Abstract
In an ever more urbanized world, animals have to cope with different challenging conditions that may shape the individual’s phenotype in the urban environment. Since body mass and body size are found to be related to fitness in many species, investigating the variation in these two morphological traits along the rural-urban gradient, is a first step to understand how animals adapt to urbanization. Here we studied two tree squirrels, the native Eurasian red squirrel (Sciurus vulgaris) and the invasive Eastern grey squirrel (Sciurus carolinensis), using a pseudo-experimental design with replicated study sites (2 rural, 2 suburban and 2 urban sites for each species). We investigated whether squirrels differed in body size and body mass along the urbanization gradient and whether the invasive alien squirrels had more marked differences along the gradient, showing a higher adaptation capacity. We did not find variation in body size in red squirrels along the gradient, but invasive grey squirrels were slightly larger in urban than in other area-types. In both species, animals of either sex were heavier in the urban than in the rural sites, while the difference between urban and suburban areas depends on species and sex. Hence, morphologically both native and invasive species showed similar changes, with higher body mass in urban habitat, which could result in higher fitness, since body mass in squirrels species is positively related to reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years cities have grown very rapidly and a further increase is expected in the coming decades (United Nations 2019, Li et al. 2021). It is known that this process has an impact on both local flora and fauna, in particular, the urban environment presents different selective forces that can shape the animals’ phenotypes, resulting in differences among conspecifics that live along the rural-urban gradient (Alberti et al. 2017; Santini et al. 2019). Adaptations, mainly by generalist species, to cope with the different conditions of the new environment have been reported for all classes of vertebrates (McDonnell and Hahs 2015). The observed changes are mostly related to the animals’ behavior (Lowry et al. 2013; Ritzel and Gallo 2020), physiology (Scheun et al. 2015; Lyons et al. 2017) and morphology (Stepkovitch et al. 2019; Putman and Tippie 2020). In particular, it has been shown that morphological traits vary between individuals of the same species that live in urban and rural areas, but the trait change direction varies among species (Iglesias-Carrasco et al. 2017; Santini et al. 2019; Ritzel and Gallo 2020). For example, house sparrows (Passer domesticus) are smaller and lighter in more urbanized habitats (Meillère et al. 2015), while there was an opposite tendency in red foxes (Vulpes vulpes), with urban individuals having larger body size and mass than foxes in rural habitats (Stepkovitch et al. 2019).
Direct relationships between body mass and/or body size with fitness components (survival or reproduction) are found in many mammal species (Wauters and Dhondt 1995; Festa-Bianchet et al. 1997; Wauters et al. 2007; Santicchia et al. 2018a; Rode et al. 2020). Therefore, investigating inter-individual variation in body size and mass along the habitat gradient from natural (rural) to urban is a first step to understand potential adaptations of a species to the human-modified environment. It is also the basis to study the impact of urbanization on that species and, where necessary, identify efficient conservation actions. Moreover, the urban environment has a role in enhancing the risk of biological invasions of invasive species: in fact, many alien species easily enter and establish in cities (Cadotte et al. 2017; Wauters and Martinoli 2018; Borden and Flory 2021). Hence, investigating also how invasive alien species adapt to urban areas will be important to target their management (Borden and Flory 2021).
Among mammals, different species of tree squirrels are known to show various levels of adaptation to and occurrence in urban environments (Partan et al. 2010; McCleery and Parker 2011; Uchida et al. 2016). Green spaces in cities, such as public parks or private gardens, seem to be relatively favorable for squirrel species, if appropriately managed (Rézouki et al. 2014; Jokimäki et al. 2017; Fingland et al. 2021). For example, Eurasian red squirrels (Sciurus vulgaris) occur in higher densities in some urban than in rural habitats in Finland (Jokimäki et al. 2017), and the same trend was described also for Eastern grey squirrels (Sciurus carolinensis) in their original distribution range (Koprowski 1994). Moreover, several tree squirrel species have been introduced to areas outside their native range, showing a tendency to become invasive (invasive alien species, hereinafter IAS, European Commission 2016; Mazzamuto et al. 2021), thus allowing comparisons of adaptations to the urban environment between native and alien species.
Here we studied two tree squirrels in Italy: the Eurasian red squirrel, the native species, and the Eastern grey squirrel, the introduced IAS. Red squirrels occur in continuous conifer forests in the Alps and the Apennines, in lowland mixed deciduous woods (Wauters et al. 2001a, 2008) and also in urban parks and gardens (Jokimäki et al. 2017; Thomas et al. 2018; Beliniak et al. 2021). Grey squirrels instead have been introduced in the United Kingdom, Ireland and Italy, where they exert negative impacts on biodiversity and replace the native congener (Teangana et al. 2000; Bertolino et al. 2014; Gurnell et al. 2015).
To examine how the native and the invasive species are adapting to the human-modified environment, we investigated if there are any differences in two morphological traits (hind foot length and body mass) among individuals along a gradient from rural, over suburban to urban areas. In tree squirrels, hind foot length is correlated with total head and body length and thus is considered a reliable proxy for animal’s body size (Wauters and Dhondt 1989a), while body mass is an indicator of body condition and correlates with several fitness parameters (Wauters and Dhondt 1989b, 1995; Wauters et al. 2007; Tranquillo et al. 2022). Furthermore, we calculated tree species diversity in each study site to account for differences in tree species composition among areas and to determine the dominant species, a factor that can affect squirrels’ body size or mass (Wauters et al. 2007; Tranquillo et al. 2022).
Since previous studies on morphological traits of red squirrels reported habitat-related changes in body size and/or body mass (Wauters et al. 2007; Tranquillo et al. 2022), and the occurrence of morphological adaptations associated with different habitats is also likely for grey squirrels (Koprowski 1994; Lurz and Lloyd 2000), we predicted: (i) differences in squirrels body size and body mass along the rural-urban gradient. The direction of this relationship is difficult to predict since each mammal species shows different responses to the urban environment (Ritzel and Gallo 2020). However, since invasive alien grey squirrels, similar to many IAS, may thrive in urban habitats, we also predict (ii) more marked differences along the rural-urban gradient in the invasive squirrel species than in the native one. If higher invasion success of the invasive species in urban environments (Cadotte et al. 2017; Santana Marques et al. 2020) would be associated with a better condition (higher body mass), then (iii) we expect an increase in body mass from rural to urban areas.
Materials and methods
Study sites
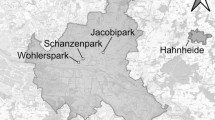
We used a pseudo-experimental study design. Overall, study sites were selected based on a combination of the surrounding landscape (Online Resource, Fig. S1 for proportion built surface per area-type), general habitat and elevation (mixed lowland woods and parks with mature seed-producing trees), and permission to access and monitor squirrels in both public and private areas. We considered urban those sites completely surrounded by an urban matrix, rural sites are separated from cities (> 1 km) and surrounded by agricultural land, while suburban sites are only partly surrounded by an urbanized matrix, thus presenting intermediate characteristics between the other two area-types. Built-up fraction was high for urban sites, low for rural, and intermediate for suburban ones (Online Resource, Fig. S1). Red squirrels were monitored by capture-mark-recapture (CMR) in six study sites in the Po plane: 2 rural sites (Golasecca, Calusco), 2 suburban sites (Pineta, Varesesub), and 2 urban sites (Vareseurban, Gallarate) (Fig. 1). Some study sites had more than one trapping grid, but since grids were within the species’ range of dispersal distances (Wauters et al. 2011), each site was considered to contain a single squirrel population (Table 1). CMR sessions were carried out seasonally (Winter from December to mid-March; Spring-summer from end of March to end of August; Autumn from September to November; Santicchia et al. 2018b) from October 2020 to April 2022. Grey squirrels were monitored by CMR and removal sampling in six study sites in the Po plane: 2 rural sites (Commande, Piobesi), 2 suburban sites (Adda Nord, RS), and 2 urban sites (ILO, Torino Giardino Botanico) (Fig. 1). Also for this species, CMR sessions were carried out seasonally, but over a longer time period (January 2011 to February 2022). For all sites we recorded the presence of only one squirrel species (Table 1).
To obtain a measure of tree species diversity, we determined the three trees closest to each trap location and then summed for all locations over the entire study site, providing an estimate of the dominant tree species for the study sites. Tree species diversity was calculated using the Simpson’s index of diversity 1-D, where D = Ʃ pi2 and pi is the proportion of species i in the community. The closer to 1, the higher the species diversity.
Trapping and handling
We trapped squirrels using Tomahawk live traps (model 202 with finer mesh, Tomahawk Live Trap Co., WI, USA) baited with hazelnuts and walnuts. About one month before each trapping session, traps blocked open or hair-tubes were pre-baited about once every 10 days. Once activated, we checked traps three times per day to reduce the time a squirrel was confined in the trap. A trapped squirrel was flushed from the trap in a zipper-tube handling bag, and, at first capture, individually marked with a numbered metal ear tag (Monel 1005 1L1 National Band and Tag Co, Newport, Kentucky, USA). Sex and reproductive condition were determined based on external genitalia and size of the nipples (Wauters and Dhondt 1993; Wauters et al. 2007; Tranquillo et al. 2022). Males were classified based on testes size and position [testes small and abdominal (non-breeding) or testes large and scrotal (breeding)]. Females were classified as anoestrus, post-oestrus/pregnant, or lactating (Wauters et al. 2007; Santicchia et al. 2022). At each capture, we measured the length of the right hind foot (claws excluded) to the nearest 0.5 mm using a thin ruler and weighed squirrels with a Pesola spring balance to the nearest 5 g (Wauters et al. 2007; Romeo et al. 2020).
Statistical analyses
Data of red and grey squirrels were analyzed separately in R version 4.1.2 (R Core Team 2021) with Linear Mixed Models (LMM). Juveniles (determined based on size of genitalia and body mass < 250 g for red squirrels and < 400 g for greys) were removed from the analysis (Wauters and Dhondt 1989b; Koprowski 1994; Wauters et al. 2007). To account for measuring errors of foot length and for fluctuations in body mass over time and with reproductive condition, we used repeated measures of both variables and estimated within and among individual variance using linear mixed models (Zuur et al. 2010). Foot length and body mass were measured on average 3.79 ± SE 0.30 times for red and 1.80 ± SE 0.07 for grey squirrels. Estimates of the among- and within-individual variance in foot length and in body mass are presented in the full models in the Online Resource (Table S1 and S2).
We first explored the effects of area-type (rural, suburban or urban) and sex as fixed effects, and the area-type by sex interaction on variation in hind foot length. To account for repeated measures and for potential variation between study sites, squirrel identity nested in study site was added as a random intercept. The LMM was fitted by REML and t-tests were calculated using Satterthwaite’s method with the R package “lme4” v 1.1–28 (Bates et al. 2015).
Next, we investigated whether area-type affected variation in body mass. Since body mass fluctuates seasonally and with reproductive condition, and since effects of reproductive condition differ between the sexes (Wauters and Lens 1995; Wauters et al. 2007), we ran a LMM for each sex separately. We used body mass as the dependent variable, hind foot length as explanatory continuous variable and area-type, season and reproductive condition as fixed effects. We further included the interaction of foot length with area-type in the full model, and added squirrel identity nested in study site as a random intercept to account for repeated measures over time.
In all LMMs, we tested interactions and fixed effects using Type-III SS and performed model selection using a backward procedure based on p-values (eliminating parameters with partial p > 0.10). Pairwise comparisons were based on differences between least square means (DLSM).
Results
Red squirrels
Mean hind foot length (± SD) of red squirrels was 57.8 ± 1.7 mm (n = 367 observations of 97 different animals, range 53.0 to 62.0 mm). Divided by area-type, the mean length of the right hind foot was 57.4 ± 2.4 mm (n = 121) in rural sites, 57.9 ± 1.2 mm (n = 146) in suburban sites, 58.2 ± 1.3 mm (n = 100) in urban sites. Foot length did not differ according to area-type (F2, 91 = 0.11; p = 0.89) or sex (F1, 91 = 1.25; p = 0.27) and there was no area*sex interaction (F2, 89 = 0.15; p = 0.86; Table 2).
Female red squirrels weighed on average 330 ± 37 g (188 measures of 45 different animals, range 250 to 420 g). Body mass of females increased with foot length (estimate ± SE = 8.35 ± 1.83, t141 = 4.56; p < 0.0001), and females were heavier in the urban than in the rural sites (estimate ± SE = 30.9 ± 10.1, t39 = 3.06; p = 0.004) while there was no body mass difference between urban and suburban populations (estimate ± SE = 6.1 ± 9.2, t39 = 0.66; p = 0.51; Table 2; Fig. 2). Lactating and post-oestrus female red squirrels were heavier than non-breeding ones (respectively: estimate ± SE = 23.0 ± 3.6; t165 = 6.39; p < 0.0001; and estimate ± SE = 20.3 ± 3.9; t156 = 5.22; p < 0.0001). Seasonal changes in body mass were small and not significant (F2, 152 = 1.98; p = 0.14; all pairwise comparisons p > 0.05). The interaction of foot length with area-type was not significant and removed during model selection (Online Resource, Table S2).
Male red squirrels weighed on average 313 ± 29 g (179 measures of 52 different animals, range 250–375 g). Body mass of males increased with foot length (estimate ± SE = 3.45 ± 1.19, t156 = 2.90; p = 0.004), and males were heavier in the urban than in the rural (estimate ± SE = 30.19 ± 8.25, t48 = 3.66; p = 0.0006) and in the suburban sites (estimate ± SE = 23.46 ± 8.41, t46 = 2.79; p = 0.008; Table 2; Fig. 2). The interaction of foot length with area-type was not significant and removed during model selection (Online Resource, Table S2). Also, reproductive condition did not affect variation in male body mass and was removed from the model. Although males tended to be heaviest in autumn (pairwise comparison with spring-summer p = 0.063), the season effect was small and not significant (F2, 147 = 1.88; p = 0.16).
Grey squirrels
Mean hind foot length (± SD) of grey squirrels was 61.9 ± 1.7 mm (n = 679 observations of 380 different animals, range 56.0 to 68.0 mm). Calculated per area-type, mean hind foot length was 61.9 ± 1.6 mm (n = 296) in rural sites, 61.7 ± 1.8 mm (n = 282) in suburban sites and 62.5 ± 1.9 mm (n = 101) in urban sites. Foot length did not differ according to sex (F1, 365 = 0.06; p = 0.81) and there was no area*sex interaction (F2, 365 = 0.23; p = 0.79). However, grey squirrels were slightly larger in the urban sites than in the suburban (estimate ± SE = 1.09 ± 0.24; t383 = 4.52; p < 0.0001) and rural sites (estimate ± SE = 0.95 ± 0.25; t370 = 3.77; p = 0.0002) (Table 3).
Female grey squirrels weighed on average 546 ± 56 g (351 measures of 191 different animals, range 400 to 685 g). Body mass of females increased with foot length (estimate ± SE = 7.46 ± 1.39, t345 = 5.38; p < 0.0001), and females were heavier in the urban than in the suburban (estimate ± SE = 60.9 ± 9.8, t187 = 6.23; p < 0.0001) and in the rural sites (estimate ± SE = 53.9 ± 10.0, t177 = 5.42; p < 0.0001; Table 3; Fig. 2). Lactating and post-oestrus female grey squirrels were heavier than non-breeding ones (respectively: estimate ± SE = 8.53 ± 4.88; t238 = 1.75; p = 0.082; and estimate ± SE = 19.8 ± 5.0; t217 = 3.96; p = 0.0001). The effects of season (partial p > 0.30) and the interaction of foot length with area-type were not significant and removed during model selection (Online Resource, Table S2).
Male grey squirrels weighed on average 528 ± 57 g (328 measures of 189 different animals, range 400–700 g). Body mass of males increased with foot length (estimate ± SE = 10.02 ± 1.57, t309 = 6.38; p < 0.0001), and males were heavier in the urban than in the rural (estimate ± SE = 46.5 ± 9.1, t174 = 5.14; p < 0.0001) and in the suburban sites (estimate ± SE = 59.8 ± 8.6, t184 = 6.96; p < 0.0001; Table 3; Fig. 2). Males with scrotal testes were heavier than non-breeding males (estimate ± SE = 30.8 ± 4.9, t248 = 6.25; p < 0.0001), and males grey squirrels were heavier in autumn than in the other seasons (spring-summer estimate ± SE = 11.7 ± 4.9, t235 = 2.38; p = 0.018; winter estimate ± SE = 18.9 ± 4.9, t200 = 3.88; p = 0.0001). The interaction of foot length with area-type was not significant and removed during model selection (Online Resource, Table S2).
Discussion
Both native red and invasive alien grey squirrels showed phenotypic differences among populations living in rural, suburban or urban areas. Hind foot length, a proxy for body size (Wauters and Dhondt 1989a), did not differ between the three area-types for red squirrels, while grey squirrels were slightly larger in urban than in other area types, even if we believe this difference is not biologically significant. In both species, animals of either sex were heavier in the urban than in the rural sites. Body mass differences between the urban and suburban sites were more subtle and varied with species and sex. Hence, both native red squirrels and invasive alien grey squirrels showed similar changes in body mass to living in urbanized green areas and the direction of the weight increase, lower in rural, higher in urban sites, was the same for both species.
Our findings of heavier animals in urban than in rural sites are similar to those from a previous study on eastern chipmunk (Tamias striatus) where a higher body condition in urban individuals than in conspecific in the natural environment was observed (Lyons et al. 2017). However, the morphological response of squirrels to the urbanization process is still unclear. In fact, our results are in contrast with those reported by Wist et al. (2022) and Beliniak et al. (2022), who found that Eurasian red squirrels in the city weighed less than conspecifics in a nearby rural habitat. Hence, the types and amount of both natural food resources and those supplemented by humans are likely to influence body mass responses in sciurids.
Red squirrels have been used as model species to investigate possible adaptations to urban environments in several European countries, but the majority of these studies monitored animals in only one urban (or urban park) and one non-urban (or non-urban park) site. Most studies about the success of squirrels in the urban environment have associated this success to the supplemental food, intentionally or inadvertently present in urban parks (Bonnington et al. 2014; Rézouki et al. 2014; Jokimaki et al. 2017; Fingland et al. 2021). In some areas, food supplemented by humans can become the principal part of the squirrels’ diet (Krauze-Gryz and Gryz 2015). For example, Reher and colleagues (2016) found higher body mass in Eurasian red squirrels with a core area located near high-mass and high-energy anthropogenically-provided food sources, and that body mass was not related to the availability of natural food sources. Furthermore, Beliniak and colleagues (2021) suggested that Eurasian red squirrels’ activity is driven by visitor frequency: in urban parks they were more active during the periods of higher human presence, thus the chances of obtaining supplementary food should increase and the time devoted to food search decrease.
In our study sites, we observed people providing additional food (hazelnuts and walnuts) to red squirrels in only one trapping site; while other sources of inadvertently left supplemental food (e.g. garbage) that red squirrels can find in our study sites are limited. In contrast, our grey squirrels readily took extra food in both urban study sites. Hence, for urban invasive alien grey squirrels, that show little fear from humans with some individuals taken nuts from the hand (LAW pers. obs.), the higher body mass of subadults and adults in the urban sites could be related to higher food-energy intake. However, for urban red squirrels in our study sites, this seems unlikely. We suggest a role for tree-species composition in urban parks and gardens (Table 1) influencing red squirrel body mass. Moreover, several tree species whose seeds are preferred by red squirrels (hornbeam seeds, hazelnuts, beechnuts, Norway spruce seeds; Wauters et al. 1992, 2001b; Molinari et al. 2006; Rubino et al. 2012; Jokimäki et al. 2017) and by grey squirrels (acorns, beechnuts, ash seeds; Koprowski 1994; Wauters et al. 2001b) were common in their respective urban sites. Hence, a wide variety of high-energy seeds, with different phenology and thus a prolonged availability in time, is likely to guarantee a relatively constant availability of mature tree seeds in urban habitats. In contrast, the rural habitats are often characterized by a lower tree species diversity and by few species of trees selected for their seeds, probably providing less predictable food resources that vary more strongly both seasonally and among years (e.g., Lurz and Lloyd 2000; Wauters et al. 2001a, 2004; Boutin et al. 2006). Also tree density and the more open, parklike structure of the urban study sites could influence the productivity of single trees, receiving more sunlight than in a closed forest stand typical of the rural sites (e.g., Dylewski et al. 2016; Mazzamuto et al. 2018; Hämäläinen et al. 2020).
Quantitative detailed studies on activity patterns and foraging (food choice) of squirrels, seed availability and seed quality (nutrient and energy content) will be necessary to explore the causal relationships between tree-species abundance, seed abundance and squirrel body mass in the different area-types. This would allow to estimate rate of energy-intake and compare it among squirrels occurring in rural, suburban and urban areas, and to what extend it explains variation in body mass (e.g. Wauters et al. 2001b). This approach would also allow to explore the role of both native (e.g. Taxus baccata) and exotic, ornamental trees (e.g. Cedrus sp., Taxodium sp.), common in parks, but absent (or rare in the case of T. baccata) in rural habitats.
In this research we considered also suburban areas, that had vegetation characteristics intermediate between the urban and rural sites (see also Table 1). Much as urban sites, they contained exotic ornamental tree species, but in common with rural sites, most of the dominant tree species typical of the natural and rural forests were also present. With this study design, we did not only evaluate the difference rural-urban, but we observed also changes along the urbanization gradient. Here, body mass patterns were species and sex-specific. In fact, both male and female grey squirrels were heavier in urban than in suburban sites, where they did not receive supplemental food. In contrast, red squirrel males were heavier in urban than in suburban sites, but this was not the case for females. Differences in body mass among sexes in red squirrels have already been noted (e.g., Tranquillo et al. 2022). First of all, body mass is a key trait for females to increase reproductive success (Mari et al. 2008), affecting several traits involved in reproduction such as the probability to enter oestrus and lactation success (Wauters and Dhondt 1989b, 1995; Wauters and Lens 1995). Thus, in the suburban environment, energy investment presumably differs among sexes with stronger pressure on females than on males to maintain a high body mass. Secondly, space use of males, that typically have larger home ranges than females (e.g., Wauters and Dhondt 1992; Mazzamuto et al. 2020) may differ between small urban and larger suburban sites, resulting in higher energy expenditure (and lower body mass) in the suburban habitats. At this stage, this is only a tentative explanation that could be verified by radio-tracking squirrels along the rural-urban gradient.
Our study also revealed a difference between the two species. The size (hind foot length) of red squirrels did not differ among area-types, while grey squirrels in the urban environment were significantly larger than in suburban and rural sites. However, the mean difference in hind foot length between urban and rural grey squirrels was only 0.5 to 0.9 mm, thus less than 1 to 1.5% of mean length (Table 3), compared to a more than 60 g, or about 10% average weight difference. Therefore, we believe that the observed difference in body size of grey squirrels along the urbanization gradient was small and biologically of little relevance.
Overall, our findings partially support the first prediction: squirrels were heavier, but not in accordance with the first hypothesis (squirrels differ in body size and body mass among the three area-types); only body mass changes along the urbanization gradient while body size is probably driven by forces present in all study areas. Moreover, these results did not support the second hypothesis, where we predicted more marked differences among area-types in the invasive species, unexpected the two squirrels showed similar responses to the urban environment. In particular, the higher weight of urban squirrels seems to indicate that both species have a fitness advantage to live in this habitat; indeed, as mentioned above, body mass is positively related to females’ reproductive success and to males’ mating success (Wauters et al. 1990; Koprowski 1993).
We studied morphological changes in body size and body mass, of a native and an invasive alien tree squirrel species, using a pseudo-experimental study design with replicates for each type of study site, along a rural-urban gradient. In conclusion, our findings give partial support to the three predictions. In agreement with prediction i, both species differed in body mass along the rural-urban gradient, but, at least in native red squirrels, this was not the case for body size. The direction of mass increase was the same in both species, with heavier animals in the urban sites. This was predicted (prediction iii) for the invasive grey squirrels, which are expected to thrive in parks and woodlots with high anthropogenic disturbance, while for native red squirrels patterns from previous studies were area-specific, with both higher and/or seasonally more constant body mass (Turner et al. 2017, this study) or lower body mass (Wist et al. 2022) in urban than in rural habitats. Finally, in contrast with prediction ii, the percentage increase in mean body mass from rural to urban sites, around 10%, was similar for the native and the invasive species. Hence, morphologically both squirrel species seem equally well adapted to the urban environment, where higher body mass could have a positive effect on reproduction, at least in females. High reproductive output in a single year, linked to high body mass (Wauters and Lens 1995; Wauters and Dhondt 1995), could be more important in urban areas where human-induced mortality (road victims, predation by domestic cats) is likely to reduce squirrel survival. However, to truly understand how squirrels benefit from the urban habitat, future long-term studies monitoring variation in survival and reproductive success along the rural-urban gradient are needed.
Data availability
Data will be made available on reasonable request.
References
Alberti M, Correa C, Marzluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y (2017) Global urban signatures of phenotypic change in animal and plant populations. Proc Natl Acad Sci USA 114:8951–8956. https://doi.org/10.1073/pnas.1606034114
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beliniak A, Krauze-Gryz D, Jasińska K, Jankowska K, Gryz J (2021) Contrast in daily activity patterns of red squirrels inhabiting urban park and urban forest. Hystrix 32:159–164. https://doi.org/10.4404/hystrix-00476-2021
Beliniak A, Gryz J, Klich D, Jasińska K, Krauze-Gryz D (2022) Body condition and breeding of urban red squirrels: comparison of two populations affected by different levels of urbanization. Animals 12:3246. https://doi.org/10.3390/ani12233246
Bertolino S, di Montezemolo NC, Preatoni DG, Wauters LA, Martinoli A (2014) A grey future for Europe: Sciurus carolinensis is replacing native red squirrels in Italy. Biol Invasions 16:53–62. https://doi.org/10.1007/s10530-013-0502-3
Bonnington C, Gaston KJ, Evans KL (2014) Squirrels in suburbia: influence of urbanisation on the occurrence and distribution of a common exotic mammal. Urban Ecosyst 17:533–546. https://doi.org/10.1007/s11252-013-0331-2
Borden JB, Flory SL (2021) Urban evolution of invasive species. Front Ecol Environ 19:184–191. https://doi.org/10.1002/fee.2295
Boutin S, Wauters LA, McAdam AG, Humphries MM, Tosi G, Dhondt AA (2006) Anticipatory reproduction and population growth in seed predators. Science 314:1928–1930. https://doi.org/10.1126/science.1135520
Cadotte MW, Yasui SLE, Livingstone S, MacIvor JS (2017) Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol Invasions 19:3489–3503. https://doi.org/10.1007/s10530-017-1586-y
Dylewski Ł, Przyborowski T, Myczko Ł (2016) Winter Habitat Choice by foraging the Red Squirrel (Sciurus vulgaris). Ann Zool Fenn 53:194–200. https://doi.org/10.5735/086.053.0408
European Commission (2016) Commission Implementing Regulation (EU) 2016/1141 of 13 July 2016. Adopting a list of invasive alien species of Union concern pursuant to regulation (EU) no 1143/2014 of the European Parliament of the Council. Official J Eur Union L 1894
Festa-Bianchet M, Jorgenson JT, Bérubé CH, Portier C, Wishart WD (1997) Body mass and survival of bighorn sheep. Can J Zool 75:1372–1379. https://doi.org/10.1139/z97-763
Fingland K, Ward SJ, Bates AJ, Bremner-Harrison S (2021) A systematic review into the suitability of urban refugia for the eurasian red squirrel Sciurus vulgaris. Mam Rev 52:26–38. https://doi.org/10.1111/mam.12264
Gurnell J, Lurz PWW, Wauters LA (2015) Years of interactions and conflict in Europe: competition between Eurasian red squirrels and North American grey squirrels. In: Shuttleworth CM, Lurz PW, Hayward M (eds) Red squirrels: ecology, conservation & management in Europe. European Squirrel Initiative, Stoneleigh Park, Warwickshire CV8 2LG, England, pp 19–37
Hämäläinen S, Fey K, Selonen V (2020) Search strategies in rural and urban environment during natal dispersal of the red squirrel. Behav Ecol Sociobiol 74:124. https://doi.org/10.1007/s00265-020-02907-z
Iglesias-Carrasco M, Martín J, Cabido C (2017) Urban habitats can affect body size and body condition but not immune response in amphibians. Urban Ecosyst 20:1331–1338. https://doi.org/10.1007/s11252-017-0685-y
Jokimäki J, Selonen V, Lehikoinen A, Kaisanlahti-Jokimäki M-L (2017) The role of urban habitats in the abundance of red squirrels (Sciurus vulgaris, L.) in finland. Urban for Urban Green 27:100–108. https://doi.org/10.1016/j.ufug.2017.06.021
Koprowski JL (1993) Alternative reproductive tactics in male eastern gray squirrels: making the best of a bad job. Behav Ecol 4:165–171. https://doi.org/10.1093/beheco/4.2.165
Koprowski JL (1994) Sciurus carolinensis. Mamm Species 480:1–9. https://doi.org/10.2307/3504224
Krauze-Gryz D, Gryz J (2015) A review of the diet of the red squirrel (Sciurus vulgaris) in different types of habitats. In: Shuttleworth CM, Lurz PW, Hayward M (eds) Red squirrels: ecology, conservation & management in Europe. European Squirrel Initiative, Stoneleigh Park, Warwickshire CV8 2LG, England, pp 39–50
Li X, Zhou Y, Hejazi M, Wise M, Vernon C, Iyer G, Chen W (2021) Global urban growth between 1870 and 2100 from integrated high resolution mapped data and urban dynamic modeling. Commun Earth Environ 2:201. https://doi.org/10.1038/s43247-021-00273-w
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments: behavioural responses to urban environments. Biol Rev 88:537–549. https://doi.org/10.1111/brv.12012
Lurz PWW, Lloyd AJ (2000) Body weights of grey and red squirrels: do seasonal weight increases occur in conifer woodland? J Zool 252:539–543. https://doi.org/10.1017/S0952836900230288
Lyons J, Mastromonaco G, Edwards DB, Schulte-Hostedde AI (2017) Fat and happy in the city: eastern chipmunks in urban environments. Behav Ecol 28:1464–1471. https://doi.org/10.1093/beheco/arx109
Mari V, Martini S, Romeo C, Molinari A, Martinoli A, Tosi G, Wauters LA (2008) Record litter size in the eurasian red squirrel (Sciurus vulgaris). Hystrix 19:61–65. https://doi.org/10.4404/hystrix-19.1-4418
Mazzamuto MV, Wauters LA, Preatoni DG, Martinoli A (2018) Behavioural and population responses of ground-dwelling rodents to forest edges. Hystrix 29:211–215. https://doi.org/10.4404/hystrix-00119-2018
Mazzamuto MV, Merrick MJ, Bisi F, Koprowski JL, Wauters LA, Martinoli A (2020) Timing of resource availability drives divergent social systems and home range dynamics in ecologically similar tree squirrels. Front Ecol Evol 8:174. https://doi.org/10.3389/fevo.2020.00174
Mazzamuto MV, Wauters LA, Koprowski JL (2021) Exotic pet trade as a cause of biological invasions: the case of tree squirrels of the genus Callosciurus. Biology 10:1046. https://doi.org/10.3390/biology10101046
McCleery RA, Parker ID (2011) Influence of the urban environment on fox squirrel range overlap. J Zool 285:239–246. https://doi.org/10.1111/j.1469-7998.2011.00835.x
McDonnell MJ, Hahs AK (2015) Adaptation and adaptedness of organisms to Urban environments. Annu Rev Ecol Evol Syst 46:261–280. https://doi.org/10.1146/annurev-ecolsys-112414-054258
Meillère A, Brischoux F, Parenteau C, Angelier F (2015) Influence of urbanization on body size, condition, and physiology in an urban exploiter: a multi-component approach. PLoS ONE 10:e0135685. https://doi.org/10.1371/journal.pone.0135685
Molinari A, Wauters LA, Airoldi G, Cerinotti F, Martinoli A, Tosi G (2006) Cone selection by eurasian red squirrels in mixed conifer forests in the italian Alps. Acta Oecol 30:1–10. https://doi.org/10.1016/j.actao.2005.11.004
Partan SR, Fulmer AG, Gounard MAM, Redmond JE (2010) Multimodal alarm behavior in urban and rural gray squirrels studied by means of observation and a mechanical robot. Curr Zool 56:313–326. https://doi.org/10.1093/czoolo/56.3.313
Putman BJ, Tippie ZA (2020) Big city living: a global meta-analysis reveals positive impact of urbanization on body size in lizards. Front Ecol Evol 8:580745. https://doi.org/10.3389/fevo.2020.580745
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reher S, Dausmann KH, Warnecke L, Turner JM (2016) Food availability affects habitat use of eurasian red squirrels (Sciurus vulgaris) in a semi-urban environment. J Mammal 97:1543–1554. https://doi.org/10.1093/jmammal/gyw105
Rézouki C, Dozières A, Le Cœur C, Thibault S, Pisanu B, Chapuis J-L, Baudry E, Rogers S (2014) A viable population of the european red squirrel in an urban park. PLoS ONE 9:e105111. https://doi.org/10.1371/journal.pone.0105111
Ritzel K, Gallo T (2020) Behavior change in urban mammals: a systematic review. Front Ecol Evol 8:576665. https://doi.org/10.3389/fevo.2020.576665
Rode KD, Atwood TC, Thiemann GW et al (2020) Identifying reliable indicators of fitness in polar bears. PLoS ONE 15:e0237444. https://doi.org/10.1371/journal.pone.0237444
Romeo C, Wauters LA, Santicchia F, Dantzer B, Palme R, Martinoli A, Ferrari N, Ferkin M (2020) Complex relationships between physiological stress and endoparasite infections in natural populations. Curr Zool 66:449–457. https://doi.org/10.1093/cz/zoaa029
Rubino FM, Martinoli Al, Pitton M, Di Fabio D, Caruso E, Banfi S, Tosi G, Wauters LA, Martinoli Ad (2012) Food choice of eurasian red squirrels and concentrations of anti-predatory secondary compounds. Mamm Bio 77:332–338. https://doi.org/10.1016/j.mambio.2012.01.003
Santana Marques P, Resende Manna L, Clara Frauendorf T, Zandonà E, Mazzoni R, El-Sabaawi R (2020) Urbanization can increase the invasive potential of alien species. J Anim Ecol 89:2345–2355. https://doi.org/10.1111/1365-2656.13293
Santicchia F, Gagnaison C, Bisi F, Martinoli A, Matthysen E, Bertolino S, Wauters LA (2018a) Habitat-dependent effects of personality on survival and reproduction in red squirrels. Behav Ecol Sociobiol 72:134. https://doi.org/10.1007/s00265-018-2546-y
Santicchia F, Dantzer B, van Kesteren F, Palme R, Martinoli A, Ferrari N, Wauters LA, Griffen B (2018b) Stress in biological invasions: introduced invasive grey squirrels increase physiological stress in native eurasian red squirrels. J Anim Ecol 87:1342–1352. https://doi.org/10.1111/1365-2656.12853
Santicchia F, Wauters LA, Tranquillo C, Villa F, Dantzer B, Palme R, Preatoni D, Martinoli A (2022) Invasive alien species as an environmental stressor and its effects on coping style in a native competitor, the eurasian red squirrel. Horm Behav 140:105127. https://doi.org/10.1016/j.yhbeh.2022.105127
Santini L, González-Suárez M, Russo D, Gonzalez‐Voyer A, Hardenberg A, Ancillotto L, Brose U (2019) One strategy does not fit all: determinants of urban adaptation in mammals. Ecol Lett 22:365–376. https://doi.org/10.1111/ele.13199
Scheun J, Bennett NC, Ganswindt A, Nowack J (2015) The hustle and bustle of city life: monitoring the effects of urbanisation in the african lesser bushbaby. Sci Nat 102:57. https://doi.org/10.1007/s00114-015-1305-4
Stepkovitch B, Martin JM, Dickman CR, Welbergen JA (2019) Urban lifestyle supports larger red foxes in Australia: an investigation into the morphology of an invasive predator. J Zool 309:287–294. https://doi.org/10.1111/jzo.12723
Teangana D, Reilly S, Montgomery WI, Rochford J (2000) Distribution and status of the Red Squirrel (Sciurus vulgaris) and Grey Squirrel (Sciurus carolinensis) in Ireland. Mamm Rev 30:45–56. https://doi.org/10.1046/j.1365-2907.2000.00054.x
Thomas LS, Teich E, Dausmann K et al (2018) Degree of urbanisation affects eurasian red squirrel activity patterns. Hystrix 29:175–180
Tranquillo C, Wauters LA, Santicchia F, Preatoni DG, Martinoli A (2022) Living on the edge: morphological and behavioral adaptations to a marginal high-elevation habitat in an arboreal mammal. Integr Zool 00:1–16. https://doi.org/10.1111/1749-4877.12679
Turner JM, Reher S, Warnecke L, Dausmann KH (2017) Eurasian red squirrels show little seasonal variation in metabolism in food-enriched habitat. Physiol Biochem Zool 90:655–662. https://doi.org/10.1086/694847
Uchida K, Suzuki K, Shimamoto T, Yanagawa H, Koizumi I (2016) Seasonal variation of flight initiation distance in eurasian red squirrels in urban versus rural habitat. J Zool 298:225–231. https://doi.org/10.1111/jzo.12306
United Nations, Department of Economic and Social Affairs, Population Division (2019) World urbanization prospects: the 2018 Revision (ST/ESA/SER.A/420). United Nations, New York
Wauters LA, Dhondt AA (1989a) Variation in length and body weight of the red squirrel (Sciurus vulgaris) in two different habitats. J Zool 217:93–106. https://doi.org/10.1111/j.1469-7998.1989.tb02477.x
Wauters LA, Dhondt AA (1989b) Body weight, longevity and reproductive success in red squirrels (sciurus vulgaris). J Anim Ecol 58:637. https://doi.org/10.2307/4853
Wauters LA, Dhondt AA (1992) Spacing behaviour of red squirrels, Sciurus vulgaris: variation between habitats and the sexes. Anim Behav 43:297–311. https://doi.org/10.1016/S0003-3472(05)80225-8
Wauters LA, Dhondt AA (1993) Immigration patterns and success in red squirrels. Behav Ecol Sociobiol 33:159–167. https://doi.org/10.1007/BF00216596
Wauters LA, Dhondt AA (1995) Lifetime reproductive success and its correlates in female eurasian red squirrels. Oikos 72:402. https://doi.org/10.2307/3546126
Wauters LA, Lens L (1995) Effects of food abundance and density on red squirrel (Sciurus vulgaris) reproduction. Ecology 76:2460–2469
Wauters LA, Martinoli A (2018) Urban biological invasions: when vertebrates come to town. In: Ossola A, Niemelä J (eds) Urban biodiversity: from research to practice. Routledge studies in urban ecology, Oxon, UK, pp 87–100
Wauters LA, Dhondt AA, De Vos R (1990) Factors affecting male mating success in red squirrels (Sciurus vulgaris). Ethol Ecol Evol 2:195–204. https://doi.org/10.1080/08927014.1990.9525486
Wauters L, Swinnen C, Dhondt AA (1992) Activity budget and foraging behaviour of red squirrels (Sciurus vulgaris) in coniferous and deciduous habitats. J Zool 227:71–86. https://doi.org/10.1111/j.1469-7998.1992.tb04345.x
Wauters LA, Gurnell J, Preatoni D, Tosi G (2001a) Effects of spatial variation in food availability on spacing behaviour and demography of eurasian red squirrels. Ecography 24:525–538. https://doi.org/10.1111/j.1600-0587.2001.tb00487.x
Wauters LA, Gurnell J, Martinoli A, Tosi G (2001b) Does interspecific competition with introduced grey squirrels affect foraging and food choice of eurasian red squirrels? Anim Behav 61:1079–1091. https://doi.org/10.1006/anbe.2001.1703
Wauters LA, Matthysen E, Adriaensen F, Tosi G (2004) Within-sex density dependence and population dynamics of red squirrels Sciurus vulgaris. J Anim Ecol 73:11–25. https://doi.org/10.1111/j.1365-2656.2004.00792.x
Wauters LA, Vermeulen M, Van Dongen S, Bertolino S, Molinari A, Tosi G, Matthysen E (2007) Effects of spatio-temporal variation in food supply on red squirrel Sciurus vulgaris body size and body mass and its consequences for some fitness components. Ecography 30:51–65. https://doi.org/10.1111/j.0906-7590.2007.04646.x
Wauters LA, Githiru M, Bertolino S, Molinari A, Tosi G, Lens L (2008) Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography 31:104–114. https://doi.org/10.1111/j.2007.0906-7590.05251.x
Wauters LA, Preatoni D, Martinoli A, Verbeylen G, Matthysen E (2011) No sex bias in natal dispersal of eurasian red squirrels. Mamm Bio 76:369–372. https://doi.org/10.1016/j.mambio.2010.04.003
Wist B, Stolter C, Dausmann KH (2022) Sugar addicted in the city: impact of urbanisation on food choice and diet composition of the eurasian red squirrel (Sciurus vulgaris). J Urban Ecol 8:juac012. https://doi.org/10.1093/jue/juac012
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
We thank Regione Lombardia, Parco Lombardo della Valle del Ticino, Parco Pineta di Appiano Gentile e Tradate, Parco Adda Nord and Città Metropolitana di Torino for permits and access to the study sites. We are also grateful to the cities of Varese and Gallarate, Ville Ponti Congress Centre, Villa Panza and Fondo Ambiente Italiano (FAI), Palace Grand Hotel Varese, Aloisianum Gallarate, Istituto Comprensivo Ponti Gallarate, Botanic garden of the Department of Life Sciences and Systems Biology University of Torino, UN campus of Turin and to the other owners of private estates for allowing fieldwork in their properties. The authors also thank the thesis students for helping with data collection.
Funding
Open access funding provided by Università degli Studi dell’Insubria within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
CT, FB, FS and LAW: conceptualization, data curation, formal analysis, methodology, writing original draft, review and editing. CT, FS, MP and LAW: investigation, methodology; DP: software, supervision, draft revision and editing; AM: project administration, supervision, draft revision and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Trapping, marking and handling of squirrels were performed under the Guidelines for the treatment of animals in behavioral research and teaching (Animal Behaviour, 2020, 159, I–XI; https://doi.org/10.1016/j.anbehav.2019.11.002). Legal requirements according to the Italian Wildlife Protection and Hunting Law L.N. 157 from 1992 and fieldwork was approved by authorization decrees n. 1938 of 18/02/2020 from Direzione Generale Agricoltura, Alimentazione E Sistemi Verdi, Regione Lombardia; authorizations n. 180–14616 of 06/07/2011 and n. 294–34,626 of 12/09/2014 from the Provincia di Torino, authorizations n. 62–3025 of 20/03/2017 and n. 1015 of 20/03/2020 from Città Metropolitana di Torino.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tranquillo, C., Wauters, L.A., Santicchia, F. et al. The advantage of living in the city: effects of urbanization on body size and mass of native and alien squirrels. Urban Ecosyst 27, 51–61 (2024). https://doi.org/10.1007/s11252-023-01435-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01435-8