Abstract
Urban ecological reserves are large green areas immersed within cities where native and exotic species of plants and animals coexist. Here, we examined the environmental features that facilitate the occurrence of nine species of native birds in an urban ecological reserve located within one of the largest cities in the world, Mexico City. We also searched for changes in occupancy rates among the three distinct climatic seasons that occur in central Mexico: warm-dry, rainy, and cold-dry. Using data collected during four years and multi-season occupancy models, we found that most of our study species prefer the urbanized sites that surround the reserve over the core conservation areas. This urban affinity can be explained by the diverse vegetation that prevails in such urban sites, which offers a high habitat heterogeneity that facilitates the presence of bird species with distinct ecological needs. In contrast, the reserve consists of a relatively homogeneous xerophytic scrubland where a few species of shrubs and small trees are dominant. We also detected seasonal changes in five species, with highest occupancy during the warm-dry season of each year, which coincides with both their breeding season and the driest period of the year. This finding indicates that these birds find in the reserve and surrounding urban areas enough food and water during this limiting season as well as safe nesting sites. Our study provides evidence that some native birds can become urban exploiters and that the benefits that they obtain from urban settings are greatest during their breeding season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban environments harbor a rich diversity of plant and animal species (Alvey 2006; Aronson et al. 2014). Many of these are non-native species that thrive in cities because urban structure provides them with refuges and breeding locations, supplementary food resources, and minimal predation (McKinney 2008). Despite the evident dominance of exotic species in urban ecosystems, some native species still inhabit cities and urban green spaces are critical for their persistence (Murgui and Hedblom 2017). These patches of vegetation offer resources and conditions that allow them to continue surviving and reproducing in the region to which they are native despite the drastic change in land cover derived from urbanization (Fuyuki et al. 2014; Morrison et al. 2016; Jensen et al. 2023). Large urban green spaces have the potential to host rich communities of native plants and animals and to sustain relatively high abundances of these species (Chamberlain et al. 2007; Barbosa de Toledo et al. 2012; Fischer et al. 2018; Southon et al. 2018).
Some urban green spaces in Mexico and elsewhere that cover a substantially large area and contain remnants of original vegetation have been designated as urban ecological reserves by local authorities (George and Crooks 2006; Camara et al. 2012; Marchionni et al. 2021). These urban reserves are protected with the main purpose of preserving the native biodiversity that still persists within cities (Kendal et al. 2017; Enedino et al. 2018). Effective management and conservation plans for native species inhabiting urban ecological reserves must be based on quantitative information about their populations. Especially, it is important to know: (1) if the species of interest are actually occupying these urban reserves, (2) the proportion of the total protected area that they are occupying, and (3) the specific ecological features of these urban reserves that facilitate their presence (i.e., the habitat traits that native species prefer within these large urban green spaces). In this way, management actions aimed at preserving the native species that still inhabit urban settings can focus on enhancing the environmental features identified as strong predictors of their occurrence. For instance, if quantitative analyses reveal that canopy cover is strongly associated with the presence of a particular native species, then managers should focus on increasing canopy cover across both the reserve and the surrounding urban matrix.
A substantial proportion of the native species that still persist within cities are now habituated to human activity and obtain benefits from human-made structures (such as nesting sites, perches, and shelters) as well as abundant food from humans, both directly (e.g., bird feeders) and indirectly (e.g., organic waste, fruits and seeds from ornamental plants) (Gray and van Heezik 2016; Johnson and Munshi-South 2017; Tryjanowski et al. 2020; Dalla Pria et al. 2022). Some of these native species can be classified as “urban exploiters” because they can successfully use these anthropogenic resources and because their fitness is likely higher in urban settings than in less disturbed areas (Kark et al. 2007; Jokimäki et al. 2017; Palacio 2020). Numerous studies have documented that native species are actively feeding and successfully breeding in urban settings (Rutz 2008; Møller 2010; Reynolds et al. 2019; Zúñiga-Vega et al. 2023), indicating that they may now depend to some extent on the resources that they can find in the urban environment. However, we still do not know if native urban exploiters have changed their habitat preferences in such a way that they use the urban matrix more frequently than the large green spaces (such as urban ecological reserves) that are available to them within cities. Comparing the rates of occurrence of native species between the urbanized area and urban green spaces can yield insight into the value of urban ecological reserves to preserve the native biodiversity. Evidence of preference for conserved areas would indicate that urban ecological reserves serve a major role in facilitating the persistence of native species within urban settings. In contrast, evidence of preference for urbanized areas would support the hypothesis that such native species have become urban exploiters and are thus less dependent on large green spaces.
Native species may occupy urban reserves only during particular periods of the year. Detecting seasonal patterns in their presence within cities would yield insight into the particular resources that urban ecosystems provide to native species. To our knowledge, no previous study has assessed whether some native species may be urban exploiters only during a particular season and then move out of the city to less disturbed sites during another season (or seasons; but see Jokimäki and Suhonen 1998). Such seasonal variation in site occupancy may result from reproductive activities because individuals may move to a different habitat when they are breeding and nesting (Betts et al. 2008; Barçante et al. 2017). For many animal species, reproductive success can be relatively high in urban settings because cities can offer a wide array of breeding and nesting sites (Reynolds et al. 2019) and predation risk for both adults and their offspring is relatively low (Gering and Blair 1999).
In addition, some critical environmental conditions that vary predictably within a year, such as ambient temperature and food availability, may promote changes in habitat preferences. For instance, individuals may move to warmer sites during cold months or to sites with sufficient sources of food during limiting periods (Boyle 2010; Boyle et al. 2010; Pageau et al. 2020). Cities can be considered heat islands because temperature is warmer all year long compared to adjacent rural or pristine areas (Yow 2007). Thus, native species may use urban ecological reserves mainly during the winter to avoid colder temperatures that occur in less disturbed sites nearby (Shochat et al. 2006). Similarly, in regions with marked seasonality in terms of rainfall, native species could prefer large urban green spaces over pristine areas during the dry season because in the former both food and water availability remain relatively constant throughout the year due to year-round irrigation and other gardening activities (Wong et al. 2023).
Quantitative information on native species that still persist in urban ecological reserves is necessary to understand their temporal and spatial patterns of habitat use. This information can be used to propose management and conservation actions such as: (1) promoting and protecting the environmental features that native species prefer, (2) removing or reducing those factors that have a negative impact on these species (e.g., the presence of human waste), and (3) informing citizens about when these native species are present in the city so they are aware of them and willing to help in their preservation. For animal species in particular, population trends can be examined by means of different approaches, all of which take into account imperfect detection (Kéry 2004; Cayuela et al. 2018; Altwegg and Nichols 2019). Spatial and temporal changes in animal populations have been analyzed through estimates of abundance (Yamaura et al. 2011; Iijima et al. 2013). However, estimating abundance can be logistically and analytically challenging for species with high mobility, such as birds, because the counts of individuals in the field may be quite imprecise (Royle et al. 2007). An alternative approach is to quantify rates of site occupancy (MacKenzie et al. 2002). In this case, the focus is placed on the proportion of a particular area (or the proportion of suitable sites within an area) that is occupied by the species of interest. Spatial and temporal (seasonal) changes in site occupancy can be examined by estimating: (1) the probability that a particular site that is occupied by the focal species during a given season becomes unoccupied the following season (this parameter has been termed “local extinction probability”), and (2) the probability that a particular site that is unoccupied during a given season becomes occupied the following season (this parameter has been termed “colonization probability”) (MacKenzie et al. 2003). These two parameters provide the basis to understand the causes of site occupancy dynamics (e.g., of seasonal changes). This approach, which explicitly estimates detection probabilities (MacKenzie et al. 2018), has been recently implemented to study population trends of species of conservation concern and, in addition, it has allowed identification of environmental features that facilitate the presence of the species of interest (Bled et al. 2013; Louvrier et al. 2018; Ortega-Álvarez et al. 2021). However, less than a handful of studies have estimated colonization and local extinction probabilities for native species inhabiting urban green spaces (Cassel et al. 2019; Rudershausen et al. 2021; Zúñiga-Vega et al. 2023).
In this study, we estimated rates of site occupancy for nine resident species of native birds in an urban ecological reserve located within one of the largest cities in the world, Mexico City. As in most cities, the urban matrix is dominated by exotic species of birds (van Heezik et al. 2008; Menon and Mohanraj 2016). However, several species of native birds can still be found in the city and little is known about their population trends (Ramírez-Cruz et al. 2019, 2020). Here, we focused on examining temporal (inter- and intra-annual) changes in the proportion of area occupied by nine non-migratory species of birds over the course of four years (2015–2018), as well as on identifying the environmental features of the reserve and surrounding urbanized areas that facilitate the occurrence of these native birds. Given that all our focal species have previously been observed inhabiting urban ecosystems (Table 1), we hypothesize that these native species have become urban exploiters and, therefore, sites where human activities are frequent (i.e., the urban matrix that surrounds the ecological reserve) would promote the presence of these birds. In contrast, sites within the core conservation areas of the reserve would be less prone to be colonized and instead should promote high rates of site abandonment. Additionally, we tested the hypothesis that these bird species occupy the urban reserve and surrounding urbanized areas mainly during the limiting seasons of the year (i.e., the cold or dry seasons) or during their breeding periods, because seasonality is dampened in cities and they can find food, water, warmer temperatures, and safe nesting sites (Shochat et al. 2006; Yow 2007; Reynolds et al. 2019; Wong et al. 2023). During the rainy, warm, or non-breeding seasons these birds should depend to a lesser extent on the urban environment and, thus, occupancy of the ecological reserve and surrounding urban matrix should be lower.
Methods
Study area
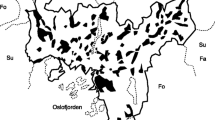
We focused this study on the Reserva Ecológica del Pedregal de San Ángel (REPSA), which is an urban ecological reserve that covers an area of 237 ha located in southern Mexico City (Fig. 1). This reserve was created in 1983 to protect the last remnants of a unique ecosystem composed of a xerophytic scrubland established over a rocky substrate that originated from the eruption of the Xitle volcano 2000 years ago (Siebe 2000, 2009). The REPSA harbors a rich biodiversity composed of 1849 native and 317 exotic species of plants and animals (SEREPSA 2013; Zambrano et al. 2016). Seasonality in the region is strong, with a rainy season from June to October, a cold-dry season from November to February, and a warm-dry season from March to May. Mean temperatures are 16.7 °C, 13.1 °C, and 18.7 °C for the rainy, cold-dry, and warm-dry seasons, respectively (Rzedowski 1954; Lot and Cano-Santana 2009).
Location in Mexico of the urban ecological reserve where we conducted our study (Reserva Ecológica del Pedregal de San Ángel) including the surrounding urban areas. This reserve is divided into core conservation areas (colored in dark blue) and buffer areas (colored in green). All the surrounding areas represent gardens, buildings, parking lots, paved walking paths, and streets. Landmarks indicate the location of our 100 observation sites and are colored according to their location in either core, buffer, or urban areas. The number of observation sites within each type of area is shown within parentheses
This urban reserve is embedded within the dense urban matrix of Mexico City, which is one of the top ten megacities in the world with a human population of more than 21 million people inhabiting its metropolitan area (Folberth et al. 2015; INEGI 2022). Our study area comprises the entire protected area, as well as the adjacent urban area, which includes a significant proportion of the campus of the Universidad Nacional Autónoma de México (National Autonomous University of Mexico; Fig. 1). The REPSA is divided into three large core conservation areas and 13 smaller buffer areas, all of them surrounded by streets, buildings, and facilities of the university (Fig. 1). The core areas are patches of original xerophytic scrubland that serve the main purpose of biodiversity conservation. Access to people is restricted in these conservation areas. The smaller buffer areas are a mix of both original scrubland and exotic vegetation that serve as connectivity corridors among the core areas. Access to people is only partially restricted in these buffer areas and, hence, human activity causes some degree of disturbance (Zambrano et al. 2016; Ramírez-Cruz et al. 2018). We were able to examine site occupancy of our nine focal species in both the undisturbed reserve and adjacent buffer areas, gardens, buildings, parking lots, paved walking paths, and streets.
Study species and field methods
We estimated rates of site occupancy for the following nine resident species of native birds that still can be found in central Mexico: Haemorhous mexicanus, Melozone fusca, Pheucticus melanocephalus, Psaltriparus minimus, Ptiliogonys cinereus, Pyrocephalus rubinus, Spinus psaltria, Thryomanes bewickii, and Toxostoma curvirostre. These species belong to eight taxonomic families and vary broadly in their diet and preferred habitats (Table 1). Therefore, we expected some differences among species in the particular features of the REPSA and surrounding urban areas that promote or preclude their presence and that cause inter- and intra-annual changes in site occupancy. However, all nine species have been frequently recorded in urban settings in distinct regions (not only in Mexico City; Table 1). For this reason, these species are appropriate study systems to examine the hypothesis that these native species, which have probably inhabited the region long before the establishment of the city, have switched habitat preferences and now they should show greater affinity for the urban area than for the ecological reserve (i.e., they could have become urban exploiters). In addition, we selected these particular nine species also because the number of detections of each of them was high enough to secure model convergence and reliable parameter estimates. Other native bird species can also be found in Mexico City, and in fact we recorded them during surveys, but their abundances (and hence our detections) were too low to obtain accurate parameter estimates (i.e., their standard errors were too large).
We established 100 observation sites distributed throughout our study area (25 located in the core conservation areas of the REPSA, 27 in the buffer areas, and 48 in adjacent urban areas; Fig. 1). The specific location of each observation site was defined randomly, with the only requirement of a minimum separation distance of 150 m from other observation sites. In this way, we ensured that the probability of detecting birds at any given site did not affect the probability of detecting them at other sites (i.e., independent detectability; MacKenzie et al. 2002; MacKenzie et al. 2018). Each observation site consisted of a circle of approximately 40 m in diameter, within which a group of trained observers recorded all the bird species that they were able to detect and accurately identify. All detections were made visually with the aid of binoculars. All observers had previous experience identifying all nine focal species and, in addition, these bird species are relatively easy to identify and differentiate from other birds that also occur in Mexico City. Thus, the probability of false-positive detections that could have biased our results (Clement 2016) was rather low. We used a smaller radius for our observation sites than the usual 50 m of point-count stations for bird surveys (Matsuoka et al. 2012) because the scrubland areas of the REPSA are located on steep and complex volcanic terrain, making it difficult to move through these areas without altering the behavior of some birds. Additionally, the dense scrub vegetation in the REPSA limits our visibility and, hence, our detectability of birds at distances greater than 40 m.
Our study spanned 11 field seasons over four years (2015–2018). Our field seasons took place during May, September, and January of each year, which represent the warm-dry, rainy, and cold-dry seasons, respectively. The first season occurred during May 2015 and the last during September 2018. We focused on these three seasons because they represent contrasting climatic conditions that may affect reproduction, physiology, and behavior of birds. Specifically, during the warm-dry season, which corresponds to spring in the Northern Hemisphere, all our nine focal species are breeding (Table 1). In addition, this is also the warmest and driest period of the year (Carrera-Hernández and Gaskin 2007) and, thus, resources such as food and water are likely scarce in the forests that surround the city. During the rainy season, juveniles of all nine species already left the nests and vegetation grows throughout the region. During the cold-dry season, rainfall is sporadic, temperatures are the lowest of the year, and our nine species are not breeding (Table 1). Given these seasonal differences in both environmental conditions and bird phenology, the habitat requirements of the nine species likely differ among these three contrasting seasons (Table 1).
During each field season, we conducted repeated surveys in all our 100 observation sites (between 6 and 8 independent surveys per site and season). Each survey consisted of 15 min of observation, searching for birds. From each field season we obtained detection histories for each species, which consisted of site-specific sequences of 0’s and 1’s, where 0 denotes that the species was not detected and 1 denotes that the species was detected during a particular survey. For each observation site, the total number of 0’s and 1’s per season was equal to the number of independent surveys that we conducted.
In addition, at each observation site we measured environmental characteristics that could have affected the probability that our focal species were present or our ability to detect them during field surveys. Also, these environmental features could have influenced whether our focal species remained at a given site or abandoned the site between field seasons. We estimated vegetation cover (as percentage) at three different layers that reflect the structural complexity of a site: trees (height > 3 m), shrubs (height between 0.5 and 3 m), and herbs (height < 0.5 m). These three vegetation strata can differentially affect our study species depending on their habitat preferences. For instance, some species depend to a greater extent on tall trees to feed and nest (e.g., Pheucticus melanocephalus and Ptiliogonys cinereus; Chu 2020; Hill 2022), other species prefer shrubs to carry out these essential activities (e.g., Thryomanes bewickii and Toxostoma curvirostre; Kennedy and White 2020; Tweit 2020), and others tend to avoid dense vegetation and forage preferentially in areas where herbs are the dominant vegetation stratum (e.g., Melozone fusca and Pyrocephalus rubinus; Johnson and Haight 2020; Ellison et al. 2021). At each visit, all observers visually estimated each type of cover and then we calculated an average across all these visual estimates, after discarding extreme estimates (as per Ramírez-Cruz et al. 2020). We used this same method to estimate urban cover as the percentage of each site that consisted of parking lots, buildings, and paved walking paths. Similarly, we estimated the percentage of the area of each site that was covered by human waste (plastics, cardboard, metal cans, organic waste, pieces of paper). We decided to examine effects of urban cover and human waste due to their potential to alter resource availability for birds (Jokimäki and Suhonen 1998; García-Arroyo et al. 2023).
Using Google Earth (Google 2017), we measured the distance from the center of each observation site to the nearest urban structure (building, sidewalk, paved road, or any other human-made structure). The distance to anthropogenic structures affects the relative exposure of birds to different levels of human noise and light pollution (Ciach and Fröhlich 2017) and can also serve as a proxy for the degree of disturbance at each site. Specifically, the shortest distances to the nearest urban structure correspond to urbanized sites with intense human activity and the longest distances correspond to sites located within the core conservation areas of the reserve (Zúñiga-Vega et al. 2019). We also recorded whether gardening activities such as watering, tree pruning, weed removal, and lawn mowing took place at each site. The presence of gardening activities is relevant for birds because they imply a sustained supply of water throughout the year to maintain the vegetation, but they are also related to artificial changes in vegetation cover that may alter the abundance of some resources for birds, such as prey availability (insects) and perching sites (Lepczyk et al. 2004; van Heezik and Adams 2016). Finally, to estimate vegetation species richness we counted the number of tree and shrub species present at each observation site. We considered plant species richness as an additional measure of the structural complexity of the vegetation. Greater habitat complexity provides a broader diversity of resources (many types of food, refuges, and nesting sites), hence meeting the diverse needs of several bird species (Ghadiri Khanaposhtani et al. 2012). Our previous ecological research in the REPSA also considered these same environmental variables (Ramírez-Cruz et al. 2019; Zúñiga-Vega et al. 2019; Ramírez-Cruz and Ortega-Álvarez 2021).
Four of these environmental variables (tree, shrub, and herb covers as well as the amount of human waste) can vary through time (i.e., among years and seasons) and, therefore, we measured them separately during each field season. The other four variables (urban cover, distance to the nearest urban structure, vegetation species richness, and presence or absence of gardening activities) did not vary through time and, therefore, we measured them only once. These environmental variables were not strongly correlated with each other (all Spearman correlation coefficients were smaller than 0.8 in absolute value; Figs. S1-S11). Therefore, we used all these variables separately in our occupancy analyses.
Dynamic occupancy models
We implemented single-species multi-season (dynamic) occupancy models using the R package unmarked (MacKenzie et al. 2003; Fiske and Chandler 2011). Based on the detection histories that we obtained for all nine focal species, these single-species dynamic occupancy models allowed us to estimate four parameters by means of maximum-likelihood routines. First, occupancy probability (denoted by ψ), which is the probability that any given site is occupied by at least one individual of a focal species. Second, detection probability (denoted by p), defined as the probability of detecting at least one individual of a focal species in sites where the species is present. Third, local extinction (denoted by ε), which is the probability that an occupied site in season i is unoccupied in season i + 1. Fourth, colonization probability (denoted by γ), defined as the probability that a site that was unoccupied in season i is occupied in season i + 1. Given that occupancy probabilities of different seasons are the result of both dynamic processes (local extinction and colonization), we estimated ε and γ for all seasons, as well as the initial occupancy probability (i.e., ψ for our first field season, which took place during May 2015) by means of maximum likelihood using unmarked and then calculated occupancy probabilities for the next 10 field seasons as derived parameters as follows:
where the subscript i represents each field season, from the second to the last (MacKenzie et al. 2003).
To identify the environmental features of the reserve that promote site occupancy and colonization as well as those that cause high rates of site abandonment, we built generalized linear models using a logit link function with which we searched for statistical effects of the following environmental variables on our parameters of interest (initial ψ, γ, ε, and p). Initial occupancy, colonization, and extinction were modeled as functions of season-specific tree cover, shrub cover, herb cover, and amount of human waste (time-varying environmental variables). In addition, these three parameters were also modeled as functions of the four variables that did not vary among years or seasons (urban cover, distance to the nearest urban structure, number of tree and shrub species, and presence or absence of gardening activities). We fitted models with each of these predictors one at a time as well as additive models, testing all possible combinations of pairs of predictors. We did not consider interactions between predictors or additive models with more than two predictors to avoid overparameterizing our models and to keep our model set reasonably small.
Detection probability was modeled as a function of tree cover, shrub cover, and the additive effect of these two predictors. We chose these two environmental variables as predictors of detection because dense vegetation could have limited our visibility during field surveys. In addition, we also compared detection probabilities between the three climatic seasons (warm-dry, rainy, and cold-dry) by combining the four field seasons that took place during May of each year (May of 2015, 2016, 2017, and 2018) into the single category of warm-dry season, the four field seasons that took place during September of each year (September of 2015, 2016, 2017, and 2018) into the category of rainy season, and the three field seasons that took place during January of each year (January of 2016, 2017, and 2018) into the category of cold-dry season. In this way, we built models in which detection probability was affected by the factor “climatic season”, which had three levels (warm-dry, rainy, and cold-dry). This factor takes into account that activity patterns of birds may vary seasonally, making them more visible to observers during those months when they carry out conspicuous activities such as territorial defense, courtship, and nest building (e.g., Zamora-Marín et al. 2021; Hackworth et al. 2022) or, alternatively, less detectable when their overall activity levels remain low, such as during cold or rainy periods (e.g., Vézina and Salvante 2010). Given that the ability to detect birds may be different among observers, we also considered a model in which p varies among observers. We also tested this observer effect on p in the following additive combinations with the other predictors: tree cover + observer, shrub cover + observer, tree cover + shrub cover + observer, and climatic season + observer. In addition, we also considered intercept-only models, in which our parameters of interest (initial ψ, γ, ε, and p) were not affected by any predictor variable.
We selected the best model for each parameter using the Akaike information criterion adjusted for small sample sizes (AICc, Burnham and Anderson 2002). We also calculated Akaike weights (wj) which are measures of the relative support for each model j (Burnham et al. 2011). In those cases where a few models (two or three) had strong support (i.e., models differing by less than two AICc units from the best-fitting model, ΔAICc < 2), we selected the model with the fewest parameters. However, in cases of high model uncertainty (i.e., four or more models having strong support), we built an average model by computing weighted averages of the regression coefficients across all competing models using the Akaike weights as the weighting factor (as per Burnham and Anderson 2002). Then, we identified the environmental variables with evident effects on our parameters of interest as those with model-averaged regression coefficients that were statistically different than zero (regression coefficients with 95% confidence intervals that did not include zero).
Testing all possible combinations of the different sources of variation for initial ψ, γ, ε, and p would have resulted in an excessively large model set (more than 250,000 competing models). To avoid this issue, we conducted our model selection procedure in a sequential manner, as suggested by Lebreton et al. (1992). First, we defined an initial model in which both γ and ε varied among field seasons (i.e., a different value of γ and ε for each season), whereas only an intercept was included for both initial ψ and p. Second, we fitted all the competing models that we defined for p and, based on the criteria that we explained above for model selection, we identified the best source of variation for this parameter or, in other words, the environmental characteristic(s) with strong effect(s) on p. Third, we used the model that we selected for p and proceeded to fit all the competing models that we defined for initial ψ, still maintaining both γ and ε varying among field seasons. Here again, we identified the best source of variation for initial ψ. In the fourth and fifth steps, we repeated this procedure for ε and γ, respectively, to find the environmental variables with strong effects on these parameters.
We evaluated goodness of fit of the models that included the environmental variables that we selected for all four parameters (ψ, γ, ε, and p) of each species. For this purpose, we used a parametric bootstrap procedure also implemented in the R package unmarked (Fiske and Chandler 2011). This procedure consisted in: (1) simulating 1000 datasets based on the selected model for each species, (2) refitting this model to each simulated dataset, and (3) calculating from each of these simulations the sum of squared errors (SSE) as a measure of model fit. We then compared the distribution of these 1000 values of SSE with the SSE obtained from fitting the selected model to the actual data of each species. An actual SSE greater than 95% of the 1000 values of SSE obtained from the simulated datasets would indicate a significant lack of fit (i.e., P < 0.05). However, in all nine species the model including the environmental variables with strong effects on ψ, γ, ε, and p provided adequate fit to the data (P > 0.05 in all cases; Figs. S12-S20).
To search for temporal trends in occupancy probabilities of our nine focal species, we calculated ψ for all field seasons as derived parameters from additional competing models that we built to estimate temporal variation in both colonization and local extinction (excepting ψ for the initial season, which we estimated through maximum likelihood). In these additional models, we included as covariates the environmental variables that we previously identified as strong predictors of initial ψ, γ, ε, and p for each species. Specifically, we built nine additional models that resulted from all the combinations of γ and ε differing among climatic seasons (i.e., using the factor “climatic season” as predictor), varying among all field seasons (i.e., a different value of γ or ε for each field season), or remaining constant across all seasons. Based on these nine competing models and their respective Akaike weights, we computed model-averaged estimates of occupancy probabilities for all field seasons (from the second to the last).
Results
The full model selection results (including ranking of models based on AICc and Akaike weights) for all the competing models that we implemented to search for effects of environmental variables on initial ψ, γ, ε, and p of our nine focal species can be found in Table S1. In addition, the average models that we built per parameter and species are reported in Table S2.
Detection probabilities
Only two environmental variables had evident effects on the detection probabilities of four bird species (Table 2). Shrub cover had fairly similar negative effects on p of three species, H. mexicanus, M. fusca, and Pyrocephalus rubinus (Fig. S21a-c). Thus, as we expected, dense shrub cover restricted our visibility in the field, limiting our ability to detect these three species. In the particular case of S. psaltria, p varied among climatic seasons, with higher detectability during the rainy season compared to the warm-dry and cold-dry seasons (Fig. S21d). In the remaining five species, we did not detect statistical relationships between environmental variables and detection probabilities (Table 2). Only in three species (Haemorhous mexicanus, Psaltriparus minimus, and Thryomanes bewickii) did detectability vary among observers (Table 2). The model-averaged regression coefficients that represent the magnitudes of these inter-observer differences can be seen in Table S2.
Initial occupancy
In four species we detected effects of three environmental variables on initial ψ (Table 3). Shrub cover had a negative effect on occupancy of two species, Haemorhous mexicanus and Melozone fusca (Fig. 2a, c) and, in contrast, a positive influence on occupancy of Ptiliogonys cinereus (Fig. 2e). Distance to the nearest urban structure negatively affected initial occupancy of H. mexicanus and P. cinereus (Fig. 2b, f). This finding indicates that occupancy of these two species is highest at urban sites. In the latter species, expected occupancy probability was essentially zero in sites located more than 100 m from the nearest urban structure (Fig. 2f). The presence of gardening activities promoted higher occupancy probabilities of M. fusca and Pyrocephalus rubinus (Fig. 2d, g). In the other five species, the confidence intervals of the model-averaged regression coefficients for all predictor variables included zero and the intercept-only model was within the models with strong support (ΔAICc < 2), indicating that considering potential effects of environmental variables did not improve substantially the model fit compared to the simplest model with no predictors (Table 3).
Colonization
Four environmental features influenced colonization probabilities of six bird species (Table 4). Again, shrub cover had contrasting effects: negative on γ of H. mexicanus (Fig. 3a) and positive on γ of P. cinereus (Fig. 3h). Colonization probabilities of five bird species (M. fusca, Pheucticus melanocephalus, Ptiliogonys cinereus, Pyrocephalus rubinus, and Toxostoma curvirostre) were negatively affected by distance to the nearest urban structure (Fig. 3b, d, f, i, k). Therefore, in these five species the highest colonization rates occurred at urban sites. With the exception of M. fusca, γ values for these species were close to zero in sites located more than 150 m from the nearest urban structure (Fig. 3d, f, i, k). The presence of gardening activities had a positive influence on the colonization probability of a single species, M. fusca (Fig. 3c). The number of tree and shrub species (vegetation species richness) had positive effects on colonization rates of three species (Pheucticus melanocephalus, Ptiliogonys cinereus, and Pyrocephalus rubinus) (Fig. 3e, g, j). We did not detect statistical relationships between environmental variables and γ of the remaining three species (Psaltriparus minimus, Spinus psaltria, and Thryomanes bewickii) (Table 4).
Local extinction
We detected effects of six environmental variables on local extinction probabilities of six bird species (Table 5). Distance to the nearest urban structure had a positive influence on ε of three species, H. mexicanus, M. fusca, and Pyrocephalus rubinus (Fig. 4a, c, g). In both M. fusca and P. rubinus, extinction probability was essentially one in sites located more than 150 m from the nearest urban structure (Fig. 4c, g). Herb cover negatively affected ε of M. fusca (Fig. 4b). In turn, tree cover had a negative influence on ε of Psaltriparus minimus and Ptiliogonys cinereus (Fig. 4d, e). In the latter species, extinction probability was higher at sites where gardening activities take place compared to sites where such activities are absent (Fig. 4f). In Pyrocephalus rubinus, ε increased as urban cover increased (Fig. 4h). In S. psaltria, ε decreased sharply as shrub cover increased (Fig. 4i). In the remaining three species (Pheucticus melanocephalus, Thryomanes bewickii, and Toxostoma curvirostre) either the intercept-only model was within the models with strong support (ΔAICc < 2) or the confidence intervals of the model-averaged regression coefficients for all predictor variables included zero (Table 5).
Seasonal changes in site occupancy
Occupancy of five species (H. mexicanus, M. fusca, Pheucticus melanocephalus, Pyrocephalus rubinus, and Toxostoma curvirostre) varied among climatic seasons (Fig. 5a-c, f, i). In H. mexicanus, Pheucticus melanocephalus, Pyrocephalus rubinus, and T. curvirostre the highest model-averaged values of ψ occurred during the warm-dry seasons (Fig. 5a, c, f, i), although in P. rubinus (Fig. 5f) differences among seasons were not as prominent as in H. mexicanus (Fig. 5a), Pheucticus melanocephalus (Fig. 5c), and T. curvirostre (Fig. 5i). During one of the rainy seasons (September 2016), ψ of H. mexicanus was substantially high and similar to the ψ values that we observed during the warm-dry seasons (Fig. 5a). In M. fusca, the highest rates of site occupancy occurred during both the warm-dry and rainy seasons (Fig. 5b). Notably, in all these five species the lowest model-averaged values of ψ were observed during the cold-dry seasons (Fig. 5a-c, f, i).
In three species (Psaltriparus minimus, Ptiliogonys cinereus, and Thryomanes bewickii) we observed inter- and intra-annual variations in site occupancy, but without consistent differences between climatic seasons (Fig. 5d, e, h). In a single species, S. psaltria, ψ remained relatively constant throughout our study period (Fig. 5g). Complete model selection results (including AICc, ΔAICc, and wj) for the nine competing models that we implemented to derive season-specific estimates of ψ (from temporal variation in γ and ε) for all nine focal species can be found in Table S3.
Discussion
In this study, we have provided evidence in support of our two hypotheses. By identifying features of the environment that cause relatively high rates of site abandonment as well as those that facilitate their presence, we have confirmed that most of the species of native birds that we studied (the only exception was S. psaltria, see below) prefer to occupy the urbanized areas that surround this unique urban ecological reserve rather than the core conservation areas. Furthermore, the temporal changes that we detected in most species in the proportion of area occupied (Fig. 5) resulted, at least to some extent, from individuals leaving predominantly conservation sites during some seasons and then returning preferentially to (choosing) urbanized sites in other seasons. These findings represent solid evidence that these species have become urban exploiters and, likely, their fitness is now higher in urban settings than in less disturbed habitats. In addition, we detected seasonal changes in habitat occupancy of five species that were evident during all four years of our study. Each year, these species occupied the largest area during the warm-dry season and the smallest during the cold-dry season. This finding indicates that these five species are using the city predominantly to place their nests and feed their chicks because all of them breed during these warm and dry months (Table 1). Also, these birds may benefit from the abundant food and water that human activities provide around this urban reserve during this driest period of the year, when these vital resources are scarce in the forests that surround the city. Below, we discuss the specific lines of evidence that provide support for both hypotheses.
In six of our nine focal species (Haemorhous mexicanus, Melozone fusca, Pheucticus melanocephalus, Ptiliogonys cinereus, Pyrocephalus rubinus, and Toxostoma curvirostre), the highest occupancy and colonization rates occurred at or near urban sites. This indicates that certain features of the urban landscape provide benefits to these species. The observed statistical effects of other environmental variables on occupancy and colonization probabilities provide clues as to what these benefits could be. In particular, the presence of gardening activities facilitates occupancy and colonization of M. fusca as well as occupancy of P. rubinus. The former species forages on the ground in open areas, searching for seeds and grains (Johnson and Haight 2020), whereas the latter is an insectivorous sit-and-wait predator that hunts in open areas, locating its prey by perching on tall branches of isolated trees (Ellison et al. 2021). Gardening activities, such as tree pruning, weed removal, and lawn mowing, occur frequently in the urban areas that surround the REPSA, increasing the proportion of green open areas (i.e., areas covered with short grass or lawn with few scattered trees), which are suitable feeding habitat for these two species.
Moreover, greater richness of trees and shrubs increases colonization probabilities of Pheucticus melanocephalus, Ptiliogonys cinereus, and Pyrocephalus rubinus likely because such plant heterogeneity offers a broad array of refuges, sources of food, and roosting and nesting sites for these and other bird species. For both Pheucticus melanocephalus and Pyrocephalus rubinus, which leave several sites before the cold-dry season (Fig. 5c, f), this positive effect of richness of shrubs and trees on colonization means that when they return to the area right before the warm-dry season (which is the season when they occupy the largest number of sites), both species choose sites with high vegetation species richness. The area occupied by Ptiliogonys cinereus also varied through time (although not consistently between climatic seasons; Fig. 5e), indicating that this species leaves some sites and then returns preferentially to unoccupied sites with high vegetation species richness. The urban areas that surround the reserve have a high richness of plants, including several species of exotic trees of very different heights and ornamental shrubs that produce fruits and flowers all year long. In contrast, the REPSA is a relatively homogeneous ecosystem, mainly composed of a xerophytic scrubland where a few species of shrubs and small trees are dominant (e.g., Buddleja cordata, Pittocaulon praecox, and Eysenhardtia polystachya; Castillo-Argüero et al. 2009). In fact, shrub cover was negatively related to occupancy and colonization of H. mexicanus as well as to occupancy of M. fusca, indicating that these two species prefer the urban areas over the core conservation areas of the reserve, where shrubs are abundant and large trees are scarce.
An interesting case was Ptiliogonys cinereus, which seems to also prefer the urban areas that surround the reserve because its occupancy decreases sharply in sites located more than 50 m from the nearest urban structure (Fig. 2f). However, this is the only species in which shrub cover had a positive influence on both occupancy and colonization probabilities (Figs. 2e and 3h). These findings indicate that, within urban areas, P. cinereus occupies sites where shrubs are abundant. This pattern of habitat use can be explained by the diet of this bird, which is partially composed of small fruits (Chu 2020) and, as we mentioned before, several species of ornamental shrubs that produce plenty of fruits all year long are abundant in the urban areas that surround the REPSA (e.g., Arctostaphylos pungens and Phytolacca icosandra; Rzedowski and Calderón de Rzedowski 2000; Moreno-Rico et al. 2014). We note here that our occupancy analyses revealed fine-scale patterns of habitat use because even though six of our nine study species have higher occupancy and colonization rates in urban sites, some of them seem to differ in the specific micro-habitats that they occupy within the urban areas. For instance, M. fusca and Pyrocephalus rubinus prefer green open areas whereas Ptiliogonys cinereus prefers sites with abundant shrubs. Such differences among species in the environmental variables that facilitate their presence are indirect evidence of niche partitioning in this urban ecosystem.
Our estimates of local extinction probabilities provide additional evidence of a preference for urban sites, in more than half of our study species. Again, in H. mexicanus, M. fusca, and Pyrocephalus rubinus urban sites are abandoned less frequently as indicated by positive relationships between local extinction rates and distance to the nearest urban structure. In both Psaltriparus minimus and Ptiliogonys cinereus, extinction probabilities decreased as tree cover increases, which also indicates fidelity to urban sites because tree cover is higher in these sites compared to the conservation sites that are dominated by shrubs. Similarly, in the particular case of M. fusca, the rates of site abandonment were highest in sites with low or no herb cover, many of which correspond to the core conservation areas of the reserve (dense shrub cover impedes the growth of herbs). In contrast, local extinction of this bird species was less frequent in sites with abundant herb cover, many of which are open green areas interspersed between buildings, walking paths, and parking lots. However, our findings also reveal that permanence in urban sites depends on the degree of habitat modification, because local extinction of Pyrocephalus rubinus was highest in sites that are predominantly covered by human-made structures, with little vegetation. Similarly, site abandonment of Ptiliogonys cinereus was more frequent in areas where gardening activities are present. Therefore, the observed relationships between environmental variables and local extinction rates also indicate that urban sites where heterogeneous vegetation is present provide the greatest benefits to more than half of our study species.
Taken together, the observed associations between habitat traits and occupancy, colonization, and local extinction probabilities of our focal species support the hypothesis that these birds have greater affinity for the urbanized areas that surround the reserve than for the core conservation areas. Therefore, we have found evidence that most of our focal species have become urban exploiters. All these species have been reported in urban areas (Table 1) and according to previous studies, some of them, in particular H. mexicanus, M. fusca, P. cinereus, and Pyrocephalus rubinus, can thrive in urban ecosystems (Badyaev et al. 2020; Chu 2020; Johnson and Haight 2020; Ellison et al. 2021). The high vegetation species richness that occurs in the urban areas that surround the REPSA provide a broad array of microhabitats for these and other bird species. In addition, human activities can provide essential resources to birds, mainly in the form of food (e.g., organic waste, bird feeders, fruits and seeds from ornamental plants) (Gray and van Heezik 2016; Johnson and Munshi-South 2017; Tryjanowski et al. 2020). One additional case of urban affinity is Thryomanes bewickii, which was the only species for which no statistical relationships were evident between environmental variables and occupancy, colonization, or extinction rates. This suggests that the spatial distribution of this insectivorous bird is relatively homogeneous across our study area, equally occupying both protected and urban sites. In fact, T. bewickii is tolerant to high levels of disturbance and is becoming a common species in urban ecosystems (Farwell and Marzluff 2013; González-Oreja 2017). The only notable exception to this pattern of urban affinity was Spinus psaltria. Local extinction of this species was highest in sites with lowest shrub cover (Fig. 4i), which largely correspond to urban sites. Hence, S. psaltria may preferentially occupy the core conservation areas of the reserve where shrubs are abundant. Apparently, this species can use shrubs as nesting sites (Watt and Willoughby 2020).
Consistent with our second hypothesis, in five species (H. mexicanus, M. fusca, Pheucticus melanocephalus, Pyrocephalus rubinus, and Toxostoma curvirostre), we observed seasonal changes in occupancy rates, with lowest occupancy occurring during the cold-dry season and highest during the warm-dry season (and also during the rainy season in H. mexicanus and M. fusca). We propose three potential explanations for this seasonal pattern. First, breeding individuals establish territories during the spring (i.e., the warm-dry season in Mexico City), thus increasing the spatial distribution of these species. Previous studies have documented that all these five species form pairs and nest during the spring (Badyaev et al. 2020; Johnson and Haight 2020; Tweit 2020; Ellison et al. 2021; Hill 2022; Table 1). Hence, these five species may be using the urbanized areas predominantly as breeding grounds likely because predation risk for nests and chicks is low (Gering and Blair 1999) and also because urban structures and the distinct species of shrubs and trees provide a broad array of nesting sites (Reynolds et al. 2019). Furthermore, availability of some insects may be higher within the city compared to the forests outside the city (e.g., flies; Chatelain et al. 2023). Given that most bird species feed their offspring with invertebrates (Schoener 1965; Badyaev et al. 2020; Hill 2022), higher abundance of these prey could contribute to their preference for urban sites during their breeding season. In the particular case of M. fusca, females extend their nesting season until September (Johnson and Haight 2020; Table 1), which explains why site occupancy of this species was highest during both the warm-dry and rainy seasons (May and September of each year).
Second, food and water availability may be higher within the city during the warm-dry season (trees and gardens are frequently watered and anthropogenic sources of food for birds are available all year long), which is when environmental conditions are driest and food sources may be scarce in the surrounding forests. Thus, numerous individuals of these species may move into the city during this limiting season, increasing the rates of site occupancy. There are records of altitudinal and short-distance migration for all nine species (Table 1). Third, mortality could be substantially high during cold months, reducing the population numbers and, consequently, the total area occupied by these species. Certainly, bird mortality can be more intense during the winter, at least at higher latitudes (Doherty and Grubb 2002). These non-mutually exclusive explanations deserve empirical research.
The lowest occupancy rates that we observed during the cold-dry season of each year in these same five species (H. mexicanus, M. fusca, Pheucticus melanocephalus, Pyrocephalus rubinus, and T. curvirostre) are not consistent with the hypothesis that native birds may use the ecological reserve and surrounding urban areas as refuge from the colder temperatures of the surrounding forests (Bonnet-Lebrun et al. 2020).
The ecological information that we have presented here allows us to propose the following management recommendations aimed at preserving the native biodiversity of this and other megacities. First, native urban exploiters seem to benefit from heterogenous vegetation. Thus, the urbanized areas that surround large green spaces such as the REPSA should include a wide array of species of shrubs and trees. Species-rich vegetation would provide diverse microhabitats and food sources for several bird species with distinct ecological needs. Native species of shrubs and trees should preferably be used. Second, tall tree species with dense canopy (e.g., the native Taxodium mucronatum; Martínez González 2008) can provide suitable habitat for forest-associated bird species (such as Psaltriparus minimus and Ptiliogonys cinereus; Fig. 4d, e) and hence facilitate their colonization of the edges of this and other urban ecological reserves. Third, open green spaces where gardening activities take place, such as lawns, may attract ground foragers. However, some trees and shrubs must be planted within and around these grass fields to provide scattered refuges and perching sites for birds. Notice that some insectivorous species, such as Pyrocephalus rubinus, prefer both open green areas and high vegetation species richness (Figs. 2g and 3j). Fourth, people living in the city must be informed that during the breeding season several species of native birds colonize the areas that surround the reserve (and probably other urban green spaces). In this way, city residents can contribute to preserve these species by avoiding factors that put them at risk. For example, minimal tree pruning during this season could prevent accidental destruction of the nests that are placed on tree branches. Similarly, control of their domestic cats, which are known to frequently prey on bird nests (Mcruer et al. 2017), could increase the survival rates of eggs and nestlings.
Finally, we highlight areas of opportunity for future research. Our study focuses only on nine species inhabiting a single city. Similar temporal and spatial examinations of occupancy in other species and other cities would strengthen the evidence in support of the ecological processes that we have inferred here. We found that, with the exception of S. psaltria, our study species prefer the urbanized areas that surround the reserve over the core conservation areas. However, the urban ecological reserve might be critical for the persistence of other birds (e.g., migratory species such as Bombycilla cedrorum and Falco sparverius) and/or other animal groups, such as native mammals (e.g., Bassariscus astutus) and reptiles (e.g., Sceloporus torquatus) (Lot and Cano-Santana 2009). Ecological studies of these other taxa in this urban ecosystem are certainly needed (but see Zúñiga-Vega et al. 2019). We only measured some of the habitat features that could facilitate or prevent the presence of these nine bird species in our study area. This means that the effects of other environmental variables, such as food availability (e.g., abundance of seeds, fruits, and invertebrates), presence of potential predators, and degree of noise and light pollution (Dominoni 2015; Arévalo et al. 2022), remain to be examined.
Data availability
Upon request from the corresponding author.
Code availability
Upon request from the corresponding author.
Change history
01 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11252-024-01570-w
References
Altwegg R, Nichols JD (2019) Occupancy models for citizen-science data. Methods Ecol Evol 10:8–21. https://doi.org/10.1111/2041-210X.13090
Alvey AA (2006) Promoting and preserving biodiversity in the urban forest. Urban For Urban Green 5:195–201. https://doi.org/10.1016/J.UFUG.2006.09.003
Arévalo C, Amaya-Espinel JD, Henríquez C et al (2022) Urban noise and surrounding city morphology influence green space occupancy by native birds in a Mediterranean-type south American metropolis. Sci Rep 12:4471. https://doi.org/10.1038/s41598-022-08654-7
Aronson MFJ, La Sorte FA, Nilon CH et al (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B Biol Sci 281:20133330. https://doi.org/10.1098/rspb.2013.3330
Badyaev AV, Belloni V, Hill GE (2020) House finch (Haemorhous mexicanus), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.HOUFIN.01
Barbosa de Toledo MC, Donatelli RJ, Teixeira Batista G (2012) Relation between green spaces and bird community structure in an urban area in Southeast Brazil. Urban Ecosyst 15:111–131. https://doi.org/10.1007/s11252-011-0195-2
Barçante L, M Vale M, S Alves MA (2017) Altitudinal migration by birds: a review of the literature and a comprehensive list of species. J Field Ornithol 88:321–335. https://doi.org/10.1111/JOFO.12234
Betts MG, Rodenhouse NL, Sillett TS et al (2008) Dynamic occupancy models reveal within-breeding season movement up a habitat quality gradient by a migratory songbird. Ecography 31:592–600. https://doi.org/10.1111/j.0906-7590.2008.05490.x
Bled F, Nichols JD, Altwegg R (2013) Dynamic occupancy models for analyzing species’ range dynamics across large geographic scales. Ecol Evol 3:4896–4909. https://doi.org/10.1002/ECE3.858
Bonnet-Lebrun A-S, Manica A, Rodrigues ASL (2020) Effects of urbanization on bird migration. Biol Conserv 244:108423. https://doi.org/10.1016/j.biocon.2020.108423
Boyle WA (2010) Does food abundance explain altitudinal migration in a tropical frugivorous bird? Can J Zool 88:204–213. https://doi.org/10.1139/Z09-133
Boyle WA, Norris DR, Guglielmo CG (2010) Storms drive altitudinal migration in a tropical bird. Proc R Soc B Biol Sci 277:2511–2519. https://doi.org/10.1098/RSPB.2010.0344
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer International Publishing, New York, USA
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Camara IA, Bony YK, Diomandé D et al (2012) Freshwater snail distribution related to environmental factors in Banco National Park, an urban reserve in the Ivory Coast (West Africa). Afr Zool 47:160–168. https://doi.org/10.3377/004.047.0106
Carrera-Hernández JJ, Gaskin SJ (2007) Spatio temporal analysis of daily precipitation and temperature in the Basin of Mexico. J Hydrol 336:231–249. https://doi.org/10.1016/j.jhydrol.2006.12.021
Cassel KW, Vanek JP, Glowacki GA et al (2019) Multiscale habitat factors influence the occupancy and turnover of the suburban herpetofauna of Chicago, Illinois, USA. Herpetol Conserv Biol 14:438–454
Castillo-Argüero S, Martínez-Orea Y, Meave JA et al (2009) Flora: susceptibilidad de la comunidad a la invasión de malezas nativas y exóticas. In: Lot A, Cano-Santana Z (eds) Biodiversidad del ecosistema del Pedregal de San Ángel. Universidad Nacional Autónoma de México, Mexico City, Mexico, pp 107–117
Cayuela H, Rougemont Q, Prunier JG et al (2018) Demographic and genetic approaches to study dispersal in wild animal populations: a methodological review. Mol Ecol 27:3976–4010. https://doi.org/10.1111/mec.14848
Chamberlain DE, Gough S, Vaughan H et al (2007) Determinants of bird species richness in public green spaces. Bird Study 54:87–97. https://doi.org/10.1080/00063650709461460
Chatelain M, Rüdisser J, Traugott M (2023) Urban-driven decrease in arthropod richness and diversity associated with group-specific changes in arthropod abundance. Front Ecol Evol 11:980387. https://doi.org/10.3389/fevo.2023.980387
Chu M (2020) Gray silky-flycatcher (Ptiliogonys cinereus), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.GRSFLY1.01
Ciach M, Fröhlich A (2017) Habitat type, food resources, noise and light pollution explain the species composition, abundance and stability of a winter bird assemblage in an urban environment. Urban Ecosyst 20:547–559. https://doi.org/10.1007/s11252-016-0613-6
Clement MJ (2016) Designing occupancy studies when false-positive detections occur. Methods Ecol Evol 7:1538–1547. https://doi.org/10.1111/2041-210X.12617
Dalla Pria C, Cawkwell F, Newton S, Holloway P (2022) City living: nest-site selection preferences in urban herring gulls, Larus argentatus. Geographies 2:161–172. https://doi.org/10.3390/geographies2020011
Doherty PF Jr, Grubb TC Jr (2002) Survivorship of permanent-resident birds in a fragmented forested landscape. Ecology 83:844–857. https://doi.org/10.2307/3071886
Dominoni DM (2015) The effects of light pollution on biological rhythms of birds: an integrated, mechanistic perspective. J Ornithol 156:409–418. https://doi.org/10.1007/s10336-015-1196-3
Ellison K, Wolf BO, Jones SL (2021) Vermilion flycatcher (Pyrocephalus rubinus), version 1.1. Birds of the World. https://doi.org/10.2173/BOW.VERFLY.01.1
Enedino TR, Loures-Ribeiro A, Santos BA (2018) Protecting biodiversity in urbanizing regions: the role of urban reserves for the conservation of Brazilian Atlantic Forest birds. Perspect Ecol Conserv 16:17–23. https://doi.org/10.1016/j.pecon.2017.11.001
Farwell LS, Marzluff JM (2013) A new bully on the block: does urbanization promote Bewick’s wren (Thryomanes bewickii) aggressive exclusion of Pacific wrens (Troglodytes pacificus)? Biol Conserv 161:128–141. https://doi.org/10.1016/J.BIOCON.2013.03.017
Fischer LK, Honold J, Cvejić R et al (2018) Beyond green: broad support for biodiversity in multicultural European cities. Glob Environ Chang 49:35–45. https://doi.org/10.1016/J.GLOENVCHA.2018.02.001
Fiske IJ, Chandler RB (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/jss.v043.i10
Fokidis HB, Deviche P (2012) Brain arginine vasotocin immunoreactivity differs between urban and desert curve-billed thrashers, Toxostoma curvirostre: relationships with territoriality and stress physiology. Brain Behav Evol 79:84–97. https://doi.org/10.1159/000332766
Folberth GA, Butler TM, Collins WJ, Rumbold ST (2015) Megacities and climate change - A brief overview. Environ Pollut 203:235–242. https://doi.org/10.1016/j.envpol.2014.09.004
Fuyuki A, Yamaura Y, Nakajima Y et al (2014) Pond area and distance from continuous forests affect amphibian egg distributions in urban green spaces: a case study in Sapporo, Japan. Urban For Urban Green 13:397–402. https://doi.org/10.1016/j.ufug.2013.11.003
García-Arroyo M, Gómez-Martínez MA, MacGregor-Fors I (2023) Litter buffet: on the use of trash bins by birds in six boreal urban settlements. Avian Res 14:100094. https://doi.org/10.1016/j.avrs.2023.100094
George SL, Crooks KR (2006) Recreation and large mammal activity in an urban nature reserve. Biol Conserv 133:107–117. https://doi.org/10.1016/J.BIOCON.2006.05.024
Gering JC, Blair RB (1999) Predation on artificial bird nests along an urban gradient: predatory risk or relaxation in urban environments? Ecography 22:532–541. https://doi.org/10.1111/j.1600-0587.1999.tb01283.x
Ghadiri Khanaposhtani M, Kaboli M, Karami M, Etemad V (2012) Effect of habitat complexity on richness, abundance and distributional pattern of forest birds. Environ Manage 50:296–303. https://doi.org/10.1007/s00267-012-9877-7
González-García F, Straub R, Lobato García JA, MacGregor-Fors I (2014) Birds of a neotropical green city: an up-to-date review of the avifauna of the city of Xalapa with additional unpublished records. Urban Ecosyst 17:991–1012. https://doi.org/10.1007/s11252-014-0370-3
González-Oreja JA (2017) Relationships of area and noise with the distribution and abundance of songbirds in urban greenspaces. Landsc Urban Plan 158:177–184. https://doi.org/10.1016/J.LANDURBPLAN.2016.05.032
Google (2017) Google Earth Mountain View, CA. https://www.google.com/earth/. Accessed 12 Sep 2017
Gray ER, van Heezik Y (2016) Exotic trees can sustain native birds in urban woodlands. Urban Ecosyst 19:315–329. https://doi.org/10.1007/s11252-015-0493-1
Hackworth ZJ, Felch JM, Murphy SM, Cox JJ (2022) Detectability of common ravens (Corvus corax) in the eastern USA: rapid assessment of a recolonizing species. Ecosphere 13:e4148. https://doi.org/10.1002/ECS2.4148
Hill GE (2022) Black-headed grosbeak (Pheucticus melanocephalus), version 2.0. Birds of the World. https://doi.org/10.2173/BOW.BKHGRO.02
Iijima H, Nagaike T, Honda T (2013) Estimation of deer population dynamics using a bayesian state-space model with multiple abundance indices. J Wildl Manage 77:1038–1047. https://doi.org/10.1002/jwmg.556
INEGI (2022) Censo población y vivienda 2020. In: Instituto Nacional de Estadıstica y geografıa (INEGI). https://www.inegi.org.mx/programas/ccpv/2020/
Jensen JK, Ekroos J, Watson H et al (2023) Urban tree composition is associated with breeding success of a passerine bird, but effects vary within and between years. Oecologia 201:585–597. https://doi.org/10.1007/s00442-023-05319-8
Johnson RR, Haight LT (2020) Canyon towhee (Melozone fusca), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.CANTOW.01
Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327. https://doi.org/10.1126/science.aam8327
Jokimäki J, Suhonen J (1998) Distribution and habitat selection of wintering birds in urban environments. Landsc Urban Plan 39:253–263. https://doi.org/10.1016/S0169-2046(97)00089-3
Jokimäki J, Suhonen J, Vuorisalo T et al (2017) Urbanization and nest-site selection of the black-billed magpie (Pica pica) populations in two Finnish cities: from a persecuted species to an urban exploiter. Landsc Urban Plan 157:577–585. https://doi.org/10.1016/J.LANDURBPLAN.2016.08.001
Kark S, Iwaniuk A, Schalimtzek A, Banker E (2007) Living in the city: can anyone become an ‘urban exploiter’? J Biogeogr 34:638–651. https://doi.org/10.1111/j.1365-2699.2006.01638.x
Kendal D, Zeeman BJ, Ikin K et al (2017) The importance of small urban reserves for plant conservation. Biol Conserv 213:146–153. https://doi.org/10.1016/j.biocon.2017.07.007
Kennedy ED, White DW (2020) Bewick’s wren (Thryomanes bewickii), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.BEWWRE.01
Kéry M (2004) Extinction rate estimates for plant populations in revisitation studies: importance of detectability. Conserv Biol 18:570–574. https://doi.org/10.1111/j.1523-1739.2004.00105.x
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118. https://doi.org/10.2307/2937171
Lepczyk CA, Mertig AG, Liu J (2004) Assessing landowner activities related to birds across rural-to-urban landscapes. Environ Manage 33:110–125. https://doi.org/10.1007/s00267-003-0036-z
Lot A, Cano-Santana Z (2009) Biodiversidad del Ecosistema del Pedregal de San Ángel. Universidad Nacional Autónoma de México, México, D.F.
Louvrier J, Duchamp C, Lauret V et al (2018) Mapping and explaining wolf recolonization in France using dynamic occupancy models and opportunistic data. Ecography 41:647–660. https://doi.org/10.1111/ECOG.02874
Mackenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
MacKenzie DI, Nichols JD, Hines JE et al (2003) Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84:2200–2207. https://doi.org/10.1890/02-3090
MacKenzie DI, Nichols JD, Royle JA et al (2018) Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence, 2nd edn. Academic Press, San Diego, USA
Marchionni V, Fatichi S, Tapper N et al (2021) Assessing vegetation response to irrigation strategies and soil properties in an urban reserve in southeast Australia. Landsc Urban Plan 215:104198. https://doi.org/10.1016/J.LANDURBPLAN.2021.104198
Martínez González L (2008) Árboles y áreas verdes urbanas de la ciudad de México y su zona metropolitana, 1st edn. Fundación Xochitla, A.C., México
Matsuoka SM, Bayne EM, Sólymos P et al (2012) Using binomial distance-sampling models to estimate the effective detection radius of point-count surveys across boreal Canada. Auk 129:268–282. https://doi.org/10.1525/auk.2012.11190
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176. https://doi.org/10.1007/s11252-007-0045-4
Mcruer DL, Gray LC, Horne L-A, Clark EE Jr (2017) Free-roaming cat interactions with wildlife admitted to a wildlife hospital. J Wildl Manage 81:163–173. https://doi.org/10.1002/jwmg.21181
Menon M, Mohanraj R (2016) Temporal and spatial assemblages of invasive birds occupying the urban landscape and its gradient in a southern city of India. J Asia-Pacific Biodivers 9:74–84. https://doi.org/10.1016/J.JAPB.2015.12.005
Møller AP (2010) The fitness benefit of association with humans: elevated success of birds breeding indoors. Behav Ecol 21:913–918. https://doi.org/10.1093/beheco/arq079
Moreno-Rico O, Groenewald JZ, Crous PW (2014) Foliicolous fungi from Arctostaphylos pungens in Mexico. IMA Fungus 5:7–15. https://doi.org/10.5598/imafungus.2014.05.01.02
Morrison JL, Gottlieb IGW, Pias KE (2016) Spatial distribution and the value of green spaces for urban red-tailed hawks. Urban Ecosyst 19:1373–1388. https://doi.org/10.1007/s11252-016-0554-0
Murgui E, Hedblom M (2017) Ecology and conservation of birds in urban environments. Springer International Publishing, Cham, Switzerland
Ortega-Álvarez R, Berrones Benítez E, Medina Mena I et al (2021) Local colonization and extinction in forestry habitats: assessing the effects of productive activities on the occupancy dynamics of bird populations. Biotropica 53:1142–1152. https://doi.org/10.1111/btp.12956
Pageau C, M Vale M, Argollo de Menezes M, et al (2020) Evolution of altitudinal migration in passerines is linked to diet. Ecol Evol 10:3338–3345. https://doi.org/10.1002/ECE3.6126
Palacio FX (2020) Urban exploiters have broader dietary niches than urban avoiders. Ibis 162:42–49. https://doi.org/10.1111/IBI.12732
Ramírez-Cruz GA, Ortega-Álvarez R (2021) Identifying management guidelines to control the invasive house sparrow (Passer domesticus) within natural protected areas through the estimation of local colonization and extinction probabilities. Biol Invasions 23:3767–3776. https://doi.org/10.1007/s10530-021-02616-2
Ramírez-Cruz GA, Mendoza-Hernández PE, Solano-Zavaleta I, Zúñiga-Vega JJ (2018) How widespread are nonnative species? Estimating habitat occupancy in an ecological reserve within a megacity. Nat Areas J 38:68–87. https://doi.org/10.3375/043.038.0108
Ramírez-Cruz GA, Solano-Zavaleta I, Mendoza-Hernández PE et al (2019) This town ain’t big enough for both of us… or is it? Spatial co-occurrence between exotic and native species in an urban reserve. PLoS ONE 14:e0211050. https://doi.org/10.1371/journal.pone.0211050
Ramírez-Cruz GA, Solano-Zavaleta I, Méndez-Janovitz M, Zúñiga-Vega JJ (2020) Demographic and spatial responses of resident bird populations to the arrival of migratory birds within an urban environment. Popul Ecol 62:105–118. https://doi.org/10.1002/1438-390X.12032
Reynolds SJ, Ibáñez-Álamo JD, Sumasgutner P, Mainwaring MC (2019) Urbanisation and nest building in birds: a review of threats and opportunities. J Ornithol 160:841–860. https://doi.org/10.1007/s10336-019-01657-8
Ríos-Chelén AA, Quirós-Guerrero E, Gil D, Macías Garcia C (2013) Dealing with urban noise: vermilion flycatchers sing longer songs in noisier territories. Behav Ecol Sociobiol 67:145–152. https://doi.org/10.1007/s00265-012-1434-0
Royle JA, Kéry M, Gautier R, Schmid H (2007) Hierarchical spatial models of abundance and occurrence from imperfect survey data. Ecol Monogr 77:465–481. https://doi.org/10.1890/06-0912.1
Rudershausen PJ, Merrell JH, Buckel JA (2021) Factors influencing colonization and survival of juvenile blue crabs Callinectes sapidus in Southeastern U.S. Tidal creeks. Diversity 13:491. https://doi.org/10.3390/d13100491
Rutz C (2008) The establishment of an urban bird population. J Anim Ecol 77:1008–1019. https://doi.org/10.1111/j.1365-2656.2008.01420.x
Rzedowski J (1954) Vegetation of Pedregal de San Angel. Esc Nac Cienc Biol 8:59–129
Rzedowski J, Calderón de Rzedowski G (2000) Notes on the genus Phytolacca (Phytolaccaceae) in Mexico. Acta Bot Mex 53:49–66
Schoener TW (1965) The evolution of bill size differences among sympatric congeneric species of birds. Evolution 19:189–213. https://doi.org/10.2307/2406374
SEREPSA (2013) Reserva Ecológica Pedregal de San Ángel. http://www.repsa.unam.mx. Accessed 30 Aug 2022
Shochat E, Warren PS, Faeth SH et al (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191. https://doi.org/10.1016/j.tree.2005.11.019
Siebe C (2000) Age and archaeological implications of Xitle volcano, southwestern Basin of Mexico-City. J Volcanol Geotherm Res 104:45–64. https://doi.org/10.1016/S0377-0273(00)00199-2
Siebe C (2009) La erupción del volcán Xitle y las lavas del Pedregal hace 1670 +/- 35 años AP y sus implicaciones. In: Lot A, Cano-Santana Z (eds) Biodiversidad del ecosistema del Pedregal de San Ángel. Universidad Nacional Autónoma de México, Mexico City, Mexico, pp 43–49
Sloane SA (2020) Bushtit (Psaltriparus minimus), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.BUSHTI.01
Southon GE, Jorgensen A, Dunnett N et al (2018) Perceived species-richness in urban green spaces: cues, accuracy and well-being impacts. Landsc Urban Plan 172:1–10. https://doi.org/10.1016/J.LANDURBPLAN.2017.12.002
Tryjanowski P, Morelli F, Møller AP (2020) Urban birds: urban avoiders, urban adapters, and urban exploiters. In: Douglas I, Anderson PML, Goode D et al (eds) The Routledge Handbook of Urban Ecology, 2nd edn. Routledge, pp 399–411. https://doi.org/10.4324/9780429506758-34
Tweit RC (2020) Curve-billed thrasher (Toxostoma curvirostre), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.CUBTHR.01
van Heezik Y, Adams AL (2016) Vulnerability of native and exotic urban birds to housing densification and changing gardening and landscaping trends. Urban Ecosyst 19:1551–1563. https://doi.org/10.1007/s11252-014-0379-7
van Heezik Y, Smyth A, Mathieu R (2008) Diversity of native and exotic birds across an urban gradient in a New Zealand city. Landsc Urban Plan 87:223–232. https://doi.org/10.1016/J.LANDURBPLAN.2008.06.004
Vézina F, Salvante KG (2010) Behavioral and physiological flexibility are used by birds to manage energy and support investment in the early stages of reproduction. Curr Zool 56:767–792. https://doi.org/10.1093/CZOOLO/56.6.767
Watt DJ, Willoughby EJ (2020) Lesser goldfinch (Spinus psaltria), version 1.0. Birds of the World. https://doi.org/10.2173/BOW.LESGOL.01
Wong JSY, Soh MCK, Low BW, Er KBH (2023) Tropical bird communities benefit from regular-shaped and naturalised urban green spaces with water bodies. Landsc Urban Plan 231:104644. https://doi.org/10.1016/j.landurbplan.2022.104644
Yamaura Y, Royle JA, Kuboi K et al (2011) Modelling community dynamics based on species-level abundance models from detection/nondetection data. J Appl Ecol 48:67–75. https://doi.org/10.1111/J.1365-2664.2010.01922.X
Yow DM (2007) Urban heat islands: observations, impacts, and adaptation. Geogr Compass 1:1227–1251. https://doi.org/10.1111/j.1749-8198.2007.00063.x
Zambrano L, Rodríguez Palacios S, Pérez Escobedo M et al (2016) La Reserva Ecológica del Pedregal de San Ángel: Atlas de Riesgos, 2nd edn. Coordinación de la Investigación Científica, Secretaría Ejecutiva de la Reserva Ecológica del Pedregal de San Ángel, UNAM, Ciudad de México, México
Zamora-Marín JM, Zamora-López A, Calvo JF, Oliva-Paterna FJ (2021) Comparing detectability patterns of bird species using multi-method occupancy modelling. Sci Rep 11:2558. https://doi.org/10.1038/s41598-021-81605-w
Zúñiga-Vega JJ, Solano-Zavaleta I, Sáenz-Escobar MF, Ramírez-Cruz GA (2019) Habitat traits that increase the probability of occupancy of migratory birds in an urban ecological reserve. Acta Oecol 101:103480. https://doi.org/10.1016/j.actao.2019.103480
Zúñiga-Vega JJ, Gutiérrez-García M, Suárez-Rodríguez M et al (2023) Raptors in the city: site occupancy and abundance of a top predator inhabiting urban green spaces within a megacity. Landsc Urban Plan 234:104725. https://doi.org/10.1016/j.landurbplan.2023.104725
Acknowledgements
P. Mendoza-Hernández, E. Serrano-García, V. Morales-Salcedo, M. Romero-Romero, and F. Sáenz-Escobar provided field and technical assistance. We thank L. Zambrano and the authorities of the Reserva Ecológica del Pedregal de San Angel for providing logistical support.
Funding
This research was funded by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) - Dirección General de Asuntos del Personal Académico - Universidad Nacional Autónoma de México through projects IN201521, IV200117,and IV210117.
Author information
Authors and Affiliations
Contributions
JJZV designed the study. JJZV and MSR performed analyses and wrote the first draft. GARC and ISZ conducted fieldwork and compiled all data. GARC curated the data and prepared figures. ISZ supervised all the steps of this study. All the authors edited the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable to this study.
Consent for publication
The authors give Urban Ecosystems consent for publication.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: There was a correction regarding the figures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zúñiga-Vega, J.J., Suárez-Rodríguez, M., Ramírez-Cruz, G.A. et al. Dynamic occupancy analyses of native birds in an urban ecological reserve reveal seasonal changes in site occupancy and preference for adjacent urbanized areas. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01538-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01538-w