Abstract
Purpose
To describe treatment patterns and outcomes in wet-overactive bladder (OAB) patients treated with anticholinergics.
Methods
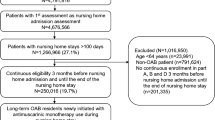
This study was a retrospective claims analysis linked to a one-time patient survey of members of a regional medical group located in California. Participants met the following criteria: received anticholinergic therapy between January 2008 and May 2012, based on pharmacy claims; had a diagnosis of OAB; and reported having ≥1 urinary incontinence (UI) episode per day at the time of the survey. Outcomes included the number of anticholinergics cycled through from treatment initiation until the end of follow-up (May 31, 2013); frequency of UI episodes; and patient requests for additional help for their OAB symptoms.
Results
A total of 620 patients were enrolled into the study. During the follow-up period, patients cycled through 1 to 6 unique anticholinergics; 65 % of the study population used only 1 anticholinergic, while 35 % used ≥2 anticholinergics. Patients reported experiencing an average of 3.5 UI episodes per day (3.6, 3.3, and 3.4 episodes for 1, 2, and ≥3 anticholinergics used, respectively), and over 80 % of patients requested additional help for their OAB symptoms, irrespective of how many anticholinergics were attempted.
Conclusion
UI symptom burden and adherence to therapy did not change as patients attempted more anticholinergic therapies. These results suggest that for patients who remain incontinent after attempting an anticholinergic, cycling on additional anticholinergics may not provide any additional benefit, resulting in sub-optimal care.



Similar content being viewed by others
References
Haylen BT, de Ridder D, Freeman RM et al (2010) International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 29:4–20
Stewart WF, Van Rooyan JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20:327–336
Litwin MS, Saigal CS (eds) (2012) Urologic diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. US Government Printing Office, Washington, DC NIH Publication No. 12-7865
D’Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R (2008) Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm 14:291–301
Gopal M, Haynes K, Bellamy SL, Arya LA (2008) Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol 112:1311–1318
Chancellor MB, Migliaccio-Walle K, Bramley TJ et al (2013) Long-term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clin Ther 35:1744–1751
Cardozo L, Staskin D, Currie B et al (2014) Validation of a bladder symptom screening tool in women with incontinence due to overactive bladder. Int Urogynecol J 25:1655–1663
Yu YF, Nichol MB, Yu AP, Ahn J (2005) Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health 8:495–505
Veenboer PW, Bosch JL (2014) Long-term adherence to antimuscarinic therapy in everyday practice: a systematic review. J Urol 191:1003–1008
Shaya FT, Blume S, Gu A, Zyczynski T, Jumadilova Z (2005) Persistence with overactive bladder pharmacotherapy in a Medicaid population. Am J Manag Care 11(4 Suppl):S121–S129
Pelletier EM, Vats V, Clemens JQ (2009) Pharmacotherapy adherence and costs versus nonpharmacologic management in overactive bladder. Am J Manag Care 15(4 Suppl):S108–S114
Balkrishnan R, Bhosle MJ, Camacho FT, Anderson RT (2006) Predictors of medication adherence and associated health care costs in an older population with overactive bladder syndrome: a longitudinal cohort study. J Urol 175(3 Pt 1):1067–1071
National Committee for Quality Assurance (NCQA) (2013). Improving quality and patient experience. State of healthcare quality 2013. http://www.ncqa.org/Portals/0/Newsroom/SOHC/2013/SOHC-web_version_report.pdf. Accessed 6 Jun 2013
HealthCare Partners Medical Group to participate as Medicare Pioneer Accountable Care Organization [press release] (2011). HealthCare Partners website. http://www.healthcarepartners.com/NewsRoom/releasedetails.aspx?rid=8. Accessed 17 Oct 2014
Acknowledgments
This study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Lisa Bloudek, PharmD, MS, and Evelyn Sarnes, PharmD, MPH of Xcenda, LLC, Palm Harbor, FL, and funded by Allergan plc, Dublin, Ireland. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Funding
This study was funded by Allergan plc, Dublin, Ireland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.B.C. has received compensation for consultant and advisory board participation from Allergan plc. A.Y., D.G., C.W., K.L.C., and M.J. are currently employees of or were employees of Allergan when this study was conducted. I.C. and R.P. are currently or were employees of HealthCare Partners when this study was conducted.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Appendix
Appendix
See Table 2.
Rights and permissions
About this article
Cite this article
Chancellor, M.B., Yehoshua, A., Waweru, C. et al. Limitations of anticholinergic cycling in patients with overactive bladder (OAB) with urinary incontinence (UI): results from the CONsequences of Treatment Refractory Overactive bLadder (CONTROL) study. Int Urol Nephrol 48, 1029–1036 (2016). https://doi.org/10.1007/s11255-016-1277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1277-0