Abstract
Some species occur in higher densities in cities than in natural habitats, despite the variety of urban stressors. Urban fragments can be extremely heterogeneous though, and species’ responses might vary between urban patches. However, only few studies examine mammalian populations from different fragments in the core of cities, where stressors are at their maximum. The Eurasian red squirrel (Sciurus vulgaris) is highly abundant in urban green spaces and readily makes use of supplemental food sources. We examined natural and supplemental food sources as well as squirrel populations in three small parks in an urban core. We found pronounced differences in natural and supplemental food availability between the parks. Squirrel population densities were generally higher at all three urban sites when compared to a geographically close located natural forest, but with variation between the parks. The squirrel density in the smallest park in the summer season was 42 times higher (index of minimum number alive: 8.8 individuals/ha) than in the forest, whereas it was only 20 or respectively 16 times higher in the two other parks. Individuals from the park with the highest population density had access to the highest amount of supplemental food sources. However, side effects from non-natural food items, e.g., related to nutrition, also need to be taken into consideration and might counteract the advantages of supplemental food sources. This study shows that urban effects can vary even at the smallest scale between nearby urban habitat patches and this needs to be considered when drawing conclusions about the effects of urbanisation and deciding about conservation actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation is a rapidly increasing threat for global biodiversity (Seto et al. 2012; McDonald et al. 2019; Simkin et al. 2022) and urban wildlife have to tolerate multiple stressors. Yet some species have successfully established dense populations in urban habitats (Luniak 2004; Hubert et al. 2011; Francis and Chadwick 2012). Urban stressors are most intense in the city centre (Grimm et al. 2008; Bateman and Fleming 2012). However, cities are composed of a heterogeneous mosaic of habitat patches and spatial scales can have considerable influence on wildlife responses (de Satgé et al. 2019; Alberti et al. 2020; Uchida et al. 2020). An important driver of high urban population densities is the higher availability of food (Shochat et al. 2006; McCleery 2010). Natural food sources in urban environments are strongly modified by humans due to vegetation cover being reduced and distributed in patches; primarily concentrated in green spaces such as parks and private gardens (Singh et al. 2018). However, urban habitats and vegetation can also offer benefits for wildlife. For example, planting of ornamental plants and neophyts as well as the heat island effect lead to higher plant diversity, earlier flowering and extended growing seasons. These processes can result in a more continuous natural food availability (Shochat et al. 2006; Pickett et al. 2011; Singh et al. 2018).
Urban habitats also provide access to various intentional or unintentional food sources from humans, further contributing to a more stable food availability throughout the year (Shochat et al. 2006). Supplemental feeding can have diverse impacts on wildlife ecology and biology – both on the individual and the community level (Robb et al. 2008; Birnie-Gauvin et al. 2016), which are not necessarily advantageous. Abundant food sources in urban systems might not meet the nutritional needs of a species or even be detrimental (Birnie-Gauvin et al. 2017). Vertebrate species that receive supplementary food show higher population densities and body masses in general (Boutin 1990). The former is likely a result from increased birth rates, litter size and survival of juveniles and adults, affecting not only density, but also structure of populations (Dantzer et al. 2020). However, possible negative effects range from increased disease transmission and loss of natural behaviour to reductions in gut microbiome or community restructuring (Robb et al. 2008; Galbraith et al. 2015; Gillman et al. 2021). Artificially increased food abundance thus might act as false cue of habitat quality, thereby creating an ecological trap for urban species (Reynolds et al. 2017; Zuñiga-Palacios et al. 2021).
Given the local heterogeneity of urban green spaces and the high proportion of non-native and ornamental plants in parks and gardens, the availability of suitable natural energy rich and nutritionally adequate food can vary substantially between patches (Pickett et al. 2011; Seress and Liker 2015; Lepczyk et al. 2017). Moreover, utilization of urban green spaces by humans and their willingness to feed wildlife can also differ between urban patches, e.g., in relation to socio-economic status (Fuller et al. 2008; Hassell et al. 2021). An increasing number of studies investigate urban wildlife, however deeper insights into how green spaces within the same city core area differ in availability and composition of food sources in combination with mammalian population parameters, remain scarce.
The Eurasian red squirrel (Sciurus vulgaris, henceforth named “squirrel”) is a synurban species; able to successfully inhibit urban habitats (Luniak 2004; Jokimäki et al. 2017; Fingland et al. 2021). This arboreal small mammal is naturally forest dependent and favours seeds and nuts in its diet. However, as a food generalist, the species can successfully exploit urban resources (Reher et al. 2016; Fingland et al. 2021; Wist et al. 2022). Food availability is known to influence population demography in squirrels, as well as several other traits, such as home range, body mass and reproduction (Wauters et al. 2007, 2008; Di Pierro et al. 2010; Reher et al. 2016; Selonen et al. 2016). Urban squirrels also show behavioural adaptations to the urban environment and the ever-abundant presence of humans, for example, by altering their anti-predator behaviour or activity patterns (Uchida et al. 2019; Beliniak et al. 2021; Fingland et al. 2021). Urban green spaces, such as parks, are expected to act as suitable refuges for squirrels (Rezouki et al. 2014; Kopij 2015; Beliniak et al. 2022). However, most of these results were obtained from comparably large parks or small cities. We therefore focused on three small urban parks located in the centre of a big city approaching 2 million inhabitants. We deliberately chose parks that were similar in many environmental conditions and investigated the availability of natural and anthropogenically provided food sources. Furthermore, we examined population parameters of Eurasian red squirrels within the parks to explore whether they were influenced by heterogeneity in food abundance. Our results help to elucidate the interplay of urbanisation and local urban food abundance with mammalian population parameters.
Methods
Study sites
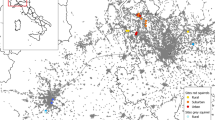
We conducted field work in northern Germany across three urban parks in the centre of Hamburg (Wohlerspark, Schanzenpark and Jacobipark; Fig. 1; Table 1). With around 1.9 million inhabitants (2,455 per km2) and an area of 755 km2, Hamburg is the second largest city in Germany. All parks were similar in several environmental factors. They were all small, with a size ranging from 4.6 to 7.4 ha, located in area with very high human population density and surrounded by a similar type of urban matrix (Table 1). In detail, we chose these parks using following criteria: (1) located in the city centre (6–9 km from the closest city limits), (2) area < 10 ha, (3) surrounded by a dense urban matrix (at least 2 large streets closer than 250 m plus large residential or commercial buildings directly adjacent to the park), and (4) high degree of diverse human utilization, such as sports, dog walking and various other outdoor activities (own observations prior to this study). In order to better assess regional conditions and population parameters, we also captured squirrels in a mixed forest about 30 km away (Hahnheide) for comparison purposes (Fig. 1; Table 1). The forest site was a nature conservation area with a high proportion of conifers and old trees (Bundesamt für Naturschutz 2019).
Natural food abundance
We compared tree species abundance and diversity per study site by using tree data obtained from Hamburg authorities (tree cadastres 2017, Freie und Hansestadt Hamburg), and the forestry office of Schleswig-Holstein (tree ledgers 2012, Schleswig-Holsteinische Landesforsten) respectively. Furthermore, we counted and classified tree seeds within two 1 × 1 m random seed plots at each trap location (20 × 20 m around the trap) at each study site in the summer season. This included fallen seeds as well as seed remains, as a measure of relative seed abundance per site (Wauters and Lens 1995). We further used literature values from the species or genus (Suppl. Table 1) for assessment of available energy-content in kJ per seed.
Supplemental food sources
For assessment of supplemental food sources, we counted and weighed anthropogenically provided food items per trapping period at each study site. We used mean values from three different days per season whenever possible as human activities varied with weather conditions and weekday. To collect these samples, we walked all paths of each park, observing the shrubbery on both sides and inspected open lawns. In the forest, we walked the whole trapping grid, but never observed supplemental food. Food was weighed to the nearest 0.5 g with a spring balance (KERN & SOHN GmbH, Balingen-Frommern, Germany). If weighing was not feasible (e.g. due to non-accessible position), the approximate weight was estimated by either comparing the amount with already measured food items (e.g. ~half fat ball) or by reproducing the set in the lab and then weighing it (e.g. a thin layer of oats 20 × 30 cm in size). We included all kinds of anthropogenically provided foods, since initial observations and a food choice experiment revealed that urban squirrels forage on a broad range of supplemental foods (Wist et al. 2022). We assigned the different food items to broader categories (e.g. bakery products or vegetables) for further analysis. The bird food category included all kinds of bird foods, such as seed mix, fat balls or scattered oats. Peanuts as high energy food were included into the “nuts or peanuts” category. We used literature values (Suppl. Table 2) to estimate the kJ content of the anthropogenic food items.
Trapping and animal handling
We trapped squirrels in all sites in summer 2017 or 2018 respectively. Furthermore, we trapped squirrels in the two most contrasting sites Wohlerspark and Hahnheide from winter 2016/2017 to autumn 2017/2018. We baited cage traps (20 × 20 × 50 cm; Tomahawk Live Trap, Hazelhurst, Wisconsin, USA) at 07:30 h with a mixture of seeds and nuts and kept them open for approximately 7 h. A longer trapping effort per day was not feasible due to the high degree of human activities in the later afternoons in the parks. Traps were placed around 100 m apart (Magris and Gurnell 2002), at approximately 1.2–1.5 m height in the park shrubbery or on trees and checked regularly. Park traps were prebaited for 1–2 days prior to each trapping period. Forest traps were prebaited every few days for one month as forest individuals are not as accustomed to search for foreign food sources.
We used a cloth cone for handling non-anesthetized trapped squirrels (Koprowski 2002). Individuals were weighed with a spring balance to the nearest 5 g (KERN & SOHN GmbH, Balingen-Frommern, Germany), sexed and aged (adult, subadult, juvenile) depending on their body mass, length and inspection of reproductive organs (Magris and Gurnell 2002; Wauters et al. 2007). The nose-anus length was determined to the nearest 0.5 cm by using a measuring tape (Wauters and Dhondt 1989a, b; Magris and Gurnell 2002; Wauters et al. 2007). Finally, the individuals were marked via subcutaneous implantation of a passive integrated transponder (ID-100B; Trovan Ltd., East Yorkshire, UK) between the shoulder blades. Animals were released immediately at the capture site after around 10 min of handling time.
Population density
We used the minimum number of individuals known to be alive (MNA) per site in one season as estimate of population density, a common method for squirrels (Magris and Gurnell 2002, Gurnell et al. 2004, Wauters et al. 2008). Furthermore, we calculated an index of relative abundance (MNAind) per site and season for adequate visualization and comparison, as exact estimates were not possible (Jokimäki et al. 2017):
Statistics
Statistics were performed in R 4.3.1 (R Core Team 2023) within the “RStudio” environment (RStudio Team 2023). Graphical data exploration was performed with the packages ‘lattice’ (Sarkar 2008) and ‘ggplot2’ (Wickham 2016). We used the ‘psych’ package (Revelle 2023) for descriptive statistics and Shapiro-Wilk test or Levene’s test respectively for testing normality and equality of variance.
We compared tree species diversity at the four study sites by using the relative abundance in percent per tree species and site since absolute tree numbers were not available for the forest. We then calculated the Shannon-Wiener Index (H’) per site (package ‘vegan’, Oksanen et al. 2013). Despite data transformation, kJ content from fallen seeds in the counted seed plots (nJakobipark = 18, nSchanzenpark = 12, nWohlerspark = 19, nHahnheide = 38) did not follow a normal distribution or equality of variance (Shapiro-Wilk test and Levene’s test, both p < 0.05). We therefore used the non-parametric Kruskal Wallis test with kJ content of fallen seeds per seed plot as dependent variable to investigate differences in natural food availability between sites. We used Dunn’s test with adjusted p values for post-hoc comparisons (package ‘FSA’) (Ogle et al. 2023). Unless otherwise stated n represents the number of individuals / seed plots per site or group. A level of p < 0.05 represents statistical significance.
Results
Natural food sources
Number of tree species was highest in the smallest park (Wohlerspark) with 45 species in total (10 per ha), followed by Schanzenpark with 36 species (5 per ha) and Jacobipark with 32 species (5 per ha). The forest study site had 11 tree species (0.3 per ha). Among the urban sites, Wohlerspark also had the highest number of trees per ha (136), which was around 1.5-times the trees per ha of Schanzenpark and 3 times that of Jacobipark. All parks were higher in tree diversity when compared to the forest site (Table 2). However, among the parks, Wohlerspark had the lowest tree diversity (Shannon-Wiener-Index H’ 2.1 vs. 2.7 in the other two parks). See Table 2 and Suppl. Table 3 for tree details. With regard to tree composition, Wohlerspark was dominated by lime (Tilia spec.) which accounted for around 55% of the park trees, whereas total proportion of conifers was only 8%. Schanzenpark was dominated by maple (Acer spec., 27%) and lime trees (Tilia spec., 26%), with only 3% conifers in the park. The most common trees in Jacobipark were European white birch (Betula pendula, 20%), maple trees (Acer spec., 20%) and oaks (18%, mostly Quercus robur). Conifers accounted for 9% of the Jacobipark trees. In the forest, European beech (Fagus sylvatica, 36%) and spruce (34%, mostly Picea abies) were the most abundant trees and the proportion of conifer trees was 59%.
kJ availability from seeds in summer differed significantly between sites (Chi2 = 41.45, df = 3, p < 0.001). Pairwise post-hoc Dunn’s test revealed higher kJ availability in the forest vs. Schanzenpark (p < 0.001), but also vs. Wohlerspark (p < 0.001). Furthermore, among the urban sites, Jacobipark had higher kJ availability from fallen seeds than Schanzenpark (p < 0.001) or Wohlerspark (p < 0.001, Fig. 2).
Available kJ from fallen seeds in the seed plots (nJakobipark = 18, nSchanzenpark = 12, nWohlerspark = 19, nHahnheide = 38) at the four different study sites. Boxes show the upper and lower quartiles (box limits) with the median as centre line. Whiskers indicate the 5 and 95% percentiles and outliers are represented as circles. Different letters indicate significant differences between sites (Dunn’s test:p ≤ 0.001)
Supplemental food sources
Anthropogenically provided food sources at the four study sites in summer ranged from zero supplementation in the forest to 1,683 g found in total on an average day in the highest supplemented park, corresponding to an available energy of 6,537 kJ (Table 3). In total, we found 13 different food items in Wohlerspark, three in Jacobipark and two in Schanzenpark (Suppl. Table 4). The availability of supplemental food sources in Wohlerspark, which was sampled throughout the year, clearly peaked in winter and was lowest in spring (Suppl. Table 5). Additionally, we observed squirrels from Wohlerspark repeatedly feeding on various foods, such as fat balls, watermelon, grapes, cupcakes, bread, rice crackers and BBQ leftovers during field work. We also observed hand feeding with walnuts, hazelnuts and peanuts in Wohlerspark on most trapping days, but not in the other two parks. This manifested itself in active begging behavior from the squirrels at Wohlerspark.
Squirrel population density and structure
In total, we trapped 67 squirrels at the urban sites (314 trap days) and 10 squirrels (428 trap days) in the forest. Within the urban sites we trapped 46 individuals in Wohlerspark (230 trap days), 12 in Jacobipark (48 trap days) and 9 in Schanzenpark (36 trap days). Summer population densities calculated as MNAind were substantially higher in the three urban parks compared to the forest. The smallest park (Wohlerspark) housed a 42-fold higher squirrel density than the forest. The density in this park was also higher than in the other two parks: 2.2 times higher compared to Jacobipark and 2.6 times higher compared to Schanzenpark. Compared to the forest, Jacobipark had a 20-fold higher and Schanzenpark a 16-fold higher population density. Specifically, we found a MNAind of 0.21 individuals per ha in summer in the forest, 4.10 in Jacobipark, 3.38 in Schanzenpark and 8.83 in Wohlerspark. We did not trap any individuals in the forest in winter.
We never trapped subadults or juveniles in the forest, whereas they were trapped in all seasons in Wohlerspark (Table 4). In summer, 23% of the trapped individuals in Wohlerspark were juvenile or subadult and in Jacobipark they accounted for 25% of the individuals. We trapped more juveniles or subadults than adults in Schanzenpark (56%), but absolute trapping numbers were low. Since all juveniles were trapped in the same corner of the park, they might have been siblings from the same litter. Apart from one exemption (Wohlerspark in winter), we always trapped more males than females, however, the proportions were neither statistically different between the four sites in summer (p = 0.40) nor between the forest and Wohlerspark in spring (p = 1.00, odds ratio 0.51, CI 0.01–6.63) or autumn (p = 0.49, odds ratio 0.00, CI 0.00-6.75). We did not recapture any of the forest individuals from one season to another during this study, but three males were recaptured in the years 2018/2019 during another study. In Wohlerspark, recapture rate was fairly high with 20 (54%) out of the 37 adult individuals being recaptured at least once in another season, thereof five individuals in two seasons, nine in three seasons and six in all four trapping seasons. Ten (53%) of the subadults were recaptured as adults in following seasons. See Table 4 as well as Figs. 3, 4 and 5 for full details on population structures and trapping success.
Squirrel density across the year for the forest site (Hahnheide, open bars) and the most contrasting urban site with regard to size and food supplementation (Wohlerspark, grey bars); shown as index of MNA (MNAind; note the logarithmic scale). We did not trap any individuals in winter in the forest despite extensive pre-baiting, 10 trapping days and squirrel feeding signs (stripped spruce cones)
Discussion
Our study highlights the heterogeneity of urban habitat patches that appear to be very similar. Urban stressors peak at urban centres, yet the spatial heterogeneity of cities can lead to distinct environmental conditions for wildlife in different green urban fragments even when located closely together (Alberti et al. 2020). The urban parks we studied showed pronounced differences in natural and supplemental food availability, though being located in the core area of the same city and sharing similar environmental conditions in terms of human population density, park size or type of urban matrix. Tree species richness as well as number of trees in total and per ha were highest in the smallest park (Wohlerspark), which might indicate higher availability of seeds and shelter for wildlife, compared to the other two parks. However, this park had the lowest tree diversity among the parks and was strongly dominated by lime trees. Lime tree seeds are not favored by squirrels, but represent secondary food items (Bertolino et al. 2004; Krauze-Gryz and Gryz 2015). Conifers, an important primary food source for squirrels, were scarce in all parks, accounting for only 3 to 9% of the local trees, thus suitability as permanent habitat could be disputable. Availability of kJ from fallen tree seeds was lower in Wohlerspark and Schanzenpark compared to the third park and the forest, but this could change throughout the year with different types of food ripening at different times. Natural seed availability might have been influenced by gardening activities in the parks where measures such as cutting of trees and cleaning of paths etc. occurred regularly during the trapping seasons. These activities might also impact the possibilities to retrieve hoarded food items.
Besides natural food sources, anthropogenically provided foods also differed between the parks in amount and variety in the summer season and were highest in Wohlerspark. Throughout the year, we observed multiple feeding spots in this park with intentionally provided wildlife food, for example, bird food or nuts. However, supplemental foods in the parks also included leftovers, e.g., processed foods or bakery products, both inappropriate food for squirrels with regard to nutrients. We received reports and witnessed first-hand that squirrels in the park foraged on various food items, e.g., fat balls, rice crackers, cookies and even cupcakes. Moreover, peanuts – a non-native food – were frequently offered to squirrels in Wohlerspark. The chemical composition of peanuts, such as a high phosphorus content, is likely to restrict the peanut intake or can lead to nutrient deficiencies in squirrels (Shuttleworth 2000). We did not take any data on trash bin content in this study, but squirrels often inspected them and food intake from trash bins was documented for grey squirrels (Rimbach et al. 2022).
Supplemental foods can make up a significant part of the diet of urban wildlife and induce substantial shifts in the feeding repertoire (Dasgupta et al. 2021; Wist et al. 2022). For example, they accounted for more than half of the stomach content of urban foxes (Contesse et al. 2004). Consequently, abundant supplemental food sources in urban habitats can increase body mass or condition (relation between body mass and a measure of body length) in species that are able to exploit them successfully, as it is the case for, e.g., gulls, chipmunks and many carnivores (Auman et al. 2008; Bateman and Fleming 2012; Lyons et al. 2017). On the other hand, effects on health and fitness of wildlife can be ambiguous and an urbanized diet can have multiple side-effects (Andersson et al. 2015; Murray et al. 2015; Pollock et al. 2017). This depends on further parameters, such as supplemental food quality and the simultaneous availability and quality of natural foods sources (Shuttleworth 2000; Birnie-Gauvin et al. 2017). We have indication for lower body condition in the highly supplemented Wohlerspark (Suppl. Table 6, Suppl. Figure 1), possibly due to unsuitable food types and a lower availability of natural food sources. Body mass in squirrels can be age dependent, but usually varies with season and food availability (Wauters and Dhondt 1989a). We do not have indications for seasonal food shortages, i.e., body mass changes over the year in the Wohlerspark population (Suppl. Table 7, Suppl. Figure 2).
A study from Poland also documented lower body condition in urban squirrels when compared to forest individuals (Beliniak et al. 2022), though another recent study found higher body masses in urban squirrels (Tranquillo et al. 2023). These ambiguous results comply with studies on other species. House sparrows (Passer domesticus), for example, ranged in studies from no difference in body condition between urban and rural individuals to a significantly decreased body condition in urban individuals (Bókony et al. 2010; Meillère et al. 2017; Jiménez-Peñuela et al. 2019). We suggest that the heterogeneity of study sites, particularly in local food abundance and quality might have contributed to these findings, as we were able to show that even closely located patches can already differ substantially. Differences in city size, park area, human population density as well as socio-economical or cultural factors can influence natural food availability and also the amount of refuse or active wildlife feeding (Fuller et al. 2008; Aronson et al. 2017; Hassell et al. 2021). Furthermore, urban wildlife might also face different levels of intra- and interspecific competition for food sources. We frequently observed other species in high numbers in the parks, such as crows, jays and doves, but also rats. Most of these species interacted with the squirrels, particularly at feeding spots, and pillaged squirrel hoards.
Increased population density is a common feature in successful urban species, including squirrels, and strongly driven by the availability of supplemental food sources (Luniak 2004; McCleery 2010; Shochat et al. 2010; Jokimäki et al. 2017). Interestingly, species densities even seem to be highest in green patches surrounded by the densest urban infrastructure. Probably because the matrix does not offer enough other habitat options, supplemental feeding occurs and numbers of natural predators are often reduced (Luniak 2004; Parker and Nilon 2012). Local squirrel densities and habitat use at rural sites usually vary with forest stand structure, tree diversity and the proportion of conifers, representing food availability (Wauters et al. 2008; Rima et al. 2010; Flaherty et al. 2012; Dylewski et al. 2021). As expected, squirrel densities in this study were higher at all urban sites when compared to the forest, but with distinct variation between the parks. The park with the highest amounts and diversity of supplemental foods also had highest squirrel population density, despite natural food sources might have been limited.
So far, studies in rural habitats, forest fragments, or larger urban parks, have found that densities of S. vulgaris rarely exceed 2.0 individuals/ha (Wauters et al. 1994, 1997; Magris and Gurnell 2002; Bosch and Lurz 2011; Rezouki et al. 2014; Haigh et al. 2015; Beliniak et al. 2021, 2022). Yet densities of grey squirrels (Sciurus carolinensis) and other synurban species can be up to 100 times higher in urban than in rural habitats (Rodewald and Gehrt 2014; Merrick et al. 2016). Our results for the Wohlerspark population even exceeded this level in the autumn trapping session. However, this might have been influenced by hoarding behaviour of squirrels in autumn, where foraging distances increase and thus individuals are more likely to be caught (Bertolino et al. 2004), together with a lower number of autumn trapping days in the forest location were trapping success was always low or even absent in winter.
Reliable conclusions about differences in population structures or breeding individuals were hampered by low absolute squirrel numbers in most sites. Generally, mammal species successful in urban habitats have larger litters (Santini et al. 2019). Yet, urbanisation can decrease the reproductive success within a species (de Satgé et al. 2019). However, the juvenile to adult ratio in all parks of our study was high and we assume that these individuals were locally born. Year-round access to abundant supplemental foods might enable squirrels to allocate more resources into reproduction (Wauters et al. 1995, 1997), although this possibly might contribute to the observed lower investment into body condition in highly supplemented parks. Higher proportions of juveniles within urban populations were also found in other studies on urban squirrels (Beliniak et al. 2022). The high recapture rate of juveniles and subadults as adults in following seasons in Wohlerspark might indicate high offspring survival and successful establishment in the parks, again possibly supported by the high amounts of supplemental foods and higher tree numbers, i.e. higher shelter availability. Furthermore, urban fragmentation can limit dispersal, and dispersal distances in urban squirrels are rather short (Selonen et al. 2018).
Our results provide insight into differences between populations living in close proximity in the same urban core area, though we acknowledge the imbalance in trapping effort and success among the sites and seasons in our study. Living in an urban park with high access to supplemental food seems to support high population densities, however might not necessarily improve other fitness relevant traits. Urban grey squirrels, for example, showed poorer physiological condition represented by higher blood glucose levels and lower levels of, e.g., potassium resulting from an anthropogenic diet (Schmidt et al. 2019). We predict that there might be some kind of threshold where positive effects of urban conditions are outweighed by the costs of other urban factors, such as malnutrition of the urban diet or negative side-effects of high population densities.
Future studies should address long-term fitness consequences, since reproductive success and survival are influenced by food availability and body mass (Wauters and Dhondt 1989b; Wauters et al. 1995) and there might be different effects on different life stages. Urban planning and conservation actions should focus on ensuring sufficient natural food availability for urban wildlife such as squirrels, e.g., by increasing tree diversity and particularly conifer numbers. It is also crucial to inform the human population about suitable supplemental feeding and possible threats to urban wildlife. Reducing littering and limiting the access to trash bins might be beneficial to prevent animals from foraging on refuse and non-natural food items. These measures might also assist in preventing the spread of wildlife diseases at clumped feeding spots and onto humans.
Conclusions
Our study highlights the importance of studying the response of wildlife to urban conditions not only on a large, but also on a small scale. Differences in population parameters between different urban habitats within the same city can be substantial, even between parks with similar area and similar type of surrounding urban matrix. Small parks can hold surprisingly high population densities, likely dependent on vegetation structure and the availability of supplemental food sources and shelters. However, we cannot exclude that these habitats might also prove to be ecological traps on a long-term basis. Further studies are necessary investigating underlying mechanisms and consequences on long-term survival at those sites. It is also crucial to consider the urban heterogeneity when drawing conclusions from urban studies and deciding about appropriate conservation actions for a certain site.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alberti M, Palkovacs EP, Roches SD, Meester LD, Brans KI, Govaert L, Grimm NB, Harris NC, Hendry AP, Schell CJ, Szulkin M, Munshi-South J, Urban MC, Verrelli BC (2020) The complexity of urban eco-evolutionary dynamics. Bioscience 70(9):772–793. https://doi.org/10.1093/biosci/biaa079
Andersson M, Wang HL, Nord A, Salmon P, Isaksson C (2015) Composition of physiologically important fatty acids in great tits differs between urban and rural populations on a seasonal basis. Front Ecol Evol 3:93. https://doi.org/10.3389/fevo.2015.00093
Aronson MF, Lepczyk CA, Evans KL, Goddard MA, Lerman SB, MacIvor JS, Nilon CH, Vargo T (2017) Biodiversity in the city: key challenges for urban green space management. Front Ecol Environ 15(4):189–196. https://doi.org/10.1002/fee.1480
Auman HJ, Meathrel CE, Richardson A (2008) Supersize me: does anthropogenic food change the body condition of silver gulls? A comparison between urbanized and remote, non-urbanized areas. Waterbirds 31(1):122–126. https://doi.org/10.1675/1524-4695(2008)31[122:Smdafc]2.0.Co;2
Bateman PW, Fleming PA (2012) Big city life: carnivores in urban environments. J Zool 287(1):1–23. https://doi.org/10.1111/j.1469-7998.2011.00887.x
Beliniak A, Krauze-Gryz D, Jasińska K, Jankowska K, Gryz J (2021) Contrast in daily activity patterns of red squirrels inhabiting urban park and urban forest. Hystrix 32(2):159–164. https://doi.org/10.4404/hystrix-00476-2021
Beliniak A, Gryz J, Klich D, Jasińska K, Krauze-Gryz D (2022) Body condition and breeding of urban red squirrels: comparison of two populations affected by different levels of urbanization. Animals 12(23):3246. https://doi.org/10.3390/ani12233246
Bertolino S, Mazzoglio PJ, Vaiana M, Currado I (2004) Activity budget and foraging behavior of introduced Callosciurus finlaysonii (Rodentia, Sciuridae) in Italy. J Mammal 85(2):254–259. https://doi.org/10.1644/bpr-009
Birnie-Gauvin K, Peiman KS, Gallagher AJ, de Bruijn R, Cooke SJ (2016) Sublethal consequences of urban life for wild vertebrates. Environ Rev 24(4):416–425. https://doi.org/10.1139/er-2016-0029
Birnie-Gauvin K, Peiman KS, Raubenheimer D, Cooke SJ (2017) Nutritional physiology and ecology of wildlife in a changing world. Conserv Physiol 5(1):cox046. https://doi.org/10.1093/conphys/cox030
Bókony V, Kulcsár A, Liker A (2010) Does urbanization select for weak competitors in house sparrows? Oikos 119(3):437–444. https://doi.org/10.1111/j.1600-0706.2009.17848.x
Bosch S, Lurz PW (2011) Das Eichhörnchen. WestarpWissenschaften.Verlagsgesellschaft mbH, Hohenwarsleben, Germany
Boutin S (1990) Food supplementation experiments with terrestrial vertebrates: patterns, problems, and the future. Can J Zool 68(2):203–220. https://doi.org/10.1139/z90-031
Bundesamt für Naturschutz (2019) Natura 2000 Gebiete in Deutschland. NSG Hahnheide. https://www.bfn.de/natura-2000-gebiet/nsg-hahnheide. Accessed 17 July 2023
Contesse P, Hegglin D, Gloor S, Bontadina F, Deplazes P (2004) The diet of urban foxes (Vulpes vulpes) and the availability of anthropogenic food in the city of Zurich, Switzerland. Mamm Biol 69(2):81–95. https://doi.org/10.1078/1616-5047-00123
Dantzer B, McAdam AG, Humphries MM, Lane JE, Boutin S (2020) Decoupling the effects of food and density on life-history plasticity of wild animals using field experiments: insights from the steward who sits in the shadow of its tail, the north American red squirrel. J Anim Ecol 89(11):2397–2414. https://doi.org/10.1111/1365-2656.13341
Dasgupta D, Banerjee A, Karar R, Banerjee D, Mitra S, Sardar P, Karmakar S, Bhattacharya A, Ghosh S, Bhattacharjee P, Paul M (2021) Altered food habits? Understanding the feeding preference of free-ranging gray langurs within an urban settlement. Front Psychol 12:649027. https://doi.org/10.3389/fpsyg.2021.649027
de Satgé J, Strubbe D, Elst J, De Laet J, Adriaensen F, Matthysen E (2019) Urbanisation lowers great tit Parus major breeding success at multiple spatial scales. J Avian Biol 50(11). https://doi.org/10.1111/jav.02108
Di Pierro E, Ghisla A, Wauters LA, Molinari A, Martinoli A, Gurnell J, Tosi G (2010) The effects of seed availability on habitat use by a specialist seed predator. Eur J Wildl Res 57(3):585–595. https://doi.org/10.1007/s10344-010-0469-7
Dylewski Ł, Jagodziński AM, Tomaś Ł, Myczko Ł, Flaherty S (2021) Forest stand structure and cone crop affect winter habitat use by eurasian red squirrel (Sciurus vulgaris). For Ecol Manage 502:119705. https://doi.org/10.1016/j.foreco.2021.119705
Fingland K, Ward SJ, Bates AJ, Bremner-Harrison S (2021) A systematic review into the suitability of urban refugia for the eurasian red squirrel Sciurus vulgaris. Mamm Rev 52(1):26–38. https://doi.org/10.1111/mam.12264
Flaherty S, Patenaude G, Close A, Lurz PWW (2012) The impact of forest stand structure on red squirrel habitat use. Forestry 85(3):437–444. https://doi.org/10.1093/forestry/cps042
Francis RA, Chadwick MA (2012) What makes a species synurbic? Appl Geogr 32(2):514–521. https://doi.org/10.1016/j.apgeog.2011.06.013
Freie H, Hamburg B, Altona (2017) Dezernat Wirtschaft, Bauen und Umwelt, Management des öffentlichen Raumes, Abteilung Stadtgrün Baumbestandspläne
Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ (2008) Garden bird feeding predicts the structure of urban avian assemblages. Divers Distrib 14(1):131–137. https://doi.org/10.1111/j.1472-4642.2007.00439.x
Galbraith JA, Beggs JR, Jones DN, Stanley MC (2015) Supplementary feeding restructures urban bird communities. Proc Natl Acad Sci 112(20):E2648–E2657. https://doi.org/10.1073/pnas.1501489112
Gillman SJ, McKenney EA, Lafferty DJR (2021) Human-provisioned foods reduce gut microbiome diversity in American black bears (Ursus americanus). J Mammal. https://doi.org/10.1093/jmammal/gyab154
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319(5864):756–760. https://doi.org/10.1126/science.1150195
Gurnell J, Wauters LA, Lurz PWW, Tosi G (2004) Alien species and interspecific competition: effects of introduced eastern grey squirrels on red squirrel population dynamics. J Anim Ecol 73(1):26–35. https://doi.org/10.1111/j.1365-2656.2004.00791.x
Haigh A, O’Riordan R, Butler F (2015) The preference for yew (Taxus baccata) by a red (Sciurus vulgaris) only squirrel population. Wildl Res 42(5):426–436. https://doi.org/10.1071/wr15009
Hassell JM, Bettridge JM, Ward MJ, Ogendo A, Imboma T, Muloi D, Fava F, Robinson TP, Begon M, Fèvre EM (2021) Socio-ecological drivers of vertebrate biodiversity and human-animal interfaces across an urban landscape. Glob Chang Biol 27(4):781–792. https://doi.org/10.1111/gcb.15412
Hubert P, Julliard R, Biagianti S, Poulle M-L (2011) Ecological factors driving the higher hedgehog (Erinaceus Europeaus) density in an urban area compared to the adjacent rural area. Landsc Urban Plan 103(1):34–43. https://doi.org/10.1016/j.landurbplan.2011.05.010
Jiménez-Peñuela J, Ferraguti M, Martínez-de la Puente J, Soriguer R, Figuerola J (2019) Urbanization and blood parasite Infections affect the body condition of wild birds. Sci Total Environ 651:3015–3022. https://doi.org/10.1016/j.scitotenv.2018.10.203
Jokimäki J, Selonen V, Lehikoinen A, Kaisanlahti-Jokimäki M-L (2017) The role of urban habitats in the abundance of red squirrels (Sciurus vulgaris, L.) in Finland. Urban for Urban Green 27:100–108. https://doi.org/10.1016/j.ufug.2017.06.021
Kopij G (2015) Distribution and abundance of the red squirrel Sciurus vulgaris in an urbanised environment. Acta Mus Sil Sci Nat 63(3):255–262. https://doi.org/10.2478/cszma-2014-0022
Koprowski JL (2002) Handling tree squirrels with a safe and efficient restraint. Wildl Soc Bull (1973–2006) 30(1):101–103. https://doi.org/10.2307/3784642
Krauze-Gryz D, Gryz J (2015) A review of the diet of the red squirrel (Sciurus vulgaris) in different types of habitats. In Shuttleworth CM, Lurz PWW, and Hayward MW (eds.) Red Squirrels: Ecology, Conservation & Management in Europe, pp. 39–50. London
Lepczyk CA, Aronson MFJ, Evans KL, Goddard MA, Lerman SB, MacIvor JS (2017) Biodiversity in the city: fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. Bioscience 67(9):799–807. https://doi.org/10.1093/biosci/bix079
Luniak M (2004) Synurbization – adaptation of animal wildlife to urban development. Proc 4th Int Symposium Urban Wildl Conserv Tucson, Citeseer: 50–55
Lyons J, Mastromonaco G, Edwards DB, Schulte-Hostedde AI (2017) Fat and happy in the city: eastern chipmunks in urban environments. Behav Ecol 28(6):1464–1471. https://doi.org/10.1093/beheco/arx109
Magris L, Gurnell J (2002) Population ecology of the red squirrel (Sciurus vulgaris) in a fragmented woodland ecosystem on the Island of Jersey, Channel Islands. J Zool 256(1):99–112. https://doi.org/10.1017/s0952836902000134
McCleery R (2010) Urban mammals. In Atikenhead-Peterson. In: Volder AV (ed) Urban ecosystem ecology, vol 55. American Society of Agronomy, Madison, WI, USA, pp 87–102
McDonald RI, Mansur AV, Ascensão F, Colbert Ml, Crossman K, Elmqvist T, Gonzalez A, Güneralp B, Haase D, Hamann M, Hillel O, Huang K, Kahnt B, Maddox D, Pacheco A, Pereira HM, Seto KC, Simkin R, Walsh B, Werner AS, Ziter C (2019) Research gaps in knowledge of the impact of urban growth on biodiversity. Nat Sustain 3(1):16–24. https://doi.org/10.1038/s41893-019-0436-6
Meillère A, Brischoux F, Henry P-Y, Michaud B, Garcin R, Angelier F (2017) Growing in a city: consequences on body size and plumage quality in an urban dweller, the house sparrow (Passer domesticus). Landsc Urban Plann 160:127–138. https://doi.org/10.1016/j.landurbplan.2016.12.014
Merrick MJ, Evans KL, Bertolino S, Shuttleworth C, Lurz P, Gurnell J (2016) Urban grey squirrel ecology, associated impacts, and management challenges. In: The Grey Squirrel: Ecology & Management of an Invasive Species in Europe, 57–77. European Squirrel Initiative, Stoneleigh Park, Warwickshire CV8 2LG UK
Murray M, Cembrowski A, Latham ADM, Lukasik VM, Pruss S, St Clair CC (2015) Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human-wildlife conflict. Ecography 38(12):1235–1242. https://doi.org/10.1111/ecog.01128
Natural Earth. (2023) Free vector and raster map data at naturalearthdata.com. Accessed 13 July 2023
Ogle DH, Doll JC, Wheeler AP, Dinno A (2023) FSA: simple fisheries stock assessment methods. R package version 0.9.4, 26. https://CRAN.R-project.org/package=FSA
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan’. Community ecology package, version 2(9): 1-295
OpenStreetMap® (2023) available under the Open Data Commons Open Database License (ODbL) by the OpenStreetMap Foundation (OSMF) at openstreetmap.org. Accessed 13 July 2023
Parker TS, Nilon CH (2012) Urban landscape characteristics correlated with the synurbization of wildlife. Landsc Urban Plann 106(4):316–325. https://doi.org/10.1016/j.landurbplan.2012.04.003
Pickett ST, Cadenasso ML, Grove JM, Boone CG, Groffman PM, Irwin E, Kaushal SS, Marshall V, McGrath BP, Nilon CH, Pouyat RV, Szlavecz K, Troy A, Warren P (2011) Urban ecological systems: scientific foundations and a decade of progress. J Environ Manage 92(3):331–362. https://doi.org/10.1016/j.jenvman.2010.08.022
Pollock CJ, Capilla-Lasheras P, McGill RAR, Helm B, Dominoni DM (2017) Integrated behavioural and stable isotope data reveal altered diet linked to low breeding success in urban-dwelling blue tits (Cyanistes caeruleus). Sc Rep 7(1):5014. https://doi.org/10.1038/s41598-017-04575-y
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reher S, Dausmann KH, Warnecke L, Turner JM (2016) Food availability affects habitat use of eurasian red squirrels (Sciurus vulgaris) in a semi-urban environment. J Mammal 97(6):1543–1554. https://doi.org/10.1093/jmammal/gyw105
Revelle WR (2023) psych: procedures for personality and psychological research. Northwestern University, Evanston, Illinois. R package version 2.3.3. https://CRAN.R-project.org/package=psych
Reynolds SJ, Galbraith JA, Smith JA, Jones DN (2017) Garden bird feeding: insights and prospects from a north-south comparison of this global urban phenomenon. Front Ecol Evol 5:24. https://doi.org/10.3389/fevo.2017.00024
Rezouki C, Dozieres A, Le Coeur C, Thibault S, Pisanu B, Chapuis JL, Baudry E (2014) A viable population of the European red squirrel in an urban park. PLoS ONE 9(8):e105111. https://doi.org/10.1371/journal.pone.0105111
Rima PC, Cagnin M, Aloise G, Preatoni D, Wauters LA (2010) Scale-dependent environmental variables affecting red squirrel (Sciurus vulgaris meridionalis) distribution. Ital J Zool 77(1):92–101. https://doi.org/10.1080/11250000902766926
Rimbach R, Butler G, Gupte PR, Jäger J, Parker C, Pontzer H (2022) Gray squirrels consume anthropogenic food waste most often during winter. Mamm Biol 103(1):69–81. https://doi.org/10.1007/s42991-022-00326-3
Robb GN, McDonald RA, Chamberlain DE, Bearhop S (2008) Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front Ecol Environ 6(9):476–484. https://doi.org/10.1890/060152
Rodewald AD, Gehrt SD (2014) Wildlife population dynamics in urban landscapes. In: McCleery RA, Moorman CE, Peterson MN (eds) Urban wildlife conservation: theory and practice. Springer, Boston, MA, USA, pp 117–147
RStudio, T. (2023). RStudio: integrated development environment for R. PBC, Boston. MA, USA.
Santini L, González-Suárez M, Russo D, Gonzalez-Voyer A, von Hardenberg A, Ancillotto L (2019) One strategy does not fit all: determinants of urban adaptation in mammals. Ecol Lett 22(2):365–376. https://doi.org/10.1111/ele.13199
Sarkar D (2008) Lattice: multivariate data visualization with R. Springer Science & Business Media, New York, USA
Schleswig-Holsteinische L (2012) Revier Hahnheide, Bestandslagerbücher
Schmidt C, Treberg JR, Kinnunen RP, Garroway CJ (2019) Serum biochemistry suggests grey squirrels (Sciurus carolinensis) have poorer physiological condition in urban settings. https://doi.org/10.1101/2019.12.16.878702. bioRxiv
Selonen V, Wistbacka R, Korpimäki E (2016) Food abundance and weather modify reproduction of two arboreal squirrel species. J Mammal 97(5):1376–1384. https://doi.org/10.1093/jmammal/gyw096
Selonen V, Fey K, Hämäläinen S (2018) Increased differentiation between individuals, but no genetic isolation from adjacent rural individuals in an urban red squirrel population. Urban Ecosyst 21(6):1067–1074. https://doi.org/10.1007/s11252-018-0794-2
Seress G, Liker A (2015) Habitat urbanization and its effects on birds. Acta Zool Acad Sci Hung 61(4):373–408. https://doi.org/10.17109/azh.61.4.373.2015
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci 109(40):16083–16088. https://doi.org/10.1073/pnas.1211658109
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21(4):186–191. https://doi.org/10.1016/j.tree.2005.11.019
Shochat E, Lerman S, Fernández-Juricic E (2010) Birds in urban ecosystems: Population dynamics, community structure, biodiversity, and conservation. In: Aitkenhead-Peterson J, Volder A (eds) Urban Ecosystem Ecology. John Wiley and Sons, New York, NY, USA, p 496
Shuttleworth CM (2000) The foraging behaviour and diet of red squirrels Sciurus vulgaris receiving supplemental feeding. Wildl Biol 6(4):149–156. https://doi.org/10.2981/wlb.2000.010
Simkin RD, Seto KC, McDonald RI, Jetz W (2022) Biodiversity impacts and conservation implications of urban land expansion projected to 2050. Proc Natl Acad Sci 119(12):e2117297119. https://doi.org/10.1073/pnas.2117297119
Singh AK, Singh H, Singh JS (2018) Plant diversity in cities call for assessment and conservation. Curr Sci 115(3):428–435. https://www.jstor.org/stable/26978227
Statistisches Bundesamt DESTATIS (2020) Experimentelle georeferenzierte Bevölkerungszahl. https://www.destatis.de/DE/Service/Statistik-Visualisiert/Bevoelkerung-Geo/_inhalt.html, Accessed 21 April 2023
Tranquillo C, Wauters LA, Santicchia F, Panzeri M, Preatoni D, Martinoli A, Bisi F (2023) The advantage of living in the city: effects of urbanization on body size and mass of native and alien squirrels. https://doi.org/10.1007/s11252-023-01435-8. Urban Ecosyst
Uchida K, Suzuki KK, Shimamoto T, Yanagawa H, Koizumi I (2019) Decreased vigilance or habituation to humans? Mechanisms on increased boldness in urban animals. Behav Ecol 30(6):1583–1590. https://doi.org/10.1093/beheco/arz117
Uchida K, Blakey RV, Burger JR, Cooper DS, Niesner CA, Blumstein DT (2020) Urban biodiversity and the importance of scale. Trends Eco Evol 36(2):123–131. https://doi.org/10.1016/j.tree.2020.10.011
Wauters L, Dhondt AA (1989a) Variation in length and body weight of the red squirrel (Sciurus vulgaris) in two different habitats. J Zool 217(1):93–106. https://doi.org/10.1111/j.1469-7998.1989.tb02477.x
Wauters L, Dhondt AA (1989b) Body weight, longevity and reproductive success in red squirrels (Sciurus vulgaris). J Anim Ecol 28(2):637–651. https://doi.org/10.2307/4853
Wauters LA, Lens L (1995) Effects of food availability and density on red squirrel (Sciurus vulgaris) reproduction. Ecology 76(8):2460–2469. https://doi.org/10.2307/2265820
Wauters LA, Hutchinson Y, Parkin DT, Dhondt AA (1994) The effects of habitat fragmentation on demography and on the loss of genetic variation in the red squirrel. Proc R Soc B: Biol Sci 255(1343):107–111. https://doi.org/10.1098/rspb.1994.0015
Wauters LA, Suhonen J, Dhondt AA (1995) Fitness consequences of hoarding behaviour in the eurasian red squirrel. Proc R Soc B: Biol Sci 262(1365):277–281. https://doi.org/10.1098/rspb.1995.0206
Wauters LA, Somers L, Dhondt A (1997) Settlement behaviour and population dynamics of reintroduced red squirrels Sciurus vulgaris in a park in Antwerp. Belgium Biol Conserv 82(1):101–107. https://doi.org/10.1016/S0006-3207(97)00007-4
Wauters LA, Vermeulen M, Van Dongen S, Bertolino S, Molinari A, Tosi G, Matthysen E (2007) Effects of spatio-temporal variation in food supply on red squirrel Sciurus vulgaris body size and body mass and its consequences for some fitness components. Ecography 30(1):51–65. https://doi.org/10.1111/j.2006.0906-7590.04646.x
Wauters L, Githiru M, Bertolino S, Molinari A, Tosi G, Lens L (2008) Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography 31(1):104–114. https://doi.org/10.1111/j.2007.0906-7590.05251.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York, USA
Wist B, Stolter C, Dausmann KH (2022) Sugar addicted in the city: impact of urbanisation on food choice and diet composition of the eurasian red squirrel (Sciurus vulgaris). J Urban Ecol 8(1). https://doi.org/10.1093/jue/juac012
Zuñiga-Palacios J, Zuria I, Castellanos I, Lara C, Sánchez-Rojas G (2021) What do we know (and need to know) about the role of urban habitats as ecological traps? Systematic review and meta-analysis. Sci Total Environ 780:146559. https://doi.org/10.1016/j.scitotenv.2021.146559
Acknowledgements
We thank the forest rangers (M. Hansen and D. Prigge) of the nature reserve Hahnheide for their support. We are grateful to the following contributors for their valuable help in field work: Bätjer V., Gandrass M., Greiche A., Kampmann M.A., Köthe J., Lindtner M.L., Marechal E., Probst B., Thomas, L. We further acknowledge the help of A. Wulff in creating the map.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge funding by the German Research Foundation (DFG, grant number DA 1013/8 − 1).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Field work and data analysis was performed by B.W. The first draft of the manuscript was written by B.W. with editorial advice by K.H.D. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We followed all applicable institutional and national guidelines for the care and use of animals. The authors complied with the ARRIVE guidelines. All procedures and animal handling were according to the German animal protection law and approved and authorized by the authorities of Hamburg and Schleswig-Holstein (“Hamburger Behörde für Gesundheit und Verbraucherschutz”, permit no. 87/16, 17 November 2016 and extension of the permit to Schleswig-Holstein by the “Ministerium für Energiewende, Landwirtschaft, Umwelt und ländliche Räume des Landes Schleswig-Holstein” (permit of 28 December 2016).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wist, B., Dausmann, K.H. Food availability and population parameters for squirrels differ even in neighbouring urban parks. Urban Ecosyst 27, 531–544 (2024). https://doi.org/10.1007/s11252-023-01468-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01468-z