. In this regard, some patients will opt for a percutaneous approach instead of a retrograde surgery in order not to bear a ureteral stent and its symptoms. When endoscopic combined intrarenal surgery is performed, the stenting decision follows the same principles as those previously detailed for ureteroscopic procedures [27].
1.1.2 Renal Transplantation
Ureteral stenting after renal transplantation should contribute for a watertight uretero-neocystostomy, preventing or minimizing urinary leakage that might lead to stricture [28]. A meta-analysis including five randomized controlled trials demonstrated that stented anastomoses have lower complication rates [29].
Due to the characteristics of the ureter in this indication, the length of the catheter used must be considerably shorter. Again, there is no optimal timing for stent removal after transplantation, being 2–4 weeks of indwelling time in the majority of series [30].
1.1.3 Reconstructive Surgery of the Upper Urinary Tract
Pyeloplasty, endopyelotomy, pyelolitectomy, ureteral stricture repair, ureteral trauma repair, etc.
Once more, the objective of the ureteral stent is to help in the healing process of the urinary tract, serving as a scaffold and preventing urinary leaks. In these indications, stents are traditionally removed after 4 weeks, although this dwelling time may be shortened reducing infection risk and morbidity to the patient [31, 32].
1.1.4 Non-urological Procedures Involving Ureteral Dissection
Placing a ureteral stent (either open-end straight or double J) before specific abdominal surgeries where a complex ureteral dissection is suspected makes it easier to identify the ureter during these maneuvers and may prevent accidental injuries. The pros and cons of this endoscopic intervention should be discussed with the patients. When the ureter has not been damaged during the surgery, these stents can be immediately removed or left overnight [33,34,35].
1.2 Therapeutic Indications
The insertion of a double J ureteral stent aims to drain an obstructed or damaged upper urinary tract.
1.2.1 Decompression of an Obstructed Collecting System
This is the most frequent indication for double J stenting, which needs to be performed in the emergency context or on a scheduled basis, depending on the severity of the case. Urinary drainage must be promptly performed in all cases of obstruction with sepsis, acute renal insufficiency or anuria due to bilateral obstruction or in solitary kidneys, as well as when there is uncontrollable pain. In some groups, percutaneous nephrostomy is preferred in infective situations, although to date there is no data to demonstrate which of these two drainage options is superior [36,37,38].
1.2.2 Conservative Treatment of Upper Urinary Tract Trauma
Depending on the severity of the damage, these injuries can be conservatively managed with a double J. Stenting provides canalization, reduces urinary leakage and might decrease the risk of strictures. In this scenario, bladder catheterization is advised to prevent backflow of urine through the double J ureteral stent into the upper tract [39, 40].
2 Ureteral Stents Complications
2.1 Intraoperative
2.1.1 Failure of Endoscopic Ureteral Stenting
On some occasions, it is not possible retrograde drainage of the upper urinary tract. It may be due to intrinsic cause (urothelial neoplasms) or extrinsic compression such us retroperitoneal fibrosis or tumours of the abdominopelvic area. It is necessary to treat it (especially if chemotherapy is required). Accordingly, the first treatment option is placing a retrograde ureteral stent However, the rate of stent failure is high, with a range failure rate between 12.2% and 34.6%. Guachetá-Bomba et al. found that cystoscopies result such as the bladder invasion or deformity of the trigone or the age >65 years old are negative factors when attempting an endoscopic urinary drainage [41]. Therefore, it should be considered percutaneous nephrostomy, whether retrograde drainage is not achieved, in order to maintain renal function until obstruction cause is resolved (Figs. 1, 2, 3, and 4).
2.1.2 Ureteral Erosion or Perforation
It’s a rarest complication of ureteral stent placement. The stent placement should be carefully. It is recommended to previously perform a retrograde pyelography, thus opacifying the upper urinary tract. Special care should be taken in cases of almost complete obstruction of the ureter where the passage of the stent can be complex and the ureteral wall more fragile. If observe any resistance during its progression, never use force, but observe what’s happening on the fluoroscopy assessment. If find urinary leak or extravasation, it means ureteral injury. The stenting should be enough to solve the complication, allowing the ureter to heal around the stent, like an internal scaffold.
2.1.3 Stent Malposition
Malposition of a stent is defined as an incorrect position relative to initial placement [42]. A badly placed stent may be in a sub-pyelic position, if the proximal end does not reach the renal pelvis, and in a supravesical position when the distal end is can be found in the ureter. The causes of this complication are mainly due to the placement technique, both endoscopy or fluroscopy placement. This is the reason that it is so important to check the correct location of the stent after it has been placed. An appropriate length is important to avoid this complication.
2.2 Early Complications (2–4 Weeks)
2.2.1 Stent Discomfort
Pain associated with ureteral stents is one of the most common symptoms in patients, with an up to 80% rate of incidence [43]. This pain can be triggered by several reasons: vesicoureteral reflux causing an upward increase in intra-ureteral pressure, related to flank pain; ureteral spasms mainly associated with the distal ureter; and irritation of the bladder mucosa associated with the presence of a bladder foreign body [44]. However, it should be highlighted that the etiology of the pain remains unknown to date.
Mainly, it is related to two separate regions in which pain is reported by patients. Up to 60–77% of patients describe the manifestation of flank pain, which is primarily but not exclusively associated with micturition and VUR caused by the stent. The incidence of suprapubic pain, with up to 38%, is associated with adverse effects at this level related to bladder pigtail and irritation of the bladder trigone [45].
2.2.2 Vesicoureteral Reflux
The UVJ (ureterovesical junction) is a fundamental structure that protects the upper urinary tract from intermittent high pressures in the bladder. The UVJ allows, through its transient opening, the passage of urine into the bladder and prevents retrograde flow into the kidneys during the micturition. A number of factors are involved in the proper working of this anti-reflux mechanism: an appropriate length of intravesical ureter, an oblique angle of insertion of the ureter into the bladder and proper smooth muscle and extracellular matrix development, able to compress the ureteral orifice. Any abnormality in these features leads to retrograde flow of urine or VUR [46].
Vesicoureteral reflux is one of the most important drawbacks in ureteral stenting. This side effect usually appears during the voiding phase of micturition, when the pressure in the bladder increases and the stent, leaving an open communication between the bladder and the ureter, causes the urine to retrograde flow of urine [47].
Regarding the overall VUR rate in stented patients, it’s 62–76%, with 80% during the voiding phase compared to 63% during the filling phase [48, 49].
In order to avoid this side effect there have been advances in stent design such as the one with anti-reflux valve, the most widely used. This stent is composed by a standard stent in which the bladder end adds a bag that encompasses the distal end of the stent. Therefore, this kind of stent just blocks the reflux that rises through the internal channel nor the one that can be produced around stent, the periprosthetic flow. Ecke et al. compare this stent with the standard ureteral stent and conclude that reduce the side effects of stents, improving quality of life, as well as being cost-effective [50]. There have been other inventions that have also incorporated a valve at the bladder end in order to prevent ureteral reflux such as McMahon et al. and Ramachandra et al. [51, 52].
2.2.3 Ureteral Smooth Muscle Spasm
A ureteral stent in the upper urinary tract, in addition to changing the dynamics of urinary flow, also has an impact on ureteral myogenic activity [53]. The increase in pressure that occurs is responded to by an increase in ureteral peristalsis during the first few hours and during this period, spasms of the smooth muscle layer of the ureter [54]. These smooth muscle spasms are triggered by the stimulation of α1-adrenergic receptors, present at the ureteral and trigone-bladder level, which causes these contractions [55]. These contractions are more important at the level of the ureterovesical junction and distal ureter, corresponding to the higher density of nerve tissue concentrated in the adventitia and smooth muscle layer in these two regions [56].
2.2.4 Lower Urinary Tract Symptoms
Lower urinary tract symptom’s (LUTS) are frequent and are clearly attributed to bladder urothelium irritation by a vesical stent end which triggers inflammation and overactivity of the bladder detrusor [57]. LUTS are classified into filling symptoms, emptying symptoms and post-mictional symptoms [58].
In a prospective analysis of the prevalence of symptoms, tolerability and complications of the ureteral stent and its impact on quality of life. Patients completed two questionnaires before stent placement, 7 days after placement, and 14 days after removal. The results concluded that 7 days after stent placement, patients experienced a significant increase symptom in terms of urinary frequency, dysuria, suprapubic pain, urgency and macroscopic hematuria, and a considerably lower quality of life. Alpha blockers, anticholinergics or beta-3 adrenergic agonists can be used to reduce the incidence of stent associated symptoms. Another strategy to achieve a decrease in associated symptoms is prevention: a smaller stent diameter and a proper stent length in order avoid distal loop crossed the bladder midline [59].
2.3 Late Complications (>2–4 Weeks)
2.3.1 Urinary Tract Infection (UTI)
Bacterial colonisation of the stents, with an overall rate of 42–90%, is a significant drawback, leading to biofilm formation and the development of bacteriuria and UTI [60]. European Association of Urology recommends, it is indicated prophylactic antibiotics either trimethoprim, trimethoprim-sulphamethoxazole, cephalosporin group 2 or 3 or aminopenicillin plus a beta-lactamase inhibitor, before the placement of a ureteral stent in order to prevent urinary tract infections, but, unfortunately, they are not enough [61]. It has been reported that colonisation occurs as early as 24 h after stent insertion, but it is not meant to cause infection [62]. The most common organisms isolated from stents are E. coli, Pseudomonas aeruginosa, Staphylococcus spp., and Enterococcus spp. [63]. Kris R et al. found that only about 25% of colonised ureteral stents are associated with positive urine cultures. They also demonstrated that dwell time of the stent is the strongest predictor of clinical urinary tract infection [64].
This susceptibility of stents to bacterial colonisation promotes the development of UTIs, which in some cases can trigger significant complications such as acute pyelonephritis, bacteriuria and renal failure [65]. A gender-related increased risk of stent colonisation has been observed, with a clear higher risk in women than in men, but with no gender-related risk in the appearance of UTIs [66].
To prevent biofilm formation on stents, there have been some innovations such us, coating of polyhydrogel poly (N,N-dimethylacrylamide) (PDMAA) with antifouling and protein repellent properties has been used by Szell et al. In vitro studies showed a five-fold decrease of bacterial load on the stent surface [67]. Unfortunately, after promising in vitro results, the human studies have not confirmed these results.
2.3.2 Stent Migration
Stent migration can occur as the ureter is a dynamic organ due to peristalsis. The precise risk factors for stent migration remain to be defined, but an appropriate selection of the stent size is not only necessary to palliate the patients’ symptoms, but also to avoid migration [68]. Despite the self-retentive design of the CDJ and appropriate placement, distal migration into the bladder or pelvic migration is a complication with an incidence of up to 9.5% [69] . Furthermore, biomaterials with low friction, such as silicones and hydrophilic coatings, will promote this event [52]. It has been recognised that polyurethane stents have better shape memory and can conform to the urinary tract when compared to silicone stents, decreasing the rate of ureteral stent migration [52] (Fig. 5).
2.3.3 Fragmentation and Breakage
Stent fracture is a very rare complication. It can be caused by mechanical stress, particularly through the lateral orifices, and by a decrease in tensile strength due to depolymerisation that can develop in long-term stenting. Interaction with the urine and extensive inflammatory reaction may promote fragmentation. The rate of ureteral stent fragmentation ranges between 0.3% and 10% [70]. The other factor related with stent fragmentation is stent material. Silicone stents may be more advantageous than polyethylene stents for the lower risk of fragmentation [70].
2.3.4 Forgotten Double-J Stent and Encrustation
The encrustation of forgotten stents is a serious problem due to recurrent urinary tract infections, hematuria, urinary tract obstructions, and renal failure. Similarly, to stent bacterial colonization, stent encrustation increases with stent duration. The aetiology of encrustation is multifactorial [71]: urine composition, stent material, surface properties, stent design, dwell time, urinary pH, urine flow dynamics and bacterial urease. The complexity of the encrustation process is clear, nowadays none of the biomaterials used are resistant to crystal deposition [72].
The definition of a forgotten stent is a device that remains in place for longer than the prescribed time without any medical monitoring. The reasons behind this complication can be attributed to inadequate counselling by the treating doctor and poor compliance of the patient (Figs. 6 and 7).
In a retrospective analysis for a period of 6 years by Adanaur et al., the mean indwelling time was 22.6 months (6–144 months). Of 54 patients, urolithiasis was the indication for stenting in 45 (83.3%) [73].
There have been some innovations to elude this complication such us the biodegradable ureteral stent. F Soria et al. designed a biodegradable antireflux stent that avoids vesicoureteral reflux and bladder trigone irritation as well as the forgotten stent syndrome. There was no ureteral obstruction due to degraded stent fragments in their experimental assessment. Consequently, morbidity secondary to ureteral stents might be reduced with intraureteral biodegradable stents [74].
2.3.5 Ureteral Stent Obstruction
Obstruction increases with stent dwell time and not stent size. Causes of obstruction are due to increased debris deposition, crystals deposited on the stent surface, as well as blood clots due to haematuria. The diagnosis is usually made by deterioration of renal function, renal fossa pain or worsening of hydronephrosis. It can be solved by replacement of the stent [75].
References
Beysens M, Tailly TO. Ureteral stents in urolithiasis. Asian J Urol. 2018;5:274–86.
Al-Awadi KA, Abdul Halim H, Kehinde EO, et al. Steinstrasse: a comparison of incidence with and without J stenting and the effect of J stenting on subsequent management. BJU Int. 1999;84(6):618–21.
Musa AA. Use of double-J stents prior to extracorporeal shock wave lithotripsy is not beneficial: results of a prospective randomized study. Int Urol Nephrol. 2008;40:19–22.
Mohayuddin N, Malik HA, Hussain M, et al. The outcome of extracorporeal shockwave lithotripsy for renal pelvic stone with and without JJ stent: a comparative study. J Pak Med Assoc. 2009;59(3):143–6.
Shen P, Jiang M, Yang J, et al. Use of ureteral stent in extracorporeal shock wave lithotripsy for upper urinary calculi: a systematic review and meta-analysis. J Urol. 2011;186:1328–35.
Chandhoke PS, Barqawi AZ, Wemecke C, Chee-Away RA. A randomized outcomes trial of ureteral stents for extracorporeal shock wave lithotripsy of solitary kidney or proximal ureteral stones. J Urol. 2020;167:1981–3.
Hubert KC, Palmer JS. Passive dilation by ureteral stenting before ureteroscopy: eliminating the need for active dilation. J Urol. 2005;174:1079–80.
Ronald A, Rubenstein RA, Zhao LC, Loeb S, Shore DM, Nadler RB. Presenting improves ureteroscopic stone-free rates. J Endourol. 2007;21:1277–80.
Jessen JP, Breda A, Brehmer M, et al. International Collaboration in Endourology: multicenter evaluation of prestenting for ureterorenoscopy. J Endourol. 2016;30:268–73.
Assimos D, Crisci A, Culkin D, et al. Preoperative JJ stent placement in ureteric and renal stone treatment: results from the Clinical Research Office of Endourological Society (CROES) ureteroscopy (URS) Global Study. BJU Int. 2016;117:648–54.
Yang Y, Tang Y, Bai Y, Wang X, Feng D, Han P. Preoperative double-J stent placement can improve the stone-free rate for patients undergoing ureteroscopic lithotripsy: a systematic review and meta-analysis. Urolithiasis. 2018;46:493–9.
Mahajan PM, Padhye AS, Bhave AA, Sovani YB, Kshirsagar YB, Bapat SS. Is stenting required before retrograde intrarenal surgery with access sheath. Indian J Urol. 2009;25:326–8.
Ambani SN, Faerber GJ, Roberts WW, Hollingsworth JM, Wolf JR. Ureteral stents for impassable ureteroscopy. J Endourol. 2013;27:549–53.
Wang H, Man L, Li G, Huang G, Liu N, Wang J. Meta-analysis of stenting versus non-stenting for the treatment of ureteral stones. PLoS One. 2017;12:e0167670.
Nabi G, Cook J, N’Dow J, McClinton S. Outcomes of stenting after uncomplicated ureteroscopy: systematic review and meta-analysis. BMJ. 2007;334:572.
Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART II. J Urol. 2016;196:1161–9.
Türk C, Neisius A, Petrik A, et al. EAU guidelines on urolithiasis. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urolithiasis-2020v4-1.pdf. Accessed 20 Dec 2020.
Song T, Liao B, Zheng S, Wei Q. Meta-analysis of postoperatively stenting or not in patients underwent ureteroscopic lithotripsy. Urol Res. 2012;40:67–77.
Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179:424–30.
Muslumanoglu AY, Fuglsig S, Frattini A, et al. Risks and benefits of postoperative double-J stent placement after ureteroscopy: results from the Clinical Research Office of Endourological Society Ureteroscopy Global Study. J Endourol. 2017;31:446–51.
Traxer O, Thomas A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J Urol. 2013;189:580–4.
Sirithanaphol W, Jitpraphai S, Taweemonkongsap T, Nualyong C, Chotikawanich E. Ureteral stenting after flexible ureterorenoscopy with ureteral access sheath; is it really needed? A prospective randomized study. J Med Assoc Thail. 2017;100:174.
Astorza G, Catalan M, Consigliere L, Selman T, Salvadó J, Rubilar F. Is a ureteral stent required after use of ureteral access sheath in presented patients who undergo flexible ureteroscopy? Cent European J Urol. 2017;70:88–92.
Shigemura K, Yasufuku T, Yamanaka K, Yamahsita M, Arakawa S, Fujisawa M. How long should double J stent be kept in after ureteroscopic lithotripsy? Urol Res. 2012;40:373–6.
Nevo A, Mano R, Baniel J, Lifshitz DA. Ureteric stent dwelling time: a risk factor for post-ureteroscopy sepsis. BJU Int. 2017;120:117–22.
Komeya M, Usui K, Asai T, et al. Outcome of flexible ureteroscopy for renal stone with overnight ureteral catheterization: a propensity score-matching analysis. World J Urol. 2018;36:1871–6.
Garofalo M, et al. Tubeless procedure reduces hospitalization and pain after percutaneous nephrolithotomy: results of a multivariable analysis. Urolithiasis. 2013;41:347–53.
Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 2013;17:CD004925.
Mangus RS, Haag BW. Stented versus nonstented extravesical ureteroneocystostomy in renal transplantation: a metanalysis. Am J Transplant. 2004;4:1889–96.
Breda A, Budde K, Figueiredo A, et al. EAU guidelines on renal transplantation. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Renal-Transplantation-2020.pdf. Accessed 20 Dec 2020.
Mandhani A, Kappor R, Zaman W, et al. Is a 2-week duration sufficient for stenting in endopyelotomy? J Urol. 2003;169:886–9.
Danuser H, Germann C, Pelzer N, Rühle A, Stucki P, Mattei A. One- vs 4-week stent placement after laparoscopic and robot-assisted pyeloplasty: results of a prospective randomized single-centre study. BJU Int. 2014;113:931–5.
Chou MT, Wang CJ, Lien RC. Prophylactic ureteral catheterization in gynecologic surgery: a 12-year randomized trial in a community hospital. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:689–93.
Speicher PJ, Goldsmith ZG, Nussbaum DP, Turley RS, Peterson AC, Mantyh CR. Ureteral stenting in laparoscopic colorectal surgery. J Surg Res. 2014;190:98–103.
Coakley KM, Kasten KR, Sims SM, Prasad T, Heniford BT, Davis BR. Prophylactic ureteral catheters for colectomy: a national surgical quality improvement program-based analysis. Dis Colon Rectum. 2018;61:84–8.
Cepeda M, Mainez JA, de la Cruz B, Amón JH. Indications and morbidity associated with double J catheters. Arch Esp Urol. 2016;69:462–70.
Lynch MF, Anson KM, Patel U. Percutaneous nephrostomy and ureteric stent insertion for acute renal deobstruction: consensus based guidance. Br J Med Surg Urol. 2008;1:120–5.
Pearle MS, Pierce HL, Miller GL, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160:1260–4.
Kitrey ND, Djakovic N, Hallscheidt P, et al. EAU guidelines on urological trauma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urological-Trauma-2020.pdf. Accessed 20 Dec 2020.
Schwartz BF, Stoller ML. Endourologic management of urinary fistulae. Tech Urol. 2000;6:193–5.
Guachetá-Bomba PL, Echeverría-García F, García-Perdomo HA. Predictors for failure of endoscopic ureteric stenting in patients with malignant ureteric obstruction: systematic review and meta-analysis. BJU Int. 2021;127(3):292–9.
Dryer RB, Chen MY, Zagoria RJ, Regan JD, Hood CH, Kavanagh PV. Complications of ureteral stent placement. Radiographics. 2002;22:1005–22.
Joshi HB, Stainthorpe A, Keeley FX, MacDonagh R, Timoney AG. Indwelling ureteral stents: evaluation of quality of life to aid outcome analysis. J Endourol. 2001;15:151–4.
Bonkat G, Rieken M, Müller G, Roosen A, Siegel FP, Frei R, et al. Microbial colonization and ureteral stent-associated storage lower urinary tract symptoms: the forgotten piece of the puzzle? World J Urol. 2013;31:541–6.
Joshi HB, Stainthorpe A, MacDonagh RP, Keeley FX, Timoney AG. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169:1065–9.
Tokhmafshan F, Brophy PD, Gbadegesin RA, Gupta IR. Vesicoureteral reflux and the extracellular matrix connection. Pediatr Nephrol. 2017;32:565–76.
Koprowski C, Kim C, Modi PK, Elsamra SE. Ureteral stent-associated pain: a review. J Endourol. 2016;30(7):744–53.
Yossepowitch O, Lifshitz DA, Dekel Y, Ehrlich Y, Gur U, Margel D, et al. Assessment of vesicoureteral reflux in patients with self-retaining ureteral stents: implications for upper urinary tract instillation. J Urol. 2005;173:890–3.
Shao Y, Shen Z, Zhuo J, Liu H, Yu S, Xia S-J. The influence of ureteral stent on renal pelvic pressure in vivo. Urol Res. 2009;37:221–5.
Ecke TH, Bartel P, Hallmann S, Ruttloff J. Evaluation of symptoms and patients’ comfort for JJ-ureteral stents with and without antireflux-membrane valve. Urology. 2010;75(1):212–6.
McMahon CW, Nief CA, Schmidt DR, Choe LJ, Aponte MG, Pelayo SL, inventor; Baylor University, assignee. Ureteral stent and method; 2017.
Ramachandra M, Mosayyebi A, Carugo D, Somani BK. Strategies to improve patient outcomes and QOL: current complications of the design and placements of ureteric stents. Res Rep Urol. 2020;12:303–14.
Janssen C, Buttyan R, Seow CY, Jäger W, Solomon D, Fazli L, et al. A role for the hedgehog effector Gli1 in mediating stent-induced ureteral smooth muscle dysfunction and aperistalsis. Urology. 2017;104:242.e1–8.
Johnson LJ, Davenport D, Venkatesh R. Effects of alpha-blockade on ureteral peristalsis and intrapelvic pressure in an in vivo stented porcine model. J Endourol. 2016;30:417–21.
Lamb AD, Vowler SL, Johnston R, Dunn N, Wiseman OJ. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int. 2011;108:1894–902.
Vernez SL, Okhunov Z, Wikenheiser J, Khoyilar C, Dutta R, Osann K, et al. Precise characterization and 3-dimensional reconstruction of the autonomic nerve distribution of the human ureter. J Urol. 2017;197:723–9.
Park HK, Paick SH, Kim HG, Lho YS, Bae S. The impact of ureteral stent type on patient symptoms as determined by the Ureteral Stent Symptom Questionnaire: a prospective, randomized, controlled study. J Endourol. 2015;29:367–71.
Andersson K. Storage and voiding symptoms: pathophysiologic aspects. Urology. 2003;62:3–10.
Scarneciu I, Lupu S, Pricop C, Scarneciu C. Morbidity and impact on quality of life in patients with indwelling ureteral stents: a 10-year clinical experience. Pak J Med Sci. 2015;31(3):522–6.
Kehinde EO, Rotimi VO, Al-hunayan A, Abdul-halim H, Boland F, Al-awadi KA. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891–6.
Urological infections. EAU guidelines. Edn. presented at the EAU Annual Congress Amsterdam the Netherlands; 2020.
Cormio L, Vuopio-varkila J, Siitonen A, Talja M, Ruutu M. Bacterial adhesion and biofilm formation on various double-J stents in vivo and in vitro. Scand J Urol Nephrol. 1996;30(1):19–24.
Liaw A, Knudsen B. Urinary tract infections associated with ureteral stents: a review. Arch Esp Urol. 2016;69(8):479–84.
Klis R, Korczak-kozakiewicz E, Denys A, Sosnowski M, Rozanski W. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23(6):1015–9.
Scotland KB, Lo J, Grgic T, Lange D. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling. 2019;35:117–27.
Kehinde EO, Rotimi VO, Al-Awadi KA, Abdul-Halim H, Boland F, Al-Hunayan A, et al. Factors predisposing to urinary tract infection after J ureteral stent insertion. J Urol. 2002;167:1334–7.
Szell TFFD, Goelz H, Bluemel B, et al. In vitro effects of a novel coating agent on bacterial biofilm development on ureteral stents. J Endourol. 2019;33(3):225–31.
Slaton JW, Kropp KA. Proximal ureteral stent migration: an avoidable complication? J Urol. 1999;155:58–61.
Leibovici D, Cooper A, Lindner A, Ostrowsky R, Kleinmann J, Velikanov S, et al. Ureteral stents: morbidity and impact on quality of life. Isr Med Assoc J. 2005;7:491–4.
El-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal stents in treatment of stone patients: morbidity related indwelling times. J Urol. 1991;146:1487–91.
Mosayyebi A, Yue QY, Somani BK, Zhang X, Manes C, Carugo D. Particle accumulation in ureteral stents is governed by fluid dynamics: in vitro study using a “stent-on-chip” model. J Endourol. 2018;32(7):639–46.
Lange D, Bidnur S, Hoah N, Chew BH. Ureteral stent-associated complications-where we are and where we are going. Nat Rev Urol. 2015;12:17–25.
Adanur S, Ozkaya F. Challenges in treatment and diagnosis of forgotten/encrusted double-J ureteral stents: the largest single-center experience. Ren Fail. 2016;38(6):920–6.
Soria F, Morcillo E, Serrano A, Budia A, Fernández I, Fernández-Aparicio T, Sánchez-Margallo FM. Evaluation of a new design of antireflux-biodegradable ureteral stent in animal model. Urology. 2018;115:59–64.
El-Faqiq SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Pérez-Fentes, D., Aranda-Pérez, J., de la Cruz, J.E., Soria, F. (2022). Indications, Complications and Side Effects of Ureteral Stents. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)