Abstract
Introduction
This study aimed to assess overactive bladder (OAB) treatment patterns and factors associated with effectiveness and persistence.
Methods
A prospective, longitudinal, observational registry study of adults starting OAB therapy with mirabegron or antimuscarinics was undertaken. Primary endpoints were time from treatment initiation to discontinuation/switching; proportion who discontinued/switched; and reasons for discontinuation/switching. Secondary endpoints included OAB Symptom Score (OABSS), OAB Questionnaire: Short Form, and OAB Bladder Assessment Tool scores; factors associated with effectiveness and persistence; and safety.
Results
In total, 556 patients initiating mirabegron and 250 initiating antimuscarinics were enrolled. There was no treatment switch, change, or discontinuation in 68.5% of the mirabegron initiator group and median time to treatment change was not reached. Mean initial treatment duration was 130.8 days. In multivariable models, baseline OABSS was the only variable significantly associated with change from baseline in OABSS, and patients with mild and moderate OAB had significantly better persistence with mirabegron than those with severe OAB. Urinary tract infection was the most common adverse event with mirabegron. There was no treatment switch, change, or discontinuation in 60.4% of the antimuscarinics initiator group and median time to treatment change was not reached. Solifenacin was the most frequent initial treatment (66.0%). Mean treatment duration was 122.2 days. In multivariable models, baseline OABSS was the only variable significantly associated with change from baseline in OABSS, while patients with OAB medication in the 12 months before enrollment had significantly better persistence with antimuscarinics than those with no previous OAB medication. Dry mouth was the most common adverse event with antimuscarinics.
Conclusions
Mirabegron and solifenacin were commonly prescribed as first-line OAB medications. There was no treatment switch, change, or discontinuation in more than 60% of the mirabegron initiator and antimuscarinics initiator groups. Mean initial treatment duration was 130.8 days and 122.2 days for mirabegron and antimuscarinics, respectively. Graphical Abstract available for this article.
Trial Registration
ClinicalTrials.gov NCT03572231.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Overactive bladder (OAB) syndrome can have a substantial negative effect on patient quality of life and can increase healthcare resource utilization. |
Limited prospective registry data are available on the use of OAB pharmacotherapy options within clinical practice, which include antimuscarinics and the β3-adrenoceptor agonist mirabegron. |
This non-interventional registry study was designed to describe OAB treatment patterns and to identify and evaluate factors associated with effectiveness and persistence in patients with OAB from Taiwan and South Korea. |
What was learned from this study? |
Over 60% of the patients who initiated mirabegron or antimuscarinic therapy did not switch, change, or discontinue treatment during the 183-day study period. |
In order to provide further explanations for the persistence data obtained, additional prospective trials are required to appreciate the treatment dynamics involved in determining OAB management in South Korea and Taiwan. |
Digital Features
This article is published with digital features, including a Graphical Abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25273000.
Introduction
The overactive bladder (OAB) syndrome has been defined as urinary urgency, with or without urgency urinary incontinence, usually with increased daytime frequency and nocturia, if there is no proven infection or other obvious pathology [1]. The condition can place a significant burden on patients in terms of their quality of life (QoL) and sexual satisfaction and can increase healthcare resource utilization (HCRU) [2, 3]. Accordingly, there is a need for effective treatments addressing outcomes important to patients.
Traditionally, antimuscarinic drugs were prescribed for OAB symptoms and, in randomized controlled trials, were found to be efficacious, generally well tolerated, and to improve health-related QoL (HRQoL) [4]. However, a known issue with OAB therapy within clinical practice, particularly with antimuscarinics, is treatment persistence, which is important for achieving reliable symptom control and preventing progression of symptom severity and associated healthcare costs [5]. Pharmacotherapy for OAB has now expanded to include β3-adrenoceptor agonists, the first of which was mirabegron [6]. With a different mechanism of action from antimuscarinics, β3-adrenoceptor agonists offer a better tolerability profile, particularly in regard to overall anticholinergic burden, with proven efficacy over placebo [6, 7].
It is important to collect data on persistence in different geographies within the clinic to assist physicians when making treatment decisions. Although several retrospective analyses using clinical practice data to evaluate OAB pharmacotherapy persistence rates have been published [8,9,10], along with data from prospective studies [11,12,13], limited prospective registry data are available, especially in Asia. This non-interventional registry study (Findings of Treatment Patterns from Overactive Bladder Initial Therapy [FAITH]) was designed to describe the treatment patterns of OAB therapies and to evaluate and identify factors associated with effectiveness and persistence in patients with OAB in Taiwan and South Korea. It also investigated QoL, treatment satisfaction, and safety profiles.
Methods
Study Design and Patient Population
This was a prospective, longitudinal, multicenter, observational registry study conducted in Taiwan and South Korea (July 2018 to September 2019; NCT03572231). Adults with OAB, whose physicians had decided to start OAB drug therapy with mirabegron or any antimuscarinic therapy as part of routine clinical practice, were enrolled (Supplemental Methods). This registry study did not provide or recommend any treatment; all decisions were made at the discretion of the treating physicians in accordance with their clinical practices.
The protocol was approved by the institutional review board or independent ethics committee at each site (Supplemental Table S1) and the study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Data Collection and Endpoints
Data were collected at baseline, prior to treatment initiation, and all subsequent follow-up visits. Assessments were done at visit 1 (week 10–14); visit 2/final visit (week 22–26); and any time when a decision to change dose or formulation or add another therapy, switch therapy, or discontinue therapy was made before week 22–26 (Supplemental Methods). Analysis time points were at visits 1 and 2 (Supplemental Methods).
Primary endpoints were time from treatment initiation to discontinuation; switch to another OAB therapy or dose change; and proportion of patients who discontinued OAB treatment or switched to another treatment or dose within 183 days of initiating treatment, along with any reasons for discontinuing or switching. Dose escalation was any increase in dose and/or frequency; discontinuation was defined as stopping the prescribed treatment for more than 30 days (day after the last day of supply to the next dispensing date). Patients were followed up until visit 2 (week 22–26) even if they discontinued the treatment earlier. Switching was defined as discontinuing the initial treatment and initiating a different therapy (including Chinese herbal medicines, onabotulinum toxin A, or surgery) either on the same day or within 30 days after the end date of initial treatment. Patients were lost to follow-up if they failed to return to the clinic for scheduled visits and did not respond to telephone or written attempts to contact them.
Secondary endpoints were changes from baseline in (1) OAB Symptom Score (OABSS; to assess effectiveness); (2) OAB Questionnaire: Short Form (OAB-Q-SF) and OAB Bladder Assessment Tool (OAB-BAT) scores [14] (to assess QoL); (3) Treatment Satisfaction Visual Analog Scale (TS-VAS) scores; (4) factors (e.g., age, OAB severity) associated with effectiveness and persistence of pharmacotherapy for OAB; (5) HCRU related to the management of OAB; and (6) safety (adverse events [AEs] and serious AEs [SAEs]). OAB severity was categorized as mild (OABSS ≤ 5), moderate (OABSS 6–11), or severe (OABSS ≥ 12). A scale of 0 to 10 was used for the TS-VAS, where a score of 10 indicated complete satisfaction and a positive change from baseline indicated improvement.
Subgroup analysis of the primary endpoint by age (≤ 65 and > 65 years) was an exploratory endpoint.
Statistical Analysis
The study was not randomized or designed to compare treatments. The safety analysis set (SAS) included all patients treated at least once with mirabegron or any antimuscarinic. The full analysis set included all patients from the SAS with data at baseline and at least one post-baseline visit.
All computations and the generation of tables, listings, and data for the figures were performed using SAS® version 9.4. The primary analysis of time to event was performed using the Kaplan–Meier method. The number and percentage of patients with treatment discontinuation, switching, or dose change within 183 days from treatment initiation were reported. Patients with no treatment discontinuation, switching, or dose change were considered treatment persistent and were censored at 183 days or at study discontinuation, death, or the last assessment/visit. Persistence rates (95% confidence intervals [CIs]) were reported at 50, 100, 150, and 183 days for the two groups and 183 days for the age subgroups (≤ 65 and > 65 years).
Changes in scores from baseline were summarized as mean (95% CI), standard deviation, median, minimum, maximum, first quartile, and third quartile.
Univariate logistic regression was used to identify factors associated with treatment discontinuation or switching. Univariate and multivariable Cox regression models were used to identify factors associated with persistence, time to treatment discontinuation and switching, or dosage change based on change in OABSS. The assessed factors for these analyses included age, sex, previous medication, severity of OAB, type of OAB, and comorbidity assessment score. Type of OAB was defined as either wet (at least one urgency incontinence episode in the 3 days before the study visit) or dry (no urgency incontinence episodes in the 3 days before the study visit).
Results
Patients and Demographics
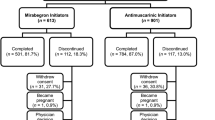
A total of 806 patients were enrolled. Overall, 570 patients (mirabegron, n = 384 patients; antimuscarinics, n = 186 patients) attended visit 1 and 507 patients (mirabegron, n = 338 patients; antimuscarinics, n = 169 patients) attended visit 2 (patients would have been counted twice if they attended both visits). In total, 58.1% of the mirabegron initiator group and 64.4% of the antimuscarinics initiator group completed the study (Fig. 1). The demographic and baseline characteristics are shown in Table 1. The most common reasons for treatment discontinuation included loss to follow-up and withdrawal (Fig. 1).
Patient disposition. AE adverse event, AM antimuscarinics, FAS full analysis set, M mirabegron, SAS safety analysis set. “Completed” was defined as patients who were not lost to follow-up for any reason, completed one or more study visits, and whose “Primary Investigational Period Status” was marked as “Complete” by the study staff, irrespective of the days spent on treatment. a“Reasons for study discontinuation” population refers to patients who were lost to follow-up for any reason and/or did not complete one or more study visits (total, n = 322 patients; M, n = 233 patients; AM, n = 89 patients). b“Reasons for treatment discontinuation” population refers to any patient who did not receive either M or AM for at least 154 days during the study (total, n = 392 patients; M, n = 277 patients; AM, n = 115 patients)
Mirabegron Initiator Group
Primary Endpoints
The proportions of the mirabegron initiator group who switched, discontinued, or changed dose are shown in Fig. 2a. Ninety-four percent of mirabegron was taken as one 50-mg dose, and the most frequent switching pattern was to solifenacin succinate. Time to treatment change for those initiating mirabegron is shown in Fig. 2b; as more than 50% of patients continued treatment, the median time to treatment discontinuation or switching could not be estimated. Sensitivity analyses were performed using all available data collected throughout the study (more than 183 days after treatment initiation); no notable differences were observed vs. the primary analyses.
For the mirabegron initiator group the a proportion of patients who discontinued OAB treatment or switched to another treatment or dose (FAS), b Kaplan–Meier curve for time to treatment change in patients with OAB (FAS), c persistence rates (FAS), and d persistence rates by age (FAS). CI confidence interval, FAS full analysis set, OAB overactive bladder. Time to treatment change was cut off at 183 days after treatment initiation. Treatment change includes treatment switch or dose escalation/reduction or discontinuation. One patient took more than one medication for OAB (i.e., mirabegron and tamsulosin) during the study
Persistence rates in the mirabegron initiator group are shown in Fig. 2c; the mean initial treatment duration was 130.8 days (including dose or frequency changes and treatment interruption within 30 days).
Secondary Endpoints
The effectiveness of mirabegron was assessed using the OABSS. Patients in the mirabegron initiator group indicated improvement in their symptoms during the study based on the OABSS (Fig. 3a).
For the mirabegron initiator group the a change from baseline in OABSS (FAS) and b unadjusted change from baseline in quality-of-life measures (FAS). CI confidence interval, FAS full analysis set, HRQoL health-related quality of life, OAB overactive bladder, OAB-BAT OAB Bladder Assessment Tool, OAB-Q-SF OAB Questionnaire: Short Form, OABSS, OAB Symptom Score, TS-VAS Treatment Satisfaction Visual Analog Scale. One patient took more than one medication for OAB
Previous OAB medication, type of OAB, and OAB severity were associated with change in OABSS at week 22–26 (univariate analysis; p ≤ 0.1). These variables were included in a multivariable regression model with baseline OABSS. Only baseline OABSS was significantly associated with change from baseline in OABSS at week 22–26 (p < 0.05) in the multivariable model (Table 2); a higher baseline OABSS indicated greater improvement in OABSS.
On the basis of univariate Cox regression analyses, significant factors (p ≤ 0.05) associated with time to treatment discontinuation or switching included baseline age and OAB severity for the mirabegron group (Supplemental Table S2). These and other variables with p ≤ 0.1 in the univariate models were included in the multivariable models, which showed patients with mild and moderate OAB had better persistence with mirabegron than those with severe OAB (hazard ratio [HR] mild vs. severe 0.58 [95% CI 0.34–0.97], p = 0.039; HR moderate vs. severe 0.51 [95% CI 0.32–0.82], p = 0.005; Supplemental Table S3).
Overall, patients indicated improvement in symptoms and QoL with mirabegron during the study period based on OAB-Q-SF (including OAB-Q-SF HRQoL total score) and OAB-BAT scores (Fig. 3b). Change from baseline results were not adjusted for any baseline characteristics; consequently, any perceived differences may be attributed to other confounders and not only to treatment effects. Post-baseline TS-VAS scores were similar to baseline or showed minimal improvement (Fig. 3b).
HCRU in patients with OAB included healthcare professional (HCP) and inpatient hospital visits associated with OAB, visits related to AEs or SAEs, and the number of incontinence pads used in the 7 days before a study visit. In the mirabegron initiator group, fewer patients reported HCP visits associated with OAB treatment at week 22–26 than at week 10–14. At each post-baseline visit (week 10–14 and week 22–26), approximately 2% of patients in the mirabegron group visited an HCP because of AEs or SAEs and fewer than 1% of patients reported a visit for a medical intervention to manage side effects or complications due to OAB. None of the patients in the mirabegron group reported an inpatient hospital visit for OAB.
The median (range) number of incontinence pads used in the 7 days prior to the visit by patients in the mirabegron group was 0.0 (0–112) pads at the week 10–14 visit (n = 398) and 0.0 (0–18) pads at the week 22–26 visit (n = 279).
The most commonly reported AE in the mirabegron treatment period was urinary tract infection (UTI; Fig. 4). Five patients experienced an SAE considered by the investigator to be related to mirabegron. One of these patients, who had a history of hospitalization and treatment for heart failure and reduced kidney function, had onset of severe “cardiac failure acute”, with a fatal outcome (Fig. 4).
AE outcomes with mirabegron (SAS). AE adverse event, SAE serious AE, SAS safety analysis set, UTI urinary tract infection. If more than one treatment group (i.e., mirabegron and antimuscarinics) overlapped with an AE, the AE was counted under both treatment groups. For the analysis of AEs, the number of patients in the mirabegron group included patients who received at least one treatment with mirabegron at any time during the study to account for treatment overlap
Exploratory Endpoint
The persistence rate at 183 days was greater for patients > 65 years of age than patients ≤ 65 years of age (Fig. 2d).
Antimuscarinic Initiator Group
Primary Endpoints
The proportions of the antimuscarinics initiator group who switched, discontinued, or changed dose are shown in Fig. 5a. The most frequent initial treatment was solifenacin succinate (66.0%); 10.8% received fesoterodine fumarate, and 10.0% received tolterodine l-tartrate. The most frequent switching pattern was to mirabegron, and there was minimal cycling of antimuscarinics (n = 3) before switching to mirabegron or discontinuing treatment.
For the antimuscarinics initiator group the a proportion of patients who discontinued OAB treatment or switched to another treatment or dose (FAS), b Kaplan–Meier curve for time to treatment change in patients with OAB (FAS), c persistence rates (FAS), and d persistence rates by age (FAS). CI confidence interval, FAS full analysis set, OAB overactive bladder. Time to treatment change was cut off at 183 days after treatment initiation. Treatment change includes treatment switch or dose escalation/reduction or discontinuation
Time to treatment change for those initiating antimuscarinics is shown in Fig. 5b; as more than 50% of patients continued treatment, the median time to treatment discontinuation or switching could not be estimated. Sensitivity analyses showed no notable differences vs. the primary analyses.
Persistence rates in the antimuscarinics initiator group are shown in Fig. 5c; the mean initial treatment duration was 122.2 days (including dose or frequency changes and treatment interruption within 30 days).
Secondary Endpoints
Effectiveness was assessed using the OABSS. Patients in the antimuscarinics initiator group indicated improvement in their symptoms during the study based on the OABSS (Fig. 6a).
For the antimuscarinics initiator group the a change from baseline in OABSS (FAS) and b unadjusted change from baseline in quality-of-life measures (FAS). CI confidence interval, FAS full analysis set, HRQoL health-related quality of life, OAB-BAT OAB Bladder Assessment Tool, OAB-Q-SF OAB Questionnaire: Short Form, OABSS OAB Symptom Score, TS-VAS Treatment Satisfaction visual analog scale
In the antimuscarinics group, sex, previous OAB medication, type of OAB, and OAB severity were associated with change in OABSS at week 22–26 (univariate analysis; p ≤ 0.1). These variables were included in a multivariable regression model with baseline OABSS. Only baseline OABSS was significantly associated with change from baseline in OABSS at week 22–26 (p < 0.05) in the multivariable model (Table 3).
On the basis of univariate Cox regression analyses, significant factors (p ≤ 0.05) associated with time to treatment discontinuation or switching included sex and previous OAB medication for the antimuscarinics group (Supplemental Table S4). These and other variables with p ≤ 0.1 in the univariate models were included in the multivariable models, which showed patients with OAB medication in the 12 months before enrollment had better persistence with antimuscarinics than those with no previous OAB medication (HR 0.57 [95% CI 0.34–0.95]; p = 0.033; Supplemental Table S5).
Overall, patients indicated symptom and QoL improvement with antimuscarinics during the study period based on OAB-Q-SF (including OAB-Q-SF HRQoL total score) and OAB-BAT scores (Fig. 6b). Post-baseline TS-VAS scores were similar to baseline or showed minimal improvement (Fig. 6b).
With regard to HCRU in the antimuscarinics group, fewer patients reported HCP visits associated with OAB treatment at week 22–26 than at week 10–14. At week 10–14, approximately 4% of patients in the antimuscarinics group visited an HCP because of AEs or SAEs. No patient visits were related to a medical intervention to manage side effects or complications due to OAB. One patient reported an inpatient hospital visit for OAB at week 10–14.
In the antimuscarinics group, the median (range) number of incontinence pads used in the 7 days prior to the visit was 0.0 (0–40) pads at the week 10–14 visit (n = 195) and 0.0 (0–70) pads at the week 22–26 visit (n = 137).
The most commonly reported AE in the antimuscarinics treatment period was dry mouth (Fig. 7).
AE outcomes with antimuscarinics (SAS). AE adverse event, SAE serious AE, SAS safety analysis set, UTI urinary tract infection. If more than one treatment group (i.e., mirabegron and antimuscarinics) overlapped with an AE, the AE was counted under both treatment groups. For the analysis of AEs, the number of patients in the antimuscarinics group included patients who received at least one treatment with antimuscarinics at any time during the study to account for treatment overlap
Exploratory Endpoint
The persistence rate at 183 days was greater for patients > 65 years of age than patients ≤ 65 years of age (Fig. 5d).
Discussion
FAITH is the first multinational study from Asia to record registry data prospectively from primary sources; it complements the clinical practice data from prospective registries involving patients with OAB in other geographies, such as BELIEVE (Europe) [12] and a Prospective, non-intErventional Registry Study of PatiEnts initiating a Course of drug Therapy for overactIVE bladder (PERSPECTIVE; North America) [15]. Studies conducted within the clinic assessing patient-reported outcomes in OAB offer insight into the elements of care important to patients and factors that may impact treatment persistence. In particular, HRQoL and treatment satisfaction can be key to optimizing persistence [12].
These data show that 68.5% of the mirabegron initiator group and 60.4% of the antimuscarinics initiator group did not change or discontinue treatment. Hence, the median time from treatment initiation to discontinuation, switching, or dose change was not estimable in either group. Persistence rates at 50 and 183 days were 91.0% and 67.4%, respectively, in the mirabegron initiator group and 85.8% and 56.9%, respectively, in the antimuscarinics initiator group. In addition, persistence was greater in older patients in both groups. These persistence data were similar to other prospective observational studies in Japanese and European populations. In a prospective post-marketing study in Japan (n = 1139), persistence with mirabegron was 77.6% at 6 months and 66.0% at 1 year [11]. A large, prospective European non-interventional study (n = 796) reported 53.8% persistence with mirabegron after a 10–12-month follow-up [12]. In addition, smaller country-specific studies reported 1-year persistence rates of 25–71% with mirabegron [16, 17]. One possible explanation for the relatively small difference in persistence and switching rates for mirabegron and antimuscarinics in our study is its design, because treatment decisions were made by the investigator rather than being randomly assigned. It is possible, therefore, that patients who previously experienced AEs with antimuscarinics were prescribed mirabegron. Persistence rates could also have been influenced by differences in healthcare systems between regions, including the cost of the drug for the patient.
The current study was not randomized or designed to compare treatments. However, a number of comparative studies have demonstrated improved persistence with mirabegron over antimuscarinics. In a prospective, open-label, 48-month observational study in Taiwan, 171 patients who switched from solifenacin to mirabegron because of continuing OAB symptoms remained on therapy for significantly longer vs. the overall group that had started on solifenacin (n = 416) [13]. Retrospective analyses of US and Canadian insurance claims databases demonstrated that median persistence was 90 days with mirabegron vs. 30 days with tolterodine [8]; discontinuation or switching occurred in 81% of patients receiving mirabegron vs. 88% receiving anticholinergics [9]; and 12-month persistence rates were 31.7% vs. 13.8–22.0% with mirabegron vs. antimuscarinics, respectively [18]. A retrospective analysis of two Japanese databases showed that median persistence with mirabegron vs. the antimuscarinic comparators was 44 vs. 21–30 days (insurance claims), respectively, and 105 vs. 62–84 days, respectively (pharmacy claims) [10]. Finally, a US screening survey found that 25% of respondents discontinued antimuscarinic treatment over a 12-month period, predominately as a result of a lack of efficacy or switching to a new medication [19].
Regarding secondary endpoints, patients in both treatment groups demonstrated similar changes from baseline in OABSS, and higher baseline OABSS was significantly associated with greater improvement in OABSS at week 22–26. Furthermore, patients in both treatment groups indicated improvement based on OAB-Q-SF, OAB-Q-SF HRQoL, and OAB-BAT scores. This is the first registry study to use the OAB-BAT, a new tool developed by a global advisory board for assessing patient-reported outcomes in OAB, which may become an alternative to bladder diaries and other patient-reported outcome measures [14]. HCRU was low across all measures for both treatment groups, in line with other studies of mirabegron [12].
In this study, the most commonly reported AE was UTI in the mirabegron treatment period and dry mouth in the antimuscarinics period. UTI, urinary retention, cardiac disorders, and increased blood pressure have been reported previously with mirabegron [6], whereas dry mouth and constipation are associated with antimuscarinics as a result of their anticholinergic effects. Anticholinergic effects are bothersome and can be concerning in older patients [20]. One explanation for the generally low incidence of dry mouth in the antimuscarinics group in this study may be that the majority were taking solifenacin, which has a lower rate of dry mouth than other antimuscarinics. The rate of UTIs was 3.3% in the antimuscarinics group and 2.5% in the mirabegron group. The overall rates of AEs were 21.6% and 17.4%, respectively, which are consistent with other reports [7, 20]. However, the overall AE rate with mirabegron in this study was about half that reported for other prospective registry studies (BELIEVE [42.8%] and PERSPECTIVE [33.1%]) [12, 15]. As these AE data reflect experiences in clinical practice, they provide valuable insight for HCPs. The “cardiac failure acute” SAE, assessed to be drug related by the investigator, was evaluated as not drug related by the sponsor and was attributed to concomitant disease. The term “cardiac failure acute” was likely selected as being closest to describing the event, but no causal relationship could be established in this non-interventional study.
Additional analyses found that patients were more likely to continue treatment if they were older (at baseline) and had mild OAB (mirabegron) or were male and had previously received OAB medication (antimuscarinics). These findings are in line with other previous persistence investigations, which have typically found that older and treatment-experienced patients persist with OAB medication for a longer period of time than younger and treatment-naïve patients [8, 10, 21,22,23]. Furthermore, some studies that have also been conducted in East Asia have found that male patients persist with treatment longer than female patients [10, 22]. Older and treatment-experienced patients may be willing to persist with treatment as they may have experienced the condition for a longer duration and might be more familiar with the benefit–risk profile of OAB therapies. In addition, AEs could be a factor associated with these findings based on the safety profile and known mechanisms of action. The safety profile of mirabegron in older patients has been established [24], with rates of dry mouth and constipation being lower than with antimuscarinics [7]. Furthermore, mirabegron does not affect cognition [25] or have an association with dementia [26], potentially because it does not have the anticholinergic burden associated with antimuscarinics [27]. Similarly, mirabegron can be considered for patients with comorbidities owing to a neutral risk for cardiac events [28] and a lower risk of urinary retention [29].
Because this registry study was an observational clinical practice investigation, some possible sources of bias may have been linked to patient selection and recall error as a result of patient self-reporting. Potential selection bias arising from a lack of complete enrollment of potentially eligible patients was reduced by maintaining screening logs at the sites. Other limitations are the relatively short duration of follow-up and the high proportion of patients lost to follow-up. Finally, the study was not randomized or designed for comparison, and results were not adjusted for any baseline characteristics. This means any perceived differences between treatment groups may be attributed to other confounders and not to treatment effects.
Conclusions
This is the first prospective clinical practice study investigating the management of OAB in daily practice in Taiwan and South Korea. Median time to treatment change was not reached and mean initial treatment time was more than 120 days for mirabegron and antimuscarinics. Overall, there was no treatment switch, change, or discontinuation in 68.5% of mirabegron initiators and 60.4% of antimuscarinics initiators, with a lower AE rate with mirabegron than previously reported. After treatment initiation, around one-third of the patients did not report back to their physician. The most common reasons for treatment discontinuation included loss to follow-up and study withdrawal. Treatment persistence was associated with increased age, mild OAB (mirabegron), and male sex and previous use of OAB medication (antimuscarinics). Further research with prospective trials would be helpful to better understand OAB management in South Korea and Taiwan.
Data Availability
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33(5):622–4.
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101(11):1388–95.
Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80(1):90–6.
Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54(3):543–62.
Kim TH, Lee K-S. Persistence and compliance with medication management in the treatment of overactive bladder. Investig Clin Urol. 2016;57(2):84–93.
Betmiga 50 mg prolonged-release tablets: summary of product characteristics. 2022. https://www.medicines.org.uk/emc/product/7540/smpc. Accessed 8 Dec 2023.
Chapple CR, Cruz F, Cardozo L, et al. Safety and efficacy of mirabegron: analysis of a large integrated clinical trial database of patients with overactive bladder receiving mirabegron, antimuscarinics, or placebo. Eur Urol. 2020;77(1):119–28.
Nitti VW, Rovner ES, Franks B, et al. Persistence with mirabegron versus tolterodine in patients with overactive bladder. Am J Pharm Benefits. 2016;8(2):e25–33.
Sussman D, Yehoshua A, Kowalski J, et al. Adherence and persistence of mirabegron and anticholinergic therapies in patients with overactive bladder: a real-world claims data analysis. Int J Clin Pract. 2017;71(3–4):e12824.
Kato D, Uno S, Van Schyndle J, Fan A, Kimura T. Persistence and adherence to overactive bladder medications in Japan: a large nationwide real-world analysis. Int J Urol. 2017;24(10):757–64.
Kato D, Tabuchi H, Uno S. Safety, efficacy, and persistence of long-term mirabegron treatment for overactive bladder in the daily clinical setting: interim (1-year) report from a Japanese post-marketing surveillance study. Low Urin Tract Symptoms. 2019;11(1):14–23.
Freeman R, Foley S, Rosa Arias J, et al. Mirabegron improves quality-of-life, treatment satisfaction, and persistence in patients with overactive bladder: a multi-center, non-interventional, real-world, 12-month study. Curr Med Res Opin. 2018;34(5):785–93.
Wang C-C, Kuo H-C. Factors associated with antimuscarinic drug persistence and increasing drug persistence after switching to mirabegron for overactive bladder patients. J Formos Med Assoc. 2019;118(1 Pt 2):279–84.
Chapple C, Kelleher C, Siddiqui E, et al. Validation of the Overactive Bladder-Bladder Assessment Tool (OAB-BAT): a potential alternative to the standard bladder diary for monitoring OAB outcomes. Eur Urol Focus. 2021;7(5):1176–83.
Carlson KV, Rovner ES, Nair KV, Deal AS, Kristy RM, Schermer CR. Factors associated with improvements in patient-reported outcomes during mirabegron or antimuscarinic treatment of overactive bladder syndrome: a registry study (PERSPECTIVE). Adv Ther. 2019;36(8):1906–21.
Duckett J, Balachandran A. Tolerability and persistence in a large, prospective case series of women prescribed mirabegron. Int Urogynecol J. 2016;27(8):1163–7.
Martan A, Masata J, Krhut J, Zachoval R, Hanus T, Svabik K. Persistence in the treatment of overactive bladder syndrome (OAB) with mirabegron in a multicenter clinical study. Eur J Obstet Gynecol Reprod Biol. 2017;210:247–50.
Wagg A, Franks B, Ramos B, Berner T. Persistence and adherence with the new beta-3 receptor agonist, mirabegron, versus antimuscarinics in overactive bladder: early experience in Canada. Can Urol Assoc J. 2015;9(9–10):343–50.
Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105(9):1276–82.
Kelleher C, Hakimi Z, Zur R, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol. 2018;74(3):324–33.
Wagg AS, Foley S, Peters J, Nazir J, Kool-Houweling L, Scrine L. Persistence and adherence with mirabegron vs antimuscarinics in overactive bladder: retrospective analysis of a UK General Practice prescription database. Int J Clin Pract. 2017;71(10):e12996.
Lee K-S, Park H, Kang D, et al. Mirabegron has longer treatment persistence than antimuscarinics: real-world data from a Korean national cohort database. Neurourol Urodyn. 2021;40(8):1972–80.
Carlson KV, Rovner ES, Nair KV, Deal AS, Kristy RM, Hairston JC. Persistence with mirabegron or antimuscarinic treatment for overactive bladder syndrome: findings from the PERSPECTIVE registry study. Low Urin Tract Symptoms. 2021;13(4):425–34.
Wagg A, Staskin D, Engel E, Herschorn S, Kristy RM, Schermer CR. Efficacy, safety, and tolerability of mirabegron in patients aged ≥65 yr with overactive bladder wet: a phase IV, double-blind, randomised, placebo-controlled study (PILLAR). Eur Urol. 2020;77(2):211–20.
Griebling TL, Campbell NL, Mangel J, et al. Effect of mirabegron on cognitive function in elderly patients with overactive bladder: MoCA results from a phase 4 randomized, placebo-controlled study (PILLAR). BMC Geriatr. 2020;20:109.
Welk B, McArthur E. Increased risk of dementia among patients with overactive bladder treated with an anticholinergic medication compared to a beta-3 agonist: a population-based cohort study. BJU Int. 2020;126(1):183–90.
Lozano-Ortega G, Walker DR, Johnston K, et al. Comparative safety and efficacy of treatments for overactive bladder among older adults: a network meta-analysis. Drugs Aging. 2020;37(11):801–16.
White WB, Siddiqui E, Tat T, Franks B, Schermer CR. Cardiovascular safety of mirabegron: analysis of an integrated clinical trial database of patients with overactive bladder syndrome. J Am Soc Hypertens. 2018;12(11):768-78.e1.
Tubaro A, Batista JE, Nitti VW, et al. Efficacy and safety of daily mirabegron 50 mg in male patients with overactive bladder: a critical analysis of five phase III studies. Ther Adv Urol. 2017;9(6):137–54.
Acknowledgements
The authors would like to thank the FAITH study investigators and all patients who took part in the study.
Medical Writing, Editorial, and Other Assistance.
Medical writing support was provided by Becky Ayles from Envision Pharma, Inc. and funded by the study sponsor.
Authorship.
In accordance with the International Committee of Medical Journal Editors (ICMJE) recommendations, each author has made substantial contributions to the conception or design of the study or the acquisition, analysis, and interpretation of the data, and drafted the manuscript or reviewed it critically for important intellectual content. All authors have approved the final version for submission and agree to be accountable for all aspects of the work.
Funding
This study was funded by Astellas Pharma, Inc., who also funded the journal’s Rapid Service and Open Access Fees.
Author information
Authors and Affiliations
Contributions
Study concept and design: Seung-June Oh, Sung Tae Cho, Hann-Chorng Kuo, Eric Chieh-Lung Chou, Yu-Chao Hsu, Kyu-Sung Lee, and Budiwan Sumarsono; acquisition of data: Seung-June Oh, Sung Tae Cho, Hann-Chorng Kuo, Eric Chieh-Lung Chou, Yu-Chao Hsu, and Kyu-Sung Lee; analysis and interpretation of data: all authors; drafting of the manuscript: all authors; critical reviewing of the manuscript for important intellectual content: all authors; statistical analysis: Yi Song; obtaining funding: Farid Hadi and Budiwan Sumarsono; administrative, technical, or material support: Farid Hadi, Yi Song, and Budiwan Sumarsono; and supervision: Kyu-Sung Lee.
Corresponding author
Ethics declarations
Conflict of Interest
Seung-June Oh has received grant support from Astellas and was a moderator of the “Astellas Asia-Oceania closed symposium” held on February 15, 2019, in Seoul, South Korea. Sung Tae Cho, Hann-Chorng Kuo, Eric Chieh-Lung Chou, Yu-Chao Hsu, and Kyu-Sung Lee have nothing to disclose outside of the submitted work. Farid Hadi is an employee of Astellas Pharma, Inc. Yi Song and Budiwan Sumarsono were employees at Astellas Pharma, Inc. at the time of the study.
Ethical Approval
The protocol was approved by the institutional review board or independent ethics committee at each site (Supplemental Table S1) and the study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Additional information
Budiwan Sumarsono and Yi Song were employees of Astellas Pharma, Inc. at the time of the study.
Prior Presentation Preliminary data from this research were presented orally at the ICS online conference, October 14–17, 2021: Oh S, Cho S, Kuo H, et al. Patients with overactive bladder remain on their treatment course longer with mirabegron than antimuscarinics: results from a real-world, prospective, registry study in Taiwan and Korea. Available at https://www.ics.org/2021/abstract/387.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Oh, SJ., Cho, S.T., Kuo, HC. et al. Treatment Patterns with Mirabegron and Antimuscarinics for Overactive Bladder: A Prospective, Registry Study in Taiwan and South Korea (FAITH). Adv Ther 41, 1652–1671 (2024). https://doi.org/10.1007/s12325-024-02784-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02784-2