Abstract
Urbanisation significantly shapes species abundance, diversity, and community structure of invertebrate taxa but the impact on orthoptera remains widely understudied. We investigated the combined effects of spatial, urban landscape and management-related parameters. Additionally, we discussed different sampling strategies. We sampled orthopteran assemblages on green infrastructure associated with the public transport system of Vienna, Austria. Sampled areas include railroad embankments, recreational areas or fallows. Using LMs, (G)LMMs and nMDS, we compared quantitative sampling using transect counts and semi-quantitative sampling which also included observations made off-transects. We found that vegetation type was the most important parameter, whereby structure-rich fallows featured highest species diversities and, together with extensive meadows, highest abundances, while intensive lawns were less suitable habitats. The semi-quantitative data set revealed an underlying species-area-relationship (SAR). Other important but highly entangled parameters were the mowing intensity, vegetational heterogeneity and cover of built-up area in a 250 m radius. Most found species have high dispersal abilities. Urban assemblages are most significantly shaped by management-related parameters on the site itself, which highlights the potential of conservation efforts in urban areas through suitable management. Sites of different vegetation types differ greatly and need adjusted management measures. Urban landscape parameters, such as the degree of soil sealing, appeared less important, likely due to the high dispersal abilities of most observed orthoptera species. The indicated species-area-relationship could be used to prioritize sites for conservation measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation is a major concern in biodiversity research (Miller and Hobbs 2002; McKinney 2002) and comes along with climate change, habitat destruction, invasive species, nutrient loading and pollution, as well as overexploitation (Puppim de Oliveira et al. 2011) as the main drivers of worldwide biodiversity loss (Millennium Ecosystem Assessment 2005; Secretariat of the Convention on Biological Diversity 2006). While 55% of the global human population is living in urban surroundings today, by 2050 approximately 70% are projected to be urban dwelling (Grimm et al. 2008; Roberts 2011; United Nations 2019). Thus, it is very likely that global biodiversity will be even more threatened by urbanisation in the future (Seto et al. 2012). Urbanisation is causing regional extinctions of native species (Czech et al. 2000) and severely affects species richness, species composition and presence of specialists of various invertebrate species such as ants (Egerer et al. 2017; Melliger et al. 2018), wild bees (Matteson et al. 2008; Hernandez et al. 2009; Fortel et al. 2014; Egerer et al. 2017; Cardoso and Gonçalves 2018), butterflies (Blair and Launer 1997; Clark et al. 2007; Lizée et al. 2011; Ramírez-Restrepo and MacGregor-Fors 2017), carabid beetles (Niemelä et al. 2002; Venn et al. 2003) or spiders (Magura et al. 2010; Egerer et al. 2017; Melliger et al. 2018). Gathering detailed knowledge on how urbanisation affects biodiversity is therefore a pre-requisite for effective species conservation in urban environments (McKinney 2002).
Research on the impact of urbanisation on orthopteran species, such as katydids, crickets and grasshoppers, parallel the general trends found in birds and other insect species (Blair and Launer 1997; Clark et al. 2007; Hernandez et al. 2009; Fortel et al. 2014; Van Nuland and Whitlow 2014; Concepción et al. 2016; Ramírez-Restrepo and MacGregor-Fors 2017; Piano et al. 2019). Urbanisation can lead to decreasing species richness (Heß 2001; Marini et al. 2008; Penone et al. 2013; Cherrill 2015; Melliger et al. 2017; Glaw and Hawlitschek 2018; Piano et al. 2019) and abundance (Heß 2001; Penone et al. 2013; Glaw and Hawlitschek 2018; Piano et al. 2019). Additionally, it affects species composition and guild structure in a way that strongly urbanised areas are more homogenised (Piano et al. 2019), feature less large carnivorous species (Hironaka and Koike 2013) and more xerothermophilous species (Heß 2001), as well as more generalists than specialists (Heß 2001; Penone et al. 2013).
Nevertheless, the overall amount of published research on this topic is, at least compared to popular insect groups like wild bees or butterflies, remarkably limited. One reason for these limitations might be that orthoptera react sensitively not only to a single, but to a long series of environmental parameters. Orthoptera in general are known to respond significantly to intensified management, which is associated with decreases in species richness and abundance (Batáry et al. 2007; Braschler et al. 2009; Marini et al. 2009; Humbert et al. 2012), although not all studies confirmed a decline in abundance (Chisté et al. 2016). At the same time, completely refraining from management measures can cause declines in species richness and abundance as well (Kolshorn and Greven 1995; Kenyeres and Szentirmai 2017). Moreover, detrimental effects of habitat fragmentation and isolation on orthopteran abundances were reported (Herrmann 1995; Sachteleben et al. 2007; Cherrill 2010; Zulka et al. 2014). Several studies postulated the underlying presence of species-area-relationships (SARs) for orthoptera (Collinge 2000; Sachteleben et al. 2007; Nufio et al. 2009; Marini et al. 2010; Melliger et al. 2017), but others could not confirm a SAR within man-made habitats (Heß 2001). An increase in orthopteran species might result from an increase in available habitat types (Herrmann 1995), which is indeed known to influence orthopteran diversity (Marini et al. 2010; Schirmel et al. 2010; Essl and Dirnböck 2012). Other postulated factors are plant species diversity (Essl et al. 2013), structural diversity (Herrmann 1995), aridity and/or precipitation (Steck et al. 2007; Lengyel et al. 2016), elevation (Fournier et al. 2017), soil composition (Fournier et al. 2017), continuity in land use (Steck et al. 2007) as well as the land cover types in surrounding areas (Herrmann 1995; Marini et al. 2008).
In consequence, it might be possible that changes in the factors mentioned above, which can be associated with urbanisation, overlay pure urbanisation effects. For example, Cherrill (2010) did not find a significant relationship between species richness and urbanisation, but a strong impact of agricultural intensification. In a later study of the same author, it was advised to characterise urbanisation by the remaining potential habitat matrix rather than by the uninhabitable environment (Cherrill 2015). Furthermore, sampling effort increases around human settlements due to the higher number of resident experts on orthopteran diversity, which leads to more frequent records of orthopteran species in areas with a dense human population (Cantarello et al. 2010).
In short, the relatively small number of studies on urban orthoptera diversity, compared to the high amount of other potential parameters, might cause severe gaps in the understanding of the underlying ecological patterns. To study orthopteran assemblages of urban green infrastructure, we sampled along several traffic lines within the European metropole Vienna (Austria). This provided a unique sampling design, as it included sampling on areas which are not open to the public and were not investigated before. As management-related parameters, we recorded the local vegetation type, mowing intensity and vegetation heterogeneity. To account for species-area-relationships, the patch area was included. To measure the local degree of urbanisation, we measured the amount of built area and green area in a 250 m radius, located around the centre of the sampling site.
By incorporating management-related, spatial and urban landscape parameters, we intended to disentangle and quantify their effects on orthopteran diversity and provide a framework for future conservation measures in urban surroundings. Additionally, we used two separate data sets. One data set was sampled in a strictly quantitative way, based on standardised transects. For the other one, quantitative data was complemented with spontaneous observations of less abundant orthopteran species that could not be observed along transects and during the standardized sampling period. Thus, our additional aim was to shed light on how different sampling approaches (quantitative vs. semi-quantitative) affect the significance of parameters tested and to determine which approach is more sensible to apply for future studies.
Material and methods
Sampling size and urban landscape parameters
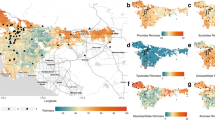
All sampling sites were situated in Vienna (48°12’N, 16°22’E, 151–542 m.a.s.l., 415 km2, 1.88 million inhabitants), the capital of Austria. We sampled 23 sites, located in various parts of the city (see Fig. 1) and covering a total area of 36,983 m2 (min. 260 m2, max. 5,217 m2, on average 1,479 m2 per site; see Table 1). All sampling sites were in close vicinity to areas of the public transport system (i.e., the ‘Wiener Linien’), including railroad embankments (n = 8), decorative and recreational areas in close vicinity to respective stations (n = 7), fallows remaining from previous construction activities (n = 5) and other areas in close vicinity to stations, or other public transport buildings (n = 3). In consequence, about half of our sampling sites (n = 12) were not open to the public.
Locations of 23 sampling sites with 36 transects in Vienna, Austria. The number of transects is indicated by colours; light green = 1 transect, medium green = 2 transects, dark green = three transects. Map credits: Processed in ArcMap 10.6 (ESRI Inc. 2017), administrational borders obtained from the Federal Office of Metrology and Surveying Austria retrieved from the Federal Ministry for Digital and Economic Affairs Austria (n.d.) in November 2020; orthophoto from Basemap Austria (n.d.)
For each sampled site and transect, we recorded data on management parameters, spatial parameters, urban landscape parameters and site-specific parameters (see Table 1). Management intensity and vegetational features varied greatly between sampling sites but formed three general vegetational categories (VEG): fallows (n = 7), which were in general structure-rich and featured ruderal and spontaneous vegetation; extensively managed meadows (n = 3), which were dominated by grasses and other flowering herbaceous plants; and intensive lawns (n = 13) with short, grass-dominated vegetation and little to no flowering aspects. Additionally, mowing frequency was documented from March to August 2019 and classified into three categories (MOW): category 1 (n = 8) with no to one mowing event during the observation period, category 2 (n = 5) with more than one but less than three mowing events (including several areas that were not mown entirely at a certain point in time, e.g. mown twice entirely and a third time only close to the railroad) and category 3 (n = 10) with three or more complete mowing events. Furthermore, to quantify structure-related vegetational features, the presence or absence was noted on each sampling site for 1) bare ground, such as pebbles, earth or sand, 2) short vegetation, i.e., grasses and herbaceous plants with less than 35 cm height, 3) high vegetation for grasses and herbaceous plants which exceeded 35 cm, 4) shrubs, and 5) trees. From that, we calculated each sampling sites vegetational heterogeneity (HET) by summing up the occurrences of the different above-mentioned vegetational characteristics, thus resulting in an ordinal score (from 1 to 5) for each site.
For all sampling sites, urban landscape parameters were analysed based on a land allocation map of the Municipal Department for Surveying and Mapping of the City of Vienna retrieved from the Federal Ministry for Digital and Economic Affairs Austria (n.d.) in April 2019. The map is digitized in 51 different land cover categories, from which we extracted the relative cover of buildings (COVERBUILT) and green areas (COVERGREEN) in a 250 m radius (approx. 196,350 m2) around the centre of the sampling site using ArcMap 10.6 (ESRI Inc. 2017). The 250 m radius included the sampling site itself which made up between 0.1 and 2.7% of the total area, thus the effect of patch area (AREA) on COVERBUILT and COVERGREEN was considered negligible. COVERBUILT and AREA were log-transformed. COVERGREEN and log(COVERBUILT) were not correlated between sampling sites (r(21) = -.65, p = .526).
Sampling methods and data sets
Sampling sites were visited three to four times in total, twice in 2019 (from mid of July to early September) and once or twice in August 2020. Quantitative sampling took place exclusively at the sampling sessions in 2019, along transects of 50 m length and 2 m width. For each sampling site, the number of transects was chosen according to the total patch area and occurrence of vegetational features; therefore, larger sites as well as sites with more vegetational features were represented by more transects. Each of the 36 transects was sampled for 10 min during the warmest hours of the day (between 9 a.m. and 5 p.m.) while avoiding rainy or cloudy days. Species composition and abundance were determined non-invasively via bioacoustic and visual classification. Sweep-netting was conducted at least four times along each transect as well as to capture individual specimen located visually.
In addition to quantitative sampling, occurrences of species observed beside the standard transects as well as species observed during the visits in 2020, when the standard transect approach was not applied, were noted to compile an exhaustive, semi-quantitative species list for each sampling site. In consequence, we gained two different data sets: one transect-based, strictly quantitative data set sampled within one year, and one less standardized but more in-depth data set per sampling site. Lastly, we gathered information on each species dispersal ability from literature (Reinhardt et al. 2005; Marini et al. 2012), categorized in three categories (high dispersal ability, intermediate dispersal ability, low dispersal ability) and deduced information for two missing species from ecologically similar species of the same genus (see Appendix, Table 5).
Statistical analysis
Data analysis was conducted using R 4.0.2 (R Core Team 2020) and confidence intervals were set at 95%, corresponding to a significance level of p ≤ 0.05. The effects of management and urban landscape parameters were investigated using two different approaches: linear methods (Linear Models (LM), Linear Mixed Models (LMM) and Generalized Linear Mixed Models (GLMM)) and non-metric multidimensional scaling (nMDS). Both methods were applied on the quantitative and the semi-quantitative data set. To determine if there was a relationship between raw abundance and prevalence in the quantitative data set, we performed Pearson’s correlations. To check if the semi-quantitative sampling provided higher species occurrences than the quantitative sampling, we performed a one-sided Student’s t-test on the square root transformed species numbers per sampled unit (sampling sites for semi-quantitative sampling, transects for quantitative sampling).
For linear regressions, we calculated the exponential Shannon Index per site for the semi-quantitative data set (exp(H’SQ)) or per transect for the quantitative data set (exp(H’Q)) as dependent variable, therefore assuming a Gaussian error distribution. As independent variables, we considered management parameters (VEG, MOW and HET) as well as urban landscape parameters (log(COVERBUILT), COVERGREEN and log(AREA)). For model selection, covariates were added one by one and models were compared by calculating the Akaike Information Criterion, corrected for small sample sizes (AICc), whereby a ΔAICc ≥ 2 was set as threshold for finally adding a covariate. Additionally, we applied the same procedure and fixed and random factors on a generalized linear mixed model (GLMM) on the total abundance of orthopteran individuals along transects, assuming a Poisson error distribution. For the semi-quantitative data set, we opted for LMs and added the number of transects per sampling site to the management and urban landscape parameters. For the quantitative data set, we used LMMs and fitted the site ID as random term. (G)LMMs were built with (g)lmer() using the lme4 package (Bates et al. 2015). The AICc was calculated with AICc() of the MuMIn package (Barton 2019), which also provided r.squaredGLMM() to calculate the conditional R2-values for the LMMs. Partial (Type III) significance values (χ2-tests) were applied to assess the significance of explanatory terms using the package car (Fox and Weisberg 2019), and LMERConvenienceFunctions (Tremblay and Ransijn 2015) was used for model validation. Furthermore, data visualization was facilitated by packages ggplot2 (Wickham 2016) and viridis (Garnier 2018). To perform the nMDS of the quantitative and semi-quantitative data set, we included a dummy species with an abundance of n = 1 on each site or transect to control for sparse samples (Clarke et al. 2006). The nMDS was performed with a Bray–Curtis similarity as distance measure and two axes, using metaMDS() of the package vegan (Oksanen et al. 2019). Management and urban landscape parameters were fit with envfit() of the vegan package, with 999 permutations. Here, we incorporated the site ID for the quantitative data set and the number of transects for the semi-quantitative data set. Additionally, we used the ordisurf() command for log(COVERBUILT) to account for its non-linear relationship with the nMDS.
Results
Species occurrence, abundance and diversity
With quantitative sampling, we detected 1,453 individuals of 21 orthopteran species, of which eleven belonged to the suborder Ensifera and ten to Caelifera. Species occurrence and abundance within each species correlated highly significantly (r(19) = .94, p < .001), in a way that species that were high in total abundance also were present on more sampling sites. The most abundant species were primarily Caeliferan species, especially Gomphocerinae, such as Euchorthippus declivus (Brisout de Barneville, 1848) (344 individuals on 31 transects), Chorthippus biguttulus (Linnaeus, 1758) (283 individuals on 32 transects), Chorthippus brunneus (Thunberg, 1815) (222 individuals on 27 transects) and Pseudochorthippus parallelus (Zetterstedt, 1821) (145 individuals on 25 transects), along with Calliptamus italicus (Linnaeus, 1758) (143 individuals on 19 transects). The most abundant Ensiferan species were Platycleis grisea (Fabricius, 1781) (48 individuals on 15 transects), followed by Bicoloriana bicolor (Philippi, 1830) (43 individuals on 11 transects) and Phaneroptera nana Fieber, 1853 (21 individuals on 7 transects). Semi-quantitative sampling enhanced the total species number to 25 species, as Aiolopus thalassinus (Fabricius, 1781), Ruspolia nitidula (Scopoli, 1786), Stenobothrus lineatus (Panzer, 1796) and Tetrix tenuicornis (Sahlberg, 1893) were observed exclusively off-transects. Due to the sampling design, quantitative sampling registered less species per sampled unit than semi-quantitative sampling (t(39.7) = -1.99, p = .027; see Fig. 2). Overall, 2,043 observations were made for the semi-quantitative sampling (590 records made aside from quantitative sampling). Of the 25 orthopteran species observed during the entire sampling process, 18 (72%) had high, 5 (20%) intermediate and 2 (8%) low dispersal abilities. All in all, they represent about 28% of all orthoptera ever observed in the metropole of Vienna (Wöss et al. 2020).
Linear regressions
For modelling Shannon diversity exp(H’Q) of the quantitative data set, the final model with a R2LMMc = .42 χ 2-tests, fitted VEG and COVERGREEN as fixed factors and site ID as random term (see Table 2). According to the χ 2-tests, VEG was significant (χ2(2,36) = 20.26, p < .001) and intensive lawn resulted in lowest exp(H’Q), structure-rich fallows featured highest exp(H’Q) and extensive meadow led to intermediate exp(H’Q). COVERGREEN was not significant (χ2(1,36) = 2.57, p = .11), but showed that lower COVERGREEN led to higher exp(H’Q). The model on the total abundance of individuals along transects fitted exclusively VEG as significant factor (χ2(2,36) = 33.23, p < .001), where extensive meadow and structure-rich fallow were associated with higher species abundances. The final model on exp(H’SQ) of the semi-qualitative data set fitted VEG and log(AREA) with an adjusted R2 = .64 (see Table 3). The factor level extensive meadow of VEG differed significantly (t(19) = .38, p < .001) from the reference category, intensive lawn, while structure-rich fallows showed no significant difference. Additionally, log(AREA) influenced exp(H’SQ) in a way that larger areas featured a significantly higher species diversity (t(19) = .86, p = .025). Illustrations of relationships between dependent variables and significant independent variables can be found in the Appendix (see Appendix, Fig. 5).
Non-metric multidimensional scaling
For the quantitative data set, a two-dimensional solution with Stress = .19 was found (see Fig. 3). The envfit analysis revealed that VEG, MOW and site ID affected species abundances per transect (see Table 4). Plotting VEG into the nMDS revealed remarkable overlapping between intensive lawn, extensive meadow and structure-rich fallow. MOW showed clearer clustering of the sampled transects, and especially intensive mowing led to more similar species compositions, while the species compositions on transects with little to no mowing were least aggregated. For the nMDS of the semi-quantitative data set with Stress = .13, envfit analysis illustrated VEG, MOW, HET, log(AREA) and log(COVERBUILT) as significant variables (see Fig. 4; Table 4). Sampling sites with different VEG were more clearly separated from each other than in the nMDS of the quantitative data set. Concerning MOW, intensive mowing regimes were again more clustered than the others but showed more data points outside the cluster than for the quantitative data set. Sampling points with lower HET were more aggregated and in general, lower to higher heterogeneity categories were ordered along a similar axis to the vector of log(AREA). The surface of log(COVERBUILT) featured two to three elliptical centres (see Fig. 4).
Two-dimensional nMDS for the species composition of each sampling site of the semi-quantitative data set, with Stress = .13. The permutation procedure of the envfit analysis featured the management parameters vegetation category (VEG), mowing intensity (MOW) and heterogeneity category (HET). Additionally, the envfit analysis revealed the urban landscape parameters log-transformed patch area (log(AREA)) and log-transformed relative cover of buildings in a 250 m radius around the sampling site (log(COVERBUILT) as significant. While the first showed a linear relationship with the nMDS results and is therefore displayed as vector, the latter was non-linear and needed to be fitted as surface using ordisurf
Discussion
Management-related parameters
Of the three tested management-related parameters, vegetation type consistently had significant effects on orthoptera species diversity and individual abundance. Structure-rich fallows were associated with high species diversity compared to intensive lawns, and extensive meadows featured intermediate species diversity. On structure-rich fallows and extensive meadows, significantly more individuals were found than on intensive lawns. Mowing intensity and vegetational heterogeneity were not included as relevant parameters in the final models explaining species diversity. In case of mowing intensity, this contradicts several previous studies, which found a significant reduction of abundance (Humbert et al. 2010, 2012; Chisté et al. 2016) and species diversity with increased mowing and management intensity (Sachteleben et al. 2007; Marini et al. 2008, 2009), or vice versa. The importance of habitat diversity within each sampling site, which we aimed to reflect by categorizing the vegetational heterogeneity, was highlighted before as well (Marini et al. 2010; Schirmel et al. 2010; Essl and Dirnböck 2012; Cherrill 2015). Despite not explaining species diversity and abundance, both mowing intensity and vegetational heterogeneity affected species composition, where they were highly entangled with each other as well as with vegetation type. We assume, that if more sampling sites of similar vegetation types were inspected, it is very likely, that one would indeed find effects of mowing intensity and vegetational heterogeneity. Additionally, to focus on the effects of mowing and habitat diversity within sampling sites of the same vegetation type, two further temporal parameters could be used in future studies: firstly, it might be sensible to estimate the time gap between the last mowing event and the sampling date, as species diversity and abundance decrease directly after mowing events due to enhanced mortality, emigration and changes in microclimate (Gardiner and Hassall 2009; Humbert et al. 2010; Kenyeres and Szentirmai 2017), and increase again with time from the last cut (Chisté et al. 2016). Secondly, species richness and/or density could be affected by the age of the specific habitat, which is proven for non-urban environments (Kohlmann 1996; Badenhausser and Cordeau 2012; Fartmann et al. 2012).
Spatial and urban landscape parameters
Of all parameters which cannot be altered through management, patch area seemed to be the most relevant parameter. Larger areas were associated with higher species diversities, at least for the semi-quantitative data set. The indicated existence of a species-area-relationship (SAR) for orthopteran species has been postulated by several other studies before (Collinge 2000; Sachteleben et al. 2007; Nufio et al. 2009; Marini et al. 2010), although only one was conducted in an urban environment (Melliger et al. 2017). However, SARs might be less obvious in urban surroundings and only become visible when very large green areas are considered (Heß 2001). Another study conducted in isolated, xerothermic habitats inferred that the rise in species numbers is less influenced by the patch area itself but rather by the increase in different vegetational structures coming along with the increase in patch area (Herrmann 1995). To discriminate between the effect of patch area and the effect of structural heterogeneity, we implemented management-related parameters. Nevertheless, we did not find a SAR for the quantitative data set but only for the semi-quantitative data set. This indicates that the relationship is only significant when species which were not found along transects are integrated into the analysis. Such species were either species occurring in lesser densities, or species that were found on specific structures, such as hedges, that did not lie within the transects.
We investigated two urban landscape parameters: the relative cover of both green area and built-up area in a 250 m radius around the sampling site. Contradicting several previous studies, these parameters appeared rather uninformative. The relative cover of green area was included into the final linear model of the quantitative data set but was not significant. The relative cover of built-up area on the other hand appeared significant in the multivariate analysis of the semi-quantitative data set, nevertheless, the relationship was not linear and featured at least two centres, contradicting Piano et al. (2019), who documented a clear loss in species diversity with higher relative cover of built-up area. But we mostly observed highly mobile species, whose distribution in urban surroundings might be less limited by the surrounding habitat matrix. This was previously demonstrated in other environments (Collinge 2000). Less mobile species might already disappear with slight urbanisation, i.e., along a broader urbanisation gradient than the range we sampled. In addition, we measured urban landscape parameters on a rather small scale, thus focusing on local factors, yet the decline in species diversity through urbanisation is stronger for mobile orthopteran species at larger spatial scales (Penone et al. 2013). For several of the most frequent species, literature provides data on which distances can be overcome: Chorthippus biguttulus (Linnaeus, 1758) for example can cover distances of 300 m, while Chorthippus albomarginatus (De Geer, 1773) can move 500 m between inhabitable patches (Laußmann 1993). The frequent Calliptamus italicus (Linnaeus, 1758) can travel several hundred meters (Detzel 1998). And some species such as Aiolopus thalassinus (Fabricius, 1781) and Chorthippus brunneus (Thunberg, 1815) possibly overcome several kilometres (Laußmann 1993; Maas et al. 2002). Even species with reduced wings can overcome surprisingly large distances by passive transport, for example Meconema meridionale (Costa, 1860) which once was documented to passively travel 360 km on a car (Maas et al. 2002). For such a species, it might be possible to passively spread along the public transport system, too. In any case, a radius of 250 m around the sampling site probably has little effect on the distribution of highly mobile species. Additionally, we see another potential reason why the relative cover of built up or green area in a 250 m radius might matter less to species diversity and abundance: Previous studies indicated, that investigating the relative cover of built-up or green area is oversimplifying the important factors of the urban landscape. Besides focusing more on inhabitable landscape parameters than man-made elements such as the relative built-up cover (Cherrill 2015), it might be more informative to sample the relative cover of identical habitat types instead of the plain relative cover of green area (Haacks 2007).
We conclude that within a radius of 250 m around the sampling sites, we could not find clear effects of urban landscape parameters, albeit there might be underlying processes visible at larger spatial scales. Most sampled species are likely to overcome barriers imposed by the urban landscape through their high mobility, which might be a pre-selection due to the urban environment affecting all our sampling sites. The resulting species pool of highly mobile orthopteran species in urban environments are therefore primarily shaped by local vegetation and management parameters, although our data indicate an underlying non-linear species-area-relationship. This is in line with a previous study by Marini et al. (2010), which demonstrated that orthopteran species richness is more correlated to habitat diversity than to patch area.
In addition, we need to stress that, at least to our knowledge, all available studies on how urbanisation affects orthopteran diversity were performed on a spatial scale (i.e., rural to urban gradients) but not on a temporal scale (i.e., before vs. after urbanisation within a specific area), which highlights the lack of long-term studies on orthoptera in urbanising areas.
Differences between the applied sampling and statistic strategies
We applied two different sampling approaches, i.e., quantitative and semi-quantitative sampling. While the first was strictly focused on pre-defined sampling transects, the latter also included non-systematic observations of further species off-transects and in the consecutive year. We found strong differences between both data sets, caused by species that occur in low densities or that require specific vegetation-related structures that were scattered non-uniformly over the sampling site. Such structures include hedgerows, shrubs, and spots with higher vegetation or bare ground. In our opinion, this stresses the importance of targeted searches on such vegetational structures. We infer, that for investigating orthopteran species diversity in urban surroundings, transect-bound sampling might come along with the high potential to miss certain specialists if transects are not placed properly, i.e., in a way, that all microhabitats of a given sampling site are covered within one transect. However, we found no significant influence of the number of transects per sampling site on its species diversity, indicating that the likelihood of finding more species when simply increasing the standardized sampling intensity was rather low. Thus, it appears like the exact placing of transects crucially affects the sampling outcome and transects placed to fit a wide range of different insect species, such as the transects used in the present study, fail to represent the total orthopteran diversity. Either transects need to be placed very sensibly, covering the specific structures with high importance to structure-bound species that can be expected in urban environments, or additional targeted searches off-transects will remain necessary.
Conclusions
Orthoptera are highly affected by several different parameters, which influence their habitat. However, our findings demonstrated that management-related parameters appear to have a stronger impact on local urban assemblages than urban landscape parameters. This might be due to a pre-selection to (highly) mobile species with strong dispersal abilities to persist in urban surroundings with fragmented and ephemeral habitats.
Especially fallows, which can occur in highly urbanised surroundings, are usually small, temporary, and mostly isolated from similar habitats, were significantly correlated with high abundance and species diversity, further emphasizing the importance of management-related parameters. These findings have strong implications for conservation efforts in urban surroundings to enhance orthopteran species richness, as management is easily influenced and adjusted than the urban landscape itself. This is in line with previous findings on orthopteran diversity (Marini et al. 2008), and arthropod diversity in general (Buchholz et al. 2018).
Despite the importance of our findings for conservation efforts, we summarize, that assemblages of different habitats (i.e., structure-rich fallows, extensive meadows, and intensive lawns) differ greatly and need adjusted management measures. Our results point towards an underlying non-linear species-area-relationship. For conservation measures, larger (and potentially more heterogenous) areas should be prioritized as they likely feature higher species diversity. For future studies on orthopteran assemblages in urban surroundings, it might be advisable to investigate different vegetation types separately. Including more specific information on management parameters, such as the time gap between the sampling date and the last cut, or the age of the specific sampling site, might help to finally disentangle the parameters affecting urban orthopteran assemblages.
Availability of data and material
The authors confirm that the data supporting the findings of this study are partly available within the article and its supplementary materials. Additionally, absolute species numbers per site and R codes for analysis of this study are available from the corresponding author, KH, upon reasonable request.
References
Badenhausser I, Cordeau S (2012) Sown grass strip-A stable habitat for grasshoppers (Orthoptera: Acrididae) in dynamic agricultural landscapes. Agric Ecosyst Environ 159:105–111. https://doi.org/10.1016/j.agee.2012.06.017
Barton K (2019) Multi-model inference. R Package Version 1:43
Basemap Austria (n.d.) basemap.at. https://www.basemap.at/. Accessed 9 Nov 2020
Batáry P, Orci KM, Báldi A et al (2007) Effects of local and landscape scale and cattle grazing intensity on Orthoptera assemblages of the Hungarian Great Plain. Basic Appl Ecol 8:280–290. https://doi.org/10.1016/j.baae.2006.03.012
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Blair RB, Launer AE (1997) Butterfly diversity and human land use: Species assemblages along an urban gradient. Biol Conserv 3207:113–125
Braschler B, Marini L, Thommen GH, Baur B (2009) Effects of small-scale grassland fragmentation and frequent mowing on population density and species diversity of orthopterans: A long-term study. Ecol Entomol 34:321–329. https://doi.org/10.1111/j.1365-2311.2008.01080.x
Buchholz S, Hannig K, Möller M, Schirmel J (2018) Reducing management intensity and isolation as promising tools to enhance ground-dwelling arthropod diversity in urban grasslands. Urban Ecosyst 21:1139–1149. https://doi.org/10.1007/s11252-018-0786-2
Cantarello E, Steck CE, Fontana P et al (2010) A multi-scale study of Orthoptera species richness and human population size controlling for sampling effort. Naturwissenschaften 97:265–271. https://doi.org/10.1007/s00114-009-0636-4
Cardoso MC, Gonçalves RB (2018) Reduction by half: the impact on bees of 34 years of urbanization. Urban Ecosyst 21:943–949. https://doi.org/10.1007/s11252-018-0773-7
Cherrill A (2015) Large-scale spatial patterns in species richness of orthoptera in the greater London area, United Kingdom: Relationships with land cover. Landsc Res 40:476–485. https://doi.org/10.1080/01426397.2014.902922
Cherrill A (2010) Species richness of Orthoptera along gradients of agricultural intensification and urbanisation. J Orthoptera Res 19:293–301. https://doi.org/10.1665/034.019.0217
Chisté MN, Mody K, Gossner MM, Simons NK, Köhler G, Weisser WW, Blüthgen N (2016) Losers, winners, and opportunists: How grassland land-use intensity affects orthopteran communities. Ecosphere 7:1–15. https://doi.org/10.1002/ecs2.1545
Clark PJ, Reed JM, Chew FS (2007) Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst 10:321–337. https://doi.org/10.1007/s11252-007-0029-4
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Bio Ecol 330:55–80. https://doi.org/10.1016/j.jembe.2005.12.017
Collinge SK (2000) Effects of grassland fragmentation on insect species loss, colonization, and movement patterns. Ecology 81(8):2211–2226
Concepción ED, Obrist MK, Moretti M, Altermatt F, Baur B, Nobis MP (2016) Impacts of urban sprawl on species richness of plants, butterflies, gastropods and birds: not only built-up area matters. Urban Ecosyst 19(1):225–242. https://doi.org/10.1007/s11252-015-0474-4
Czech B, Krausman PR, Devers PK (2000) Economic associations among causes of species endangerment in the United States. Bioscience 50(7):593–601
Detzel P (1998) Die Heuschrecken Baden-Württembergs. Ulmer, Stuttgart, Germany
Egerer MH, Arel C, Otoshi MD et al (2017) Urban arthropods respond variably to changes in landscape context and spatial scale. J Urban Ecol 3(1):1–10. https://doi.org/10.1093/jue/jux001
ESRI Inc. (2017) ArcGIS Desktop 10.6
Essl F, Dirnböck T (2012) What determines Orthoptera species distribution and richness in temperate semi- natural dry grassland remnants? Biodivers Conserv 21(10):2525–2537. https://doi.org/10.1007/s10531-012-0315-1
Essl F, Moser D, Dirnböck T et al (2013) Native, alien, endemic, threatened, and extinct species diversity in European countries. Biol Conserv 164:90–97. https://doi.org/10.1016/j.biocon.2013.04.005
Fartmann T, Krämer B, Stelzner F, Poniatowski D (2012) Orthoptera as ecological indicators for succession in steppe grassland. Ecol Indic 20:337–344. https://doi.org/10.1016/j.ecolind.2012.03.002
Federal Ministry for Digital and Economic Affairs Austria (n.d.) Open Data Austria. https://www.data.gv.at/
Fortel L, Henry M, Guilbaud L et al (2014) Decreasing abundance, increasing diversity and changing structure of the wild bee community (hymenoptera: anthophila) along an urbanization gradient. PLoS ONE 9(8):e104679. https://doi.org/10.1371/journal.pone.0104679
Fournier B, Mouly A, Moretti M, Gillet F (2017) Contrasting processes drive alpha and beta taxonomic, functional and phylogenetic diversity of orthopteran communities in grasslands. Agric Ecosyst Environ 242:43–52. https://doi.org/10.1016/j.agee.2017.03.021
Fox J, Weisberg S (2019) An R companion to applied regression. R package version 3.0‐3
Gardiner T, Hassall M (2009) Does microclimate affect grasshopper populations after cutting of hay in improved grassland? J Insect Conserv 13(1):97–102. https://doi.org/10.1007/s10841-007-9129-y
Garnier S (2018) viridis: Default color maps from “matplotlib”. R package version 051
Glaw F, Hawlitschek O (2018) Beobachtungen zur Phänologie und Bestandsentwicklung einer allochthonen Population der Küstenstrauchschrecke (Pholidoptera littoralis) in München. Articulata 33:57–64
Grimm NB, Faeth SH, Golubiewski NE et al (2008) Global change and the ecology of cities. Science 319(5864):756–760. https://doi.org/10.1126/science.1150195
Haacks M (2007) Untersuchungen zu Heuschreckengemeinschaften auf urbanen Brachflächen innerhalb der Freien und Hansestadt Hamburg. Articulata 22:1–16
Hernandez JL, Frankie GW, Thorp RW (2009) Ecology of urban bees: A review of current knowledge and directions for future study. Cities Environ 2(1):1–15. https://doi.org/10.15365/cate.2132009
Herrmann M (1995) Die heuschrecken-gemeinschaften verinselter trockenstandorte in nordwestniedersachsen. Articulata 10:119–139
Heß CH (2001) Habitatwahl und Artenzusammensetzung von Arthropodenpopulationen im urbanen Bereich am Beispiel des Rhein-Main-Ballungsraumes unter besonderer Berücksichtigung der Saltatoria (Doctoral dissertation). Johannes Gutenberg-Universität Mainz
Hironaka Y, Koike F (2013) Guild structure in the food web of grassland arthropod communities along an urban—rural landscape gradient. Écoscience 20(2):148–160. https://doi.org/10.2980/20-2-3575
Humbert JY, Ghazoul J, Richner N, Walter T (2012) Uncut grass refuges mitigate the impact of mechanical meadow harvesting on orthopterans. Biol Conserv 152:96–101. https://doi.org/10.1016/j.biocon.2012.03.015
Humbert JY, Ghazoul J, Richner N, Walter T (2010) Hay harvesting causes high orthopteran mortality. Agric Ecosyst Environ 139(4):522–527. https://doi.org/10.1016/j.agee.2010.09.012
Kenyeres Z, Szentirmai I (2017) Effects of different mowing regimes on orthopterans of Central-European mesic hay meadows. J Orthoptera Res 26(1):29–37. https://doi.org/10.3897/jor.26.14549
Kohlmann T (1996) Zur Heuschreckenfauna auf Ackerbrachen - Veränderungen nach 4 Jahren. Articulata 11:29–35
Kolshorn P, Greven H (1995) Die Heuschreckenfauna auf Grünland- und Heideflächen des Naturschutzgebietes “Lüsekamp und Boschbeek” (Kreis Viersen, NRW) und ihre Beeinflussung durch Nutzung und Pflegemaßnahmen. Articulata 10:141–159
Laußmann H (1993) Die Besiedelung neu entstandener Windwurfflächen durch Heuschrecken. Articulata 8:53–59
Lengyel S, Déri E, Magura T (2016) Species richness responses to structural or compositional habitat diversity between and within grassland patches: A multi-taxon approach. PLoS ONE 11(2):e0149662. https://doi.org/10.1371/journal.pone.0149662
Lizée MH, Mauffrey JF, Tatoni T, Deschamps-Cottin M (2011) Monitoring urban environments on the basis of biological traits. Ecol Indic 11(2):353–361. https://doi.org/10.1016/j.ecolind.2010.06.003
Maas S, Detzel P, Staudt A (2002) Gefährdungsanalyse der Heuschrecken Deutschlands. Verbreitungsatlas, Gefährdungseinstufung und Schutzkonzepte. Bundesamt für Naturschutz, Bonn, Germany
Magura T, Horváth R, Tóthmérész B (2010) Effects of urbanization on ground-dwelling spiders in forest patches. Hungary Landsc Ecol 25(4):621–629
Marini L, Bommarco R, Fontana P, Battisti A (2010) Disentangling effects of habitat diversity and area on orthopteran species with contrasting mobility. Biol Conserv 143(9):2164–2171. https://doi.org/10.1016/j.biocon.2010.05.029
Marini L, Fontana P, Battisti A, Gaston KJ (2009) Agricultural management, vegetation traits and landscape drive orthopteran and butterfly diversity in a grassland-forest mosaic: A multi-scale approach. Insect Conserv Divers 2(3):213–220. https://doi.org/10.1111/j.1752-4598.2009.00053.x
Marini L, Fontana P, Scotton M, Klimek S (2008) Vascular plant and Orthoptera diversity in relation to grassland management and landscape composition in the European Alps. J Appl Ecol 45(1):361–370. https://doi.org/10.1111/j.1365-2664.2007.01402.x
Marini L, Öckinger E, Battisti A, Bommarco R (2012) High mobility reduces beta-diversity among orthopteran communities - implications for conservation. Insect Conserv Divers 5(1):37–45. https://doi.org/10.1111/j.1752-4598.2011.00152.x
Matteson KC, Ascher JS, Langellotto GA (2008) Bee richness and abundance in New York City urban gardens. Ann Entomol Soc Am 101(1):140–150
McKinney ML (2002) Urbanization, Biodiversity, and Conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52(10):883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
Melliger RL, Braschler B, Rusterholz H-P, Baur B (2018) Diverse effects of degree of urbanisation and forest size on species richness and functional diversity of plants, and ground surface-active ants and spiders. PLoS ONE 13(6):e0199245
Melliger RL, Rusterholz HP, Baur B (2017) Habitat- and matrix-related differences in species diversity and trait richness of vascular plants, Orthoptera and Lepidoptera in an urban landscape. Urban Ecosyst 20(5):1095–1107. https://doi.org/10.1007/s11252-017-0662-5
Millennium Ecosystem Assessment MEA (2005) Ecosystems and human well-being. Synthesis (Stuttg)
Miller JR, Hobbs RJ (2002) Conservation where people live and work. Conserv Biol 16(2):330–337. https://doi.org/10.1046/j.1523-1739.2002.00420.x
Niemelä J, Kotze DJ, Venn S et al (2002) Carabid beetle assemblages (Coleoptera, Carabidae) across urban- rural gradients: an international comparison. Landsc Ecol 17(5):387–401
Nufio RC, McClenahan LJ, Thurston GE (2009) Determining the effects of habitat fragment area on grasshopper species density and richness: A comparison of proportional and uniform sampling methods. Insect Conserv Divers 2(4):295–304. https://doi.org/10.1111/j.1752-4598.2009.00065.x
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Vegan: Community ecology package. R package version 2.5–6
Penone C, Kerbiriou C, Julien JF et al (2013) Urbanisation effect on Orthoptera: Which scale matters? Insect Conserv Divers 6(3):319–327. https://doi.org/10.1111/j.1752-4598.2012.00217.x
Piano E, Souffreau C, Merckx T et al (2019) Urbanization drives cross- taxon declines in abundance and diversity at multiple spatial scales. Glob Chang Biol 26(3):1196–1211. https://doi.org/10.1111/gcb.14934
Puppim de Oliveira JA, Balaban O, Doll CNH et al (2011) Cities and biodiversity: Perspectives and governance challenges for implementing the convention on biological diversity (CBD) at the city level. Biol Conserv 144(5):1302–1313. https://doi.org/10.1016/j.biocon.2010.12.007
R Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria
Ramírez-Restrepo L, MacGregor-Fors I (2017) Butterflies in the city: a review of urban diurnal Lepidoptera. Urban Ecosyst 20(1):171–182. https://doi.org/10.1007/s11252-016-0579-4
Reinhardt K, Köhler G, Maas S, Detzel P (2005) Low dispersal ability and habitat specificity promote extinctions in rare but not in widespread species: The Orthoptera of Germany. Ecography (cop) 28(5):593–602. https://doi.org/10.1111/j.2005.0906-7590.04285.x
Roberts L (2011) 9 Billion? Science 333(6042):540–544. https://doi.org/10.1787/9789264202085-graph4-en
Sachteleben J, Hartmann P, Marschalek H et al (2007) Reagieren Heuschrecken auf die Aushagerung von Grünlandflächen? Ergebnisse einer neunjährigen Studie im Alpenvorland. Articulata 22:192–152
Schirmel J, Blindow I, Fartmann T (2010) The importance of habitat mosaics for Orthoptera (Caelifera and Ensifera) in dry heathlands. Eur J Entomol 107(1):129–132. https://doi.org/10.14411/eje.2010.017
Secretariat of the Convention on Biological Diversity (2006) Global Biodiversity Outlook 2. Montreal
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA 109(40):16083–16088. https://doi.org/10.1073/pnas.1211658109
Steck CE, Bürgi M, Coch T, Duelli P (2007) Hotspots and richness pattern of grasshopper species in cultural landscapes. Biodivers Conserv 16(7):2075–2086. https://doi.org/10.1007/s10531-006-9089-7
Tremblay A, Ransijn J (2015) Model Selection and Post-hoc Analysis for (G)LMER Models. R Package Version 2:10
United Nations (2019) World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420). United Nations, New York
Van Nuland ME, Whitlow WL (2014) Temporal effects on biodiversity and composition of arthropod communities along an urban–rural gradient. Urban Ecosyst 17(4):1047–1060. https://doi.org/10.1007/s11252-014-0358-z
Venn S, Kotze DJ, Niemela J (2003) Urbanization effects on carabid diversity in boreal forests. Eur J Entomol 100(1):73–80
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis
Wöss G, Denner M, Forsthuber L et al (2020) Insekten in Wien - Heuschrecken. In: Zettel H, Gaal-Haszler S, Rabitsch W, Christian E (eds) Insekten in Wien. Österreichische Gesellschaft für Entomofaunistik (ÖGEF), Wien, p 288
Zulka KP, Abensperg-Traun M, Milasowszky N, Bieringer G, Gereben-Krenn BA, Holzinger W, Hölzler G, Rabitsch W, Reischütz A, Querner P, Sauberer N (2014) Species richness in dry grassland patches of eastern Austria: A multi-taxon study on the role of local, landscape and habitat quality variables. Agric Ecosyst Environ 182:25–36. https://doi.org/10.1016/j.agee.2013.11.016
Acknowledgements
We would like to thank the Wiener Linien for financial support and their employees for assistance during sampling. Additionally, we would like to thank Friedrich Leisch (BOKU Vienna) for statistical advice.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). This research received financial support from our co-operation partner Wiener Linien, the company running the Viennese public transport system.
Author information
Authors and Affiliations
Contributions
KH, BP and MK conceptualized the project and methodology; field work and species identification was conducted by KH und MK; KH wrote the manuscript and performed the statistical analysis. All authors discussed the results and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors have no relevant conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix
Appendix
Illustrations of relationships between dependent variables and significant independent variables: a Exponential Shannon Index of the quantitative data set on vegetation category; b Total abundance of the quantitative data set on vegetation category; c Exponential Shannon Index of the semi-quantitative data set on vegetation category; d Exponential Shannon Index of the semi-quantitative data set on logarithmic patch area
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huchler, K., Pachinger, B. & Kropf, M. Management is more important than urban landscape parameters in shaping orthopteran assemblages across green infrastructure in a metropole. Urban Ecosyst 26, 209–222 (2023). https://doi.org/10.1007/s11252-022-01291-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01291-y