Abstract
Multi-host pathogens that infect various animal species and humans are considered of great importance for public and animal health. Leishmania spp. parasites are a characteristic example of such pathogens. Although leishmaniosis in humans is endemic for about 100 countries around the world it is classified as a neglected tropical disease. There are three main forms of leishmaniosis in humans: cutaneous (CL), visceral (VL) and mucocutaneous leishmaniosis (MCL). Each year, about 30,000 new cases of VL and more than 1 million new cases of CL are recorded. In Europe L. infantum is the dominant species with dogs being reservoir hosts. Apart from dogs, infection has been recorded in various animals, which suggests that other species could play a role in the maintenance of the parasite in nature. Herein we provide an in-depth review of the literature with respect to studies that deal with Leishmania infantum infections in domestic and wild animal species in Europe. Given the fact that domesticated and wild animals could contribute to the incidences of leishmaniosis in humans, the aim of this paper is to provide a comprehensive review which could potentially be used for the development of measures when it comes to the control of the Leishmania infantum parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogens that infect different animal species and humans are of great importance for public health. Among of all human pathogens, 61% are zoonotic, infecting both humans and animals (Taylor et al., 2001). A large proportion of pathogens that use specific animal species as hosts in their biological cycle can also be transmitted and infect other domestic or wild animals, influencing about 77% of livestock species and 90% of carnivores (Cleaveland et al., 2001). Given the notion that these pathogens can spread widely amongst humans or emerge constantly from their reservoirs in nature, zoonotic infections could have a major impact both in public health and the economy (Bowden and Drake, 2013). According to the World Organization for Animal Health, 75% of emerging infectious diseases in humans have their origins in domestic and wild animals (Fong, 2017). Even though many of these diseases are considered to have more than one reservoir hosts in nature, these hosts in many cases are not yet identified (Daszak et al., 2000). Leishmania is a genus of parasites that represents a typical example of a multi-host pathogen, important for both public and animal health (Haydon et al., 2002).
Parasite and transmission
The genus Leishmania consists of dimorphic, vector-borne protozoan-parasites that belong to the order Kinetoplasida and the family Trypanosomatidae and are responsible for the mammalian parasitic disease of leishmaniosis (Dedet, 2002). The species of the genus Leishmania have a complex biological life cycle and use a vertebrate host, bearing the amastigote form of the parasite, that multiplies into the macrophages and into the mononuclear phagocytes and an invertebrate vector (i.e. phlebotomine sand fly) that carries the promastigote form of the parasite (CDC, 2017). Worldwide there are more than 800 known sand fly species, 78 which are proven vectors of the parasite (Killick-Kendrick, 1990; Banuls et al., 2007; Ready, 2010; Akhoundi et al., 2016). Two out of the six described sand fly genera, namely Phlebotomus (Old World) and Lutzomyia (New World) are proven vectors of Leishmania spp. and thus important for human and animal health (Killick-Kendrick, 1999; Zavitsanou et al., 2008). Transmission in nature among the parasite’s vertebrate hosts occurs in most cases by the bite of the female infected sand fly (order Diptera, family Psychodidae; subfamily Phlebotominae) (Ready, 2014; Maroli et al. 2013). Apart from this, there are some exceptional ways of transmission. In humans, the parasites can be transmitted congenitally, sexually, by blood transfusion, by transplants and finally by the sharing of infected needles, a fact that could explain the large number of Leishmania/HIV co-infections (Desjeux, 1999; W.H.O., 2000; Pagliano et al., 2005; Boehme et al., 2006). In dogs, it is believed that transmission can take place by biting, by blood transfusion, sexually and congenitally, with the last two also proven experimentally (Rosypal et al., 2005; Quinnell and Courtenay, 2009; Silva et al., 2009; CFSPH, 2022).
Leishmania species
Globally, there are about 30 species of Leishmania parasites that are transmitted to mammals. These species are divided into 2 subgenera: (1) Leishmania, in which the parasites develop in the midgut and foregut of the vector; and (2) Viannia, in which the parasites undergo further development in the hindgut (Desjeux, 1996). At least 20 of these species are transmitted and can cause disease to humans. Humans are the primary reservoir host for two of these species, i.e., Leishmania donovani and Leishmania. tropica that are recognized as strictly anthroponotic, while the rest of the species are considered zoonotic (Desjeux, 2004; WHO, 2010). Most of the animals do not show any obvious sign of disease, in many cases the parasitic load is very low, and the host response is minimal or cannot be detected. The dog represents an exception to this general rule, as it is a very susceptible animal to the infection. Dogs are considered to be the main reservoir host of L. infantum in nature, and suffer from a severe and fatal disease, canine leishmaniosis (CanL) (WHO, 2010; Gramiccia, 2011). In humans, leishmaniosis (HumL) has three main clinical forms: cutaneous leishmaniosis-CL (localized or diffuse), mucocutaneous leishmaniosis-ML and visceral leishmaniosis-VL. This variability of clinical features is due to the diversity of Leishmania spp. and the immune response of the hosts. On the other hand, infected people can have a silent infection, without any development of symptoms (Ready, 2010; CDC, 2020).
Disease prevalence
Leishmaniosis is endemic in around 100 countries in tropical, subtropical and temperate territories of the world (Alvar et al., 2012; Faiman et al., 2013). Human population that could encounter aa risk in these endemic areas are estimated to be more than one billion (Alvar et al., 2006; Torres-Guerrero et al., 2017). The disease is the third most important vector-borne disease after malaria and lymphatic filariosis (Pennisi, 2015) and although leishmaniosis is estimated to be the cause of the ninth largest burden among individual infectious diseases, it is still one of the world’s most neglected and underreported disease (WHO, 2010; Gramiccia, 2011). Each year, about 30 000 new cases of VL and more than 1 million new cases of CL occur in the endemic zones of the word i.e. Latin America, Africa, India, Middle East and Mediterranean region. The occurrence of leishmaniosis is not uniformly distributed: 90% of VL clinical cases occur in only six countries (i.e. India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil) (Alvar et al., 2012; Gradoni, 2013). On the other hand, CL is more widely distributed, and 90% of the cases occur every year in Afghanistan, Algeria, Iran, Saudi Arabia, Syria, Bolivia, Brazil, Colombia, Nicaragua and Peru (WHO, 2010; Rezvan, 2014). In the Mediterranean area, four Leishmania spp. are present: (1) L. infantum, is the most common one and the causative pathogen for the human VL and CL and the CanL of dogs, (2) L. major causes CL in the North Africa and the Middle East, (3). L. tropica causes CL in Greece, Turkey, the Middle East and North Africa and finally, (4) L. donovani, which is a recently reported species in Cyprus and can cause both VL and CL (Antoniou et al., 2013). Leishmaniosis is endemic in all of the southern European countries and is the only tropical vector-borne disease that has been endemic in that area for decades (Dujardin et al., 2008). There are two major epidemiological entities in Europe: (1) zoonotic VL and CL due to L. infantum infection, being present in all the southern European countries, where dogs are the main reservoir hosts of the parasite, and (2) anthroponotic CL, due to L. tropica infection, occurring sporadically in Greece, with most reported cases being of zoonotic visceral leishmaniοsis (ZVL) (Gradoni, 2013). In the endemic areas of Europe reported clinical cases of HumL range between 0.02 and 0.49/100,000; this means that annually about 700 new clinical cases occur in Europe (Dujardin et al., 2008; Di Muccio et al., 2015). It should be noted that there are many human carriers of the parasite (Michel et al., 2011). In addition, it is estimated that for each reported clinical case of VL there are in addition 30 to 100 subclinical cases which are not reported due to the benign clinical manifestations and the lack of need for hospitalization (Christodoulou et al., 2012; Gradoni, 2013). In Greece, the mean annual incidence for the period 1998–2011 was 0.36/100,000 (Gkolfinopoulou et al., 2013). As for dogs, CanL which is endemic in more than 70 countries worldwide (Solano-Gallego et al., 2011), seroprevalence ranges between 2 and 37% depending on the region and the methods used for detecting the infection (Solano-Gallego et al., 2009; Athanasiou et al., 2012). With the more extensive use of molecular methods for the detection of Leishmanial DNA in canine tissues, has been shown a prevalence of infection between 67 to80% in some foci (Solano-Gallego et al., 2001; Leontides et al., 2002; Moreno and Alvar, 2002).
Wild and synanthropic reservoir hosts of Leishmania spp. in nature
A possible definition for a reservoir host of an infectious agent in nature, is the ecological system where this agent can be maintained permanently, which system, in the case of a vector-borne agent involves one or more vectors and one or more mammalian hosts. Thus, we can say that a reservoir is a mammalian host that is responsible for the long-term preservation of this agent in nature (Ashford, 1996; Haydon et al., 2002). In the case of Leishmania spp., apart from the primary reservoir host, in the same area it is possible for other animals to be infected incidentally- incidental hosts. Incidental hosts are not related to the long-term preservation of the parasite, but under circumstances these host may become secondary reservoir hosts and be the source of infection for the both the vectors and humans (Shaw, 1988; Rotureau, 2006). The distinction between a primary and a secondary reservoir host is not easy (Quinnell and Courtenay, 2009). For a mammal to be incriminated as a primary reservoir host of Leishmania spp. parasites, it must be shown that this mammal is necessary for the preservation of the parasite in nature, which requires extensive ecological studies. Practically, for an animal species to serve as a primary reservoir host, a number of criteria are set by the WHO(1984): Specifically, (1). Such species should have long lifespan and must be of a minimum population in a certain area to serve as a good source of blood in order sand fly vectors to get infected. (2). In a such population, a large proportion of animals should become infected, in some cases over 20% of the population, even though the prevalence of infection varies with season (WHO, 2010). (3). The animals of the population should also be exposed to infection for prolonged periods of time, without particularly showing severe signs of the disease, but should allow for the vectors to become infected though skin or blood (4). Sand flies should have an intense contact with the host animal to facilitate the transmission of the parasite, (5) The parasite species of the animal population should have the potential to infect humans (WHO, 1990). One of the most impressive achievements of the Leishmania spp. parasites is the fact that they successfully parasitize the host’s macrophages, which are in fact the cells that are responsible for killing invaders (Shaw, 1997). These extremely successful parasites infect several mammalian species that belong to several orders: Carnivora, Chiroptera, Cingulata, Hyracoidea, Marsupialia, Perissodactyla, Pilosa, Primata and, Rodentia, (Dantas-Torres, 2007; Roque and Jansen, 2014). Thus, infection with L. donovani has been reported in Sudan in Egyptian mongooses (Herpestes ichneumon) and in the rodent species Mastomys natalensis and Arvicanthis niloticus (Elnaiem et al., 2001) and in Ethiopia in the rodent species Arvicanthis spp., Mastomys erythroleucus and Gerbilliscus nigricaudus (Kassahun et al., 2015). Infected with L. tropica were reported in Ethiopia the rodent species Gerbillus nanus and Acomys spp. (Kassahun et al., 2015), in Egypt the rodent species Gerbillus pyramidum floweri (Shehata et al., 2009) and with L. gerbilli και L. turanica in great gerbils (Rhombomys opimus) in Iran (Akhavan et al., 2010). L infαntum infection has been reported in several rodent species (Meriones persicus, Cricetalus migratorius, Mesocricetus auratus etc.), foxes (Vulpes vulpes), jacals (Canis aureus) and wolves (Canis lupus) in Iran (Mohebali et al., 2005; Fallah et al., 2006), in foxes and jacals in Israel (Baneth et al., 1998), in Norwegian rats (Rattus norvegicus) in Brazil (Lara-Silva et al., 2014). Similarly, several rodent species (R. rattus, Sigmodon hispidus, Thrichomys apereoides etc.) were reported infected with L. mexicana and L. braziliensis in Venezuela (De Lima et al., 2002), Brazil (Oliveira et al., 2005; Marcelino et al., 2011) and Mexico (Canto-Lara et al., 1999).
Reservoir hosts of L. infantum in Europe
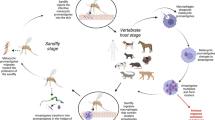
In Europe, the domestic dog is the proven to be the main reservoir host for L. infantum parasites while other animal species can serve as potential hosts. Figure 1 illustrates different host species that have been identified in different European countries. However, the rising incidence of ZVL in endemic areas of the world, is an indication that the currently applied control measures focused on sand fly and dog control, with the latter based on culling of seropositive dogs, or treatment of dogs with permethrin and deltamethrin (Ferroglio et al., 2008), are not entirely effective (Quinnell and Courtenay, 2009; WHO, 2010; Millán et al., 2011). Apart from the problems associated with the implementation of dog culling, the failure of leishmaniosis control, when the measures were focused on the dogs, led to the assumption that there might be alternative reservoir hosts of the parasite and it is possible that a peri-domestic and sylvatic cycle, with different mammalian species as primary reservoirs, can exist concurrently (Sobrino et al., 2008; Maia and Campino, 2011). Various animal species, domestic and wild, have been recorded to be naturally infected with L. infantum. Infection in domestic animals was reported in cats, equids, sheep and goats. A summary of the studies conducted across European countries in different wild and domesticated species that serve as hosts on L. infantum is presented in Table 1.
Host species of Leishmania infantum that have been identified in different European countries: (1) Cat, (2) Horse, (3) Sheep, (4) Goat, (5) Red fox, (6) Grey wolf, (7) Golden jackal, (8) European wildcat, (9) Barbary lion, (10) Tiger, 11. Eurasian otter, 12. Pole cat, 13. European pine marten, 14. Stone marten, 15. Common genet, 16. Iberian lynx, 17. Egyptian mongoose, 18. European badger, 19. European mink, 20. American mink, 21. Brown bear, 22. Common pipistrelle bat 23. Wild rabbit, 24. Hare, 25. Black rat, 26. Norwegian rat, 27. House mouse, 28. Algerian mouse, 29. Wood mouse, 30. Lizard
CYPRUS: 25, 26; FRANCE: 1, 5, 6, 9; GEORGIA: 5, 7; GERMANY: 2; GREECE: 1, 2, 5, 20, 23, 24, 25,26, 27; ITALY: 1, 2, 5, 23, 24, 25, 26, 27, 30; PORTUGAL: 1, 2, 5, 6, 26, 27; SPAIN: 1, 2, 3,4, 5, 6, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 23, 24, 25, 26, 27, 28, 29
Domestic animals
Cats
Over the last century, domestic cats (Felis catus) were generally considered as unusual hosts for Leishmania spp. parasites, with a relatively high natural resistance to infection, possibly due to genetic factors and not strictly related to cell mediated immunity (Mancianti, 2004) and though these animals live in the same habitat with dogs and humans, only sporadic clinical cases of feline leishmaniosis had been reported (Tabar et al., 2008). Recently, advances in feline medicine and developments of sensitive diagnostic tools in serology and molecular analyses, showed that reported clinical and asymptomatic cases of leishmaniosis in cats were underestimated, nevertheless the detected prevalence of leishmaniosis in cats seems to be lower than in dogs as studies indicate in the same areas (Diakou et al., 2009; Maia et al., 2010; Penisi et al., 2015). As a result, there are a few surveys, based on serology and/or PCR that show infection in several European countries, were CanL and HumL are endemic, with prevalence ranging between 0.3 and 60% (Martín-Sánchez et al., 2007; Ayllon et al., 2008; Cardoso et al., 2010; Maia and Campino, 2011; Vilhena et al., 2013; Chatzis et al., 2014). Infected cats were reported in Portugal (Maia et al., 2008), Spain (Miró et al., 2014), Italy (Poli et al., 2002; Iatta et al., 2019), France (Ozon et al., 1998) and Greece (Diakou et al., 2009). The recent results on the susceptibility and the role of cats in the epidemiology of L. infantum in endemic areas remain controversial. There are studies which point out that cats appear to have natural resistance to infection (Diakou et al., 2009), are rare hosts of the parasite and consequently are not a serious threat for public health (Duarte et al., 2010; Miró et al., 2014), or can act as a secondary reservoir host which cannot maintain infection in an area unless there are also present infected dogs (Penisi et al., 2015). On the other hand, there are studies suggesting that cats are susceptible to the parasite, infectious to sand flies and thus, can have a role not as an accidental host but as an alternative reservoir host of the parasite (Maroli et al., 2007; Martín-Sánchez et al., 2007; Maia et al., 2010; Chatzis et al., 2014; Pennisi and Persichetti, 2018) In any case, the role of cats in the maintenance and transmission of the parasite needs to be further investigated (Spada et al., 2020; Cardoso et al., 2021).
Equids
Leishmania spp. infection in horses, is not uncommon in areas of South and Central America, with L. braziliensis being the identified species (Koehler et al., 2002; Madeira et al., 2006), where it was proposed that horses can acts as reservoirs in peri-urban areas of Brazil (Rolao et al., 2005). In Europe, equine leishmaniosis caused by L. infantum, has been reported in Spain, Portugal, Germany, Italy and Greece, with cutaneous leishmaniosis being the only clinical form described and seroprevalence ranging between 0.3% to14% (Koehler et al., 2002; Solano-Gallego et al., 2003; Rolao et al., 2005; Kouam et al., 2010; Sgorbini et al., 2014). Equids are incidental hosts of the parasite as evidenced by the the rare reported clinical cases, the low rate of infection (Kouam et al., 2010) and the spontaneous healing of the lesions (Koehler et al., 2002). All of these indicate that the immune response of these animals can prevent the progressing of disease (Fernández-Bellón et al., 2006). Nevertheless, leishmaniosis in equids, requires further investigation to clarify the clinical form of infection and the role of these animals in the epidemiology of the disease (Solano-Gallego et al., 2003; Rolao et al., 2005).
Sheep and goats
Leishmania spp. infection in sheep and goats is uncommon and only a few cases have been reported worldwide, as it was described in a sheep in Eastern Transvaal (Leishmania spp.), in a goat in Kenya (most likely L. aethiopica), in goats in Sudan (L. donovani) and in sheep and goats in China (L. infantum) (Williams et al., 1991; Van der Lugt et al., 1992; Mukhtar et al., 2000; Gao et al., 2015). In Europe, infection of sheep and goats was reported in Spain, with seroprevalence of 13.9% and 10.2% respectively (Fisa et al., 1999). As these farm animals share the same bio habitat with humans, their role in the epidemiology of the disease needs to by further investigated and elucidated.
Wild animals
Natural infection in wild animals was reported in various animal species which belonged to the orders of carnivores, chiroptera, lagomorphs, rodents, and squamata reptiles.
Carnivores
Out of carnivores, the species that was the most extensively studied is the red fox (Vulpes vulpes) and a possible reason is that both foxes and dogs are canids, which means that they belong the same family of classification (Canidae) and moreover the red fox is the wild carnivore that is in greater numbers in the European continent (Millán et al., 2014). Red foxes were found positive for Leishmania spp. infection in Spain (Criado-Fornelio et al., 2000; Sobrino et al., 2008), Italy (Verin et al., 2010 ), France (Davoust et al., 2014), Portugal (Semião-Santos et al., 1996; Cardoso, et al., 2015), Georgia (Babuadze et al., 2014) and Greece (Karayiannis et al., 2015), with seroprevalence up to 60% amongst the aforementioned countries. Similarly, molecular analyses indicated a prevalence of infection up to 74.6% (Millán et al., 2014; Piantedosi et al., 2016; Abbate et al., 2019).
Leishmaniosis has also been reported in grey wolves (Canis lupus), golden jackals (Canis aureus), European wildcats (Felis silvestris silvestris), barbary lions (Panthera leo), tigers (Panthera tigris), Eurasian otters (Lutra lutra), pole cats (Mustela putorius), European pine martens (Martes martes), stone martens (Martes foina), common genets (Genetta genetta), Iberian lynxes (Lynx pardinus), Egyptian mongooses (Herpestes ichneumon), European badgers (Meles meles), European minks (Mustela lutreola), American minks (Neovison vison) and in brown bear (Ursus arctos) (Sastre et al., 2008; Millán et al., 2011; Libert et al., 2012; Babuadze et al. 2014; Del Río et al., 2014; Ortuño et al., 2019; Tsakmakidis, et al., 2019; Iatta et al., 2020; Cantos-Barreda et al., 2020). In most of the studies conducted on red foxes, most of the researchers postulate the notion that red foxes could be considered as wild reservoir hosts for Leishmania spp. (Criado-Fornelio et al., 2000; Dipineto et al., 2007; Davoust et al., 2014; Karayiannis et al., 2015). Red foxes live near urban and agricultural areas and are frequently exposed to sand flies. Moreover, studies in Europe showed a preference of Phlebotomus pernicious sand flies to them for their blood meal (Veronesi et al., 2023). Further studies are necessary to assess the role of red foxes and other wild carnivores in the epidemiology of the disease (Sobrino et al., 2008; Cardoso et al., 2021) and therefore the existence of other reservoir hosts, apart from dogs, should not be underestimated (Millán et al., 2011).
Chiroptera
Infection has been recorded in common pipistrelle bats (Pipistrellus pipistrellus) in Spain, when 59.3% were found infected with L. infantum, performing a molecular method (Azami-Conesa, et al., 2020).
Lagomorphs
The lagomorphs that were reported with infection were the wild rabbit (Oryctolagus cuniculus) and the hare species Lepus europaeus, Lepus granatensis and Lepus castroviejoi (Ruiz-Fons et al., 2013; Díaz-Sáez et al., 2014). Infected wild rabbits were found in Spain, Italy and Greece, with a seroprevalence up to 75.4%. Molecular analyses indicated prevalence of infection up to 20.7% (Chitimia et al., 2011; García et al., 2014; Jiménez et al., 2014; Moreno et al., 2014; Abbate et al., 2019; Tsakmakidis, et al., 2019). Infection in hares was reported in Spain, Italy and Greece, with rates reaching 74.1% when serology was used for diagnosis and 43.6% when a molecular method was used (Ruiz-Fons et al., 2013; Moreno et al., 2014; Tsokana et al., 2015; Rocchigiani et al., 2018; Tsakmakidis, et al., 2019).
Research studies on the role of these species implicated, for the first time, the hares as reservoirs in the epidemiology of leishmaniosis and more specifically, in the human leishmaniosis outbreak reported in the area of Madrid, Spain, during July 2009 and December 2012 (Molina et al., 2012; Acre et al., 2013). The high rates of infection reported in hares, combined with their proven ability, under xenodiagnostic studies, to infect sandflies, the fact that they are apparently healthy while infected and thus can sustain a chronic infection and the fact that they inhabit large areas in European continent, makes them a potential wild reservoir for Leishmania spp. in Europe (Molina et al., 2012; Ruiz-Fons et al., 2013; Jiménez et al., 2014). In rabbits on the other hand, results seem to be controversial, were there are studies suggesting that rabbits cannot possibly play the role as reservoirs of L. infantum (Chitimia et al., 2011), were others that rabbits show some of the characteristics expected in a possible reservoir host, as, like the hares they are long lived, at least they survive through one non-transmission period, show no apparent signs of an acute disease (Díaz-Sáez et al., 2014) and can be infective to sandflies, as it was proven with xenodiagnostic studies (Jiménez et al., 2014). In conclusion, most of the researchers agree that the impact of lagomorphs in the epidemiology of leishmaniosis in Europe needs to be further investigated.
Rodents
Infection of wild rodents was reported in black rats (Rattus rattus), Norwegian rats (Rattus norvegicus), house mice (Mus musculus), wood mice (Apodemus sylvaticus) and Algerian mice (Mus spretus). Infected black rats were reported in Italy, Spain, Cyprus and Greece, with a seroprevalence up to 57.5%, while molecular analysis indicated a rate of infection up to45% (Di Bella et al., 2003; Psaroulaki et al., 2010; Zanet et al., 2014; Navea-Pérez et al., 2015; Tsakmakidis et al., 2017). Infection of the Norwegian rat has been reported in Italy, Spain, Portugal, Cyprus and Greece with a seroprevalence up to 70%. In a molecular study conducted in Greece, the prevalence of infection was found to be 6.25% while in a parasitological study conducted in Portugal the prevalence of infection was determined as33.33% (Papadogiannakis et al., 2010; Psaroulaki et al., 2010; Helhazar et al., 2013; Tsakmakidis et al., 2017; Ortuño et al., 2019). House mice were found positive to infection with prevalence up to 50% as indicated in studies conducted in Italy, Portugal, Spain and Greece (Di Bella et al. 2003; Helhazar et al., 2013; Navea-Pérez et al., 2015; Tsakmakidis et al., 2017). Infected wood and Algerian mice were recorded in Spain when serology showed rates of infection 42.85% for Algerian mice and in in molecular studies the rate of infection for the wood mice was 20.8% (Navea-Pérez et al., 2015; Alcover et al., 2020). Most of the researchers agree to the fact that rodents show some of the characteristics of a reservoir host and possibly can have a role in the preservation of the parasite in nature and that it is necessary that research studies should be continued on the field of xenodiagnosis in order to evaluate the ability of these animals to infect the sand fly vectors (Helhazar et al., 2013; Zanet et al., 2014; Navea-Pérez et al., 2015). It must be noted here that high infection rates alone do not ensure that these animals can act as a reservoir host and detection of the parasite’s DNA in the examined tissues does not show conclusively active infection or the ability of the animal to infect sandflies, therefore xenodiagnostic studies are necessary to be applied (Del Río et al., 2014). So far, the ability to infect the sand fly vectors has already been confirmed by xenodiagnostic studies in domestic cats, hares, rabbits and black rats, a fact that increases for these animal species the possibility to act as reservoir hosts (Quinnell and Courtenay, 2009; Molina et al., 2012; Díaz-Sáez et al., 2014; Jiménez et al., 2014).
Squamata reptiles
Confirmed for L. infantum infection were identified by a molecular method 3,1% of examined lizards in Italy (Mendoza-Roldan et al., 2022).
Conclusions
An emerging infectious disease can be described as a previously known or recently recognized infectious disease that showed a recent and fast increase in occurrence, geographic range, or recently moved into new host populations (Morse, 1995; Daszak et al., 2000). Under that definition are included several diseases that pose a serious threat to public health and a burden on global economies. This emergence is mostly resulting from socio-economic, environmental and ecological changes (CDC, 1998; Jones et al., 2008). The main event in most cases of emergence is a change in ecology between the host and the infectious agent. Wild animals are considered an important feature of this emergence, because in many cases are the reservoirs from which zoonotic pathogens can emerge (Daszak et al. 2001).
Human leishmaniosis due to L. infantum infection, is an important emerging zoonosis that shows an increasing frequency and a greater geographic distribution than the previous decades and although the domestic dog is the animal of the greatest importance and the main reservoir host in nature, there are several animal species, domestic and wild that are susceptible to the parasite’s infection (Maia et al., 2015). Even though there are a few studies in which the presence of L. infantum in various animal species has been demonstrated, the importance of these species as reservoirs remains largely unclear (Souza et al., 2014). This role of potential alternative host needs to be further explored, because, as it has been shown in previous cases, under certain circumstances can change and lead to serious public health problems, as it happened in the outbreak of leishmaniosis in Madrid (Arce et al., 2013). Epidemiology of the parasite is more complicated than we believed in the past (Navea-Pérez et al., 2015) and assessment of the possibility of other animals participating in Leishmania’s life circle is important.
Data availability
This is a review article and no experimental data were generated or analysed during the study. The bibliography based on which this manuscript was prepared on, is listed at the end of the manuscript.
References
Abbate JM, Arfuso F, Napoli E, Gaglio G, Giannetto S, Latrofa MS, Otranto D and Brianti E (2019). Leishmania infantum in wild animals in endemic areas of southern Italy. Comparative Immunology, Microbiology & Infectious Diseases, 67: 101374. https://doi.org/10.1016/j.cimid.2019.101374
Acre A, Estirado A, Ordobas M, Sevilla S, García N, Moratilla L, de la Fuente S, Martínez AM, Pérez AM, Aránguez E, Iriso A, Sevillano O and Bernal J (2013). Re-emergence of leishmaniasis in Spain: Community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveillance, 18(30):20546. https://doi.org/10.2807/1560-7917.ES2013.18.30.20546
Akhavan A, Mirhendi H, Khamesipour A, Alimohammadian M, Rassi Y, Bates P, Kamhawi S, Valenzuela JG, Arandian MH, Abdoli H, Jalali-zand N, Jafari R, Shareghi N, Ghanei M and Yaghoobi-Ershadi MR (2010). Leishmania species: Detection and identification by nested PCR assay from skin samples of rodent reservoirs. Experimental Parasitology, 126(4):552–556. https://doi.org/10.1016/j.exppara.2010.06.003
Akhoundi M, Kuhls K, Cannet Votýpka J, Marty P, Delaunay P and Sereno D (2016). A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Neglected Tropical Diseases, 10(3), e0004349. https://doi.org/10.1371/journal.pntd.0004770
Alcover MM, Ribas A, Guillén MC, Berenguer D, Tomás-Pérez M, Riera C, Fisa R (2020). Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area. Preventive Veterinary Medicine, 175:104874. https://doi.org/10.1016/j.prevetmed.2019.104874
Alvar J, Yactayo S and Bern C (2006). Leishmaniasis and poverty. Trends in Parasitology, 22 (12): 552–557. https://doi.org/10.1016/j.pt.2006.09.004
Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J and Den Boer M (2012). Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE, 7(5):e35671. https://doi.org/10.1371/journal.pone.0035671
Antoniou M, Gramiccia M, Molina R, Dvorak V and Volf P (2013). The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Euro Surveillance, 18(30):20540. https://doi.org/10.2807/1560-7917.ES2013.18.30.20540
Ashford RW (1996). Leishmaniasis reservoirs and their significance in control. Clinics in Dermatology, 14(5): 523–532. https://doi.org/10.1016/0738-081X(96)00041-7
Athanasiou LV, Kontos VI, Saridomichelakis MN, Rallis TS and Diakou A (2012). A cross-sectional sero-epidemiological study of canine leishmaniasis in Greek mainland. Acta Tropica, 122(3): 291–295. https://doi.org/10.1016/j.actatropica.2012.02.003
Ayllon T, Tesouro MA, Amusategui I, Villaescusa A, Rodriguez-Franco F and Sainz A (2008). Serologic and molecular evaluation of Leishmania infantum in cats from Central Spain. Annals of the New York Academy of Sciences, 1149(1):361–364. https://doi.org/10.1196/annals.1428.019
Azami-Conesa I, Martínez-Díaz RA, González F and Gómez-Muñoz MT (2020). First detection of Leishmania infantum in common urban bats Pipistrellus pipistrellus in Europe. Research in Veterinary Science, 132: 172–176. https://doi.org/10.1016/j.rvsc.2020.06.019
Babuadze G, Alvar J, Argaw D, de Koning HP, Iosava M, Kekelidze M, Tsertsvadze N, Tsereteli D, Chakhunashvili G, Mamatsashvili T, Beria N, Kalandadze I, Ejov M and Imnadze P (2014). Epidemiology of visceral leishmaniasis in Georgia. PLoS Neglected Tropical Diseases, 8(3):e2725. https://doi.org/10.1371/journal.pntd.0002725
Baneth G, Dank G, Keren-Kornblatt E, Sekeles E, Adini I, Eisenberger CL, Schnur LF, King R and Jaffe CL (1998). Emergence of visceral leishmaniasis in central Israel. The American Journal of Tropical Medicine and Hygiene, 59(5):722–725. https://doi.org/10.4269/ajtmh.1998.59.722
Banuls AL, Hide M and Prugnolle F (2007). Leishmania and the Leishmaniases: A Parasite Genetic Update and Advances in Taxonomy, Epidemiology and Pathogenicity in Humans. Advances in Parasitology, 64:1–109. https://doi.org/10.1016/S0065-308X(06)64001-3
Boehme CC, Hain U, Novosel A, Eichenlaub S, Fleischmann E and Löscher T (2006). Congenital visceral leishmaniasis. Emerging Infectious Diseases, 12(2): 359–360. https://doi.org/10.3201/eid1202.050449
Bowden SE and Drake JM (2013). Ecology of multi-host pathogens of animals. Nature Education Knowledge, 4(8): 5. Available at: https://www.nature.com/scitable/knowledge/library/ecology-of-multi-host-pathogens-of-animals-105288915/
Canto-Lara SB, Van Wynsberghe NR, Vargas-González A, Ojeda-Farfán FF and Andrade-Narváez FJ (1999). Use of monoclonal antibodies for the identification of Leishmania spp. isolated from humans and wild rodents in the State of Campeche, Mexico. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro, 94(3):305–309. https://doi.org/10.1590/S0074-02761999000300005
Cantos-Barreda, A., Navarro, R., Pardo-Marín, L., Martínez-Subiela, S., Ortega, E., Cerón, J. J., Tecles, F., & Escribano, D. (2020). Clinical leishmaniosis in a captive Eurasian otter (Lutra lutra) in Spain: A case report. BMC Veterinary Research, 16: 312. https://doi.org/10.1186/s12917-020-02509-x
Cardoso L, Lopes AP, Sherry K, Schallig H and Solano-Gallego L. (2010). Low seroprevalence of Leishmania infantum infection in cats from northern Portugal based on DAT and ELISA. Veterinary Parasitology, 174(1–2):37–42. https://doi.org/10.1016/j.vetpar.2010.08.022
Cardoso L, Gilad M, Cortes HC, Nachum-Biala Y, Lopes AP, Vila-Viçosa MJ, Simões M, Rodrigues P and Baneth G (2015). First report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasites & Vectors, 8: 144. https://doi.org/10.1186/s13071-015-0756-y
Cardoso L, Schallig H, Persichetti MF and Pennisi MG (2021). New epidemiological aspects of animal leishmaniosis in Europe: The role of vertebrate hosts other than dogs. Pathogens, 10(3):307. https://doi.org/10.3390/pathogens10030307
Center for Food Security and Public Health (CFSPH). (2022). Leishmaniasis (cutaneous and visceral). Iowa State University, College of Veterinary Medicine, Iowa. Available at: https://www.cfsph.iastate.edu/Factsheets/pdfs/leishmaniasis.pdf
Centers for Disease Control and Prevention (CDC). (1998). Preventing emerging infectious diseases: A strategy for the 21st century, overview of the updated CDC plan. MMRW- September 11/Vol. 47/No. RR-15. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00054779.htm
Centers for Disease Control and Prevention (CDC) (2017). Leishmaniasis: Biology. Atlanta: CDC. Available at: http://www.cdc.gov/parasites/leishmaniasis/biology.html
Centers for Disease Control and Prevention (CDC). (2020). Leishmaniasis: Disease. Atlanta: CDC. Available at: http://www.cdc.gov/parasites/leishmaniasis/disease.html
Chatzis MK, Andreadou M, Leontides L, Kasabalis D, Mylonakis M, Koutinas AF, Rallis T, Ikonomopoulos J and Saridomichelakis MN (2014). Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Veterinary Parasitology, 202(3–4): 217–225. https://doi.org/10.1016/j.vetpar.2014.02.044
Chitimia L, Muñoz-García CI, Sánchez-Velasco D, Lizana V, Del Río L, Murcia L, Fisa R, Riera C, Giménez-Font P, Jiménez-Montalbán P, Martínez-Ramírez A, Meseguer-Meseguer JM, García-Bacete I, Sánchez-Isarria MA, Sanchis-Monsonís G, García-Martínez JD, Vicente V, Segovia M and Berriatua E (2011). Cryptic leishmaniasis by Leishmania infantum, a feature of canines only? A study of natural infection in wild rabbits, humans, and dogs in southeastern Spain. Veterinary Parasitology, 181(1): 12–16. https://doi.org/10.1016/j.vetpar.2011.04.016
Christodoulou V, Antoniou M, Ntais P, Messaritakis I, Ivovic V, Dedet JP, Pratlong F, Dvorak V and Tselentis Y (2012). Re-emergence of visceral and cutaneous leishmaniasis in the Greek island of Crete. Vector-Borne and Zoonotic Diseases, 12(3), 214–222. https://doi.org/10.1089/vbz.2011.0004
Cleaveland S, Laurenson MK and Taylor LH (2001). Diseases of humans and their domestic mammals; pathogen characteristics, host range, and the risk of emergence. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 356(1411): 991–999. https://doi.org/10.1098/rstb.2001.0889
Criado-Fornelio A, Gutierrez-Garcia L, Rodriguez-Caabeiro F, Reus-Garcia E, Roldan-Soriano MA and Diaz-Sanchez MA (2000). A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara, Spain. Veterinary Parasitology, 92(4):, 245–251. https://doi.org/10.1016/s0304-4017(00)00329-0
Dantas-Torres F (2007). The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Veterinary Parasitology, 149 (3–4): 139–146. https://doi.org/10.1016/j.vetpar.2007.07.007
Daszak P, Cunningham AA and Hyatt AD (2000). Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science, 287(5452): 443–449. https://doi.org/10.1126/science.287.5452.443
Daszak P, Cunningham AA and Hyatt AD (2001). Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica, 78(2):103–116. https://doi.org/10.1016/S0001-706X(00)00179-0
Davoust B, Mary C and Marié JL (2014). Detection of Leishmania in red foxes (Vulpes vulpes) from Southeastern France using real-time quantitative PCR. Journal of Wildlife Diseases, 50(1): 130–132. https://doi.org/10.7589/2013-07-190
De Lima, H., De Guglielmo, Z., Rodríguez, A., Convit, J., & Rodriguez, N. (2002). Cotton Rats (Sigmodon hispidus) and Black Rats (Rattus rattus) as Possible Reservoirs of Leishmania spp. in Lara State, Venezuela. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro, 97(2): 169–174. https://doi.org/10.1590/S0074-02762002000200004
Dedet JP (2002). Current status of epidemiology of leishmaniases. In J. P. Farrell (Ed.), World class parasites Volume 4 Leishmania (pp. 1–10). Kluwer Academic Publishers.
Del Río L, Chitimia L, Cubas A, Victoriano I, De la Rúa P, Gerrikagoitia X, Barral M, Muñoz-García CI, Goyena E, García-Martínez D, Fisa R, Riera C, Murcia L, Segovia M and Berriatua E. (2014). Evidence for widespread Leishmania infantum infection among wild carnivores in L. infantum periendemic northern Spain. Preventive Veterinary Medicine, 113(4): 430–435. https://doi.org/10.1016/j.prevetmed.2013.12.001
Desjeux P (1996). Leishmaniasis: public health aspects and control. Clinics in Dermatology, 14(5): 417–423. https://doi.org/10.1016/0738-081X(96)00057-0
Desjeux P (1999). Global Control and Leishmania HIV Co-Infection. Clinics in Dermatology, 17(3): 317–325. https://doi.org/10.1016/S0738-081X(99)00050-4
Desjeux P (2004). Leishmaniasis: current situation and new perspectives. Comparative Immunology, Microbiology & Infectious Diseases, 27(5):305–318. https://doi.org/10.1016/j.cimid.2004.03.004
Di Bella C, Vitale F, Russo G, Greco A, Milazzo C, Aloise G and Cagnin M (2003). Are rodents a potential reservoir for Leishmania infantum in Italy? Journal of Mountain Ecology, 7: 125–129. Available at: https://api.semanticscholar.org/CorpusID:54854972
Di Muccio T, Scalone A, Bruno A, Marangi M, Grande R, Armignacco O, Gradoni L and Gramiccia M (2015). Epidemiology of Imported Leishmaniasis in Italy: Implications for a European Endemic Country. PLoS ONE, 10(6):e0129418. https://doi.org/10.1371/journal.pone.0129418
Diakou A, Papadopoulos E and Lazarides K (2009). Specific anti-Leishmania spp. antibodies in stray cats in Greece. Journal of Feline Medicine and Surgery, 11(8):728–730. https://doi.org/10.1016/j.jfms.2008.01.009
Díaz-Sáez V, Merino-Espinosa G, Morales-Yuste M, Corpas-López V, Pratlong F, Morillas-Márquez F and Martín-Sánchez J (2014). High rates of Leishmania infantum and Trypanosoma nabiasi infection in wild rabbits (Oryctolagus cuniculus) in sympatric and syntrophic conditions in an endemic canine leishmaniasis area: Epidemiological consequences. Veterinary Parasitology, 202(3–4): 119–127. https://doi.org/10.1016/j.vetpar.2014.03.029
Dipineto L, Manna L, Baiano A, Gala M, Fioretti A, Gravino AE and Menna LF (2007). Presence of Leishmania infantum in red foxes (Vulpes vulpes) in southern Italy. Journal of Wildlife Diseases, 43(3):518–520. https://doi.org/10.7589/0090-3558-43.3.518
Duarte A, Castro I, Pereira da Fonseca IM, Almeida V, Madeira de Carvalho LM, Meireles J, Fazendeiro MI, Tavares L and Vaz Y (2010). Survey of infectious and parasitic diseases in stray cats at the Lisbon Metropolitan Area, Portugal. Journal of Feline Medicine and Surgery, 12(6): 441–446. https://doi.org/10.1016/j.jfms.2009.11.003
Dujardin JC, Campino L, Cañavate C, Dedet JP, Gradoni L, Soteriadou K, Mazeris A, Ozbel Y and Boelaert M (2008). Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerging Infectious Diseases, 14(7): 1013–1018. https://doi.org/10.3201/eid1407.071589
Elnaiem DA, Hassan MM, Maingon R, Nureldin GH, Mekawi AM, Miles M and Ward RD (2001). The Egyptian mongoose, Herpestes ichneumon, is a possible reservoir host of visceral leishmaniasis in eastern Sudan. Parasitology, 122(5): 531–536. https://doi.org/10.1017/s0031182001007594
Faiman R, Abbasi I, Jaffe C, Motro Y, Nasereddin A, Schnur LF, Torem M, Pratlong F, Dedet JP and Warburg A (2013). A newly emerged cutaneous leishmaniasis focus in northern Israel and two new reservoir hosts of Leishmania major. PLoS Neglected Tropical Diseases, 7(2): e2058. https://doi.org/10.1371/journal.pntd.0002058
Fallah E, Farshchian M, Mazlomi A, Majidi J, Kusha A, Mardi A and Mahdipoorzareh N (2006). Study on the prevalence of visceral leishmaniasis in rodents of Azarshahr district (new focus), northwest of Iran. Archives of Razi Institute, 61(1): 27–33. Available at: https://api.semanticscholar.org/CorpusID:54510394
Fernández-Bellon H, Solano-Gallego L, Bardagí M, Alberola J, Ramis A and Ferrer L (2006). Immune response to Leishmania infantum in healthy horses in Spain. Veterinary Parasitology, 135(2): 181–185. https://doi.org/10.1016/j.vetpar.2005.09.007
Ferroglio E., Poggi M and Trisciuoglio A (2008). Evaluation of 65% Permethrin spot-on and Deltamethrin-impregnated collars for canine Leishmania infantum infection prevention. Zoonoses and Public Health, 55, 145–148. https://doi.org/10.1111/j.1863-2378.2007.01092.x
Fisa R, Gállego M, Castillejo S, Aisa MJ, Serra T, Riera C, Carrió J, Gállego J and Portús M (1999). Epidemiology of canine leishmaniasis in Catalonia (Spain): The example of the Priorat focus. Veterinary Parasitology, 83(2): 87–97. https://doi.org/10.1016/S0304-4017(99)00074-6
Fong IW (2017). Animals and mechanisms of disease transmission. Emerging Zoonoses, 8: 15–38. https://doi.org/10.1007/978-3-319-50890-0
Gao CH, Wang JY, Zhang S, Yang YT and Wang Y (2015). Survey of wild and domestic mammals for infection with Leishmania infantum following an outbreak of desert zoonotic visceral leishmaniasis in Jiashi, People’s Republic of China. PLoS ONE, 10(7): e0132493. https://doi.org/10.1371/journal.pone.0132493
García N, Moreno I, Alvarez J, de la Cruz ML, Navarro A, Pérez-Sancho M, García-Seco T, Rodríguez-Bertos A, Conty ML, Toraño A, Prieto A, Domínguez L and Domínguez M (2014). Evidence of Leishmania infantum infection in rabbits (Oryctolagus cuniculus) in a natural area in Madrid, Spain. BioMed Research International, 2014:318254. https://doi.org/10.1155/2014/318254
Gkolfinopoulou K, Bitsolas N, Patrinos S, Veneti L, Marka A, Dougas G, Pervanidou D, Detsis M, Triantafillou E, Georgakopoulou T, Billinis C, Kremastinou J and Hadjichristodoulou C (2013). Epidemiology of human leishmaniasis in Greece, 1981–2011. Euro Surveillance, 18(29):20532. https://doi.org/10.2807/1560-7917.ES2013.18.28.20532
Gradoni L (2013). Epidemiological surveillance of leishmaniasis in the European Union: operational and research challenges. Euro Surveillance, 18(30):20539. https://doi.org/10.2807/1560-7917.ES2013.18.30.20539
Gramiccia M (2011). Recent advances in leishmaniosis in pet animals: Epidemiology, diagnostics and anti-vectorial prophylaxis. Veterinary Parasitology, 181(1):23–30. https://doi.org/10.1016/j.vetpar.2011.04.019
Haydon DT, Cleaveland S, Taylor LH and Laurenson MK (2002). Identifying reservoirs of infection: a conceptual and practical challenge. Emerging Infectious Diseases, 8(12):1468–1473. https://doi.org/10.3201/eid0812.010317
Helhazar M, Leitão J, Duarte A, Tavares L andda Fonseca IP (2013). Natural infection of synanthropic rodent species Mus musculus and Rattus norvegicus by Leishmania infantum in Sesimbra and Sintra—Portugal. Parasites & Vectors, 6, 88. https://doi.org/10.1186/1756-3305-6-88
Iatta R, Furlanello T, Colella V, Tarallo VD, Latrofa MS, Brianti E, Trerotoli P, Decaro N, Lorusso E, Schunack B, Mirò G, Dantas-Torres F and Otranto D (2019). A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Neglected Tropical Diseases, 13(7): e0007594. https://doi.org/10.1371/journal.pntd.0007594
Iatta R, Zatelli A, Laricchiuta P, Legrottaglie M, Modry D, Dantas-Torres F and Otranto D (2020). Leishmania infantum in Tigers and Sand Flies from a Leishmaniasis-Endemic Area, Southern Italy.Emerging Infectious Diseases, 26(6): 1311–1314. https://doi.org/10.3201/EID2606.191668
Jiménez M, González E, Martín-Martín I, Hernández S, and Molina R (2014). Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania infantum in the focus of Madrid, Spain? Veterinary Parasitology, 202(3–4): 296–300. https://doi.org/10.1016/j.vetpar.2014.03.027
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL and Daszak P (2008). Global trends in emerging infectious diseases.Nature, 451(7181): 990–993. https://doi.org/10.1038/nature06536
Karayiannis S, Ntais P, Messaritakis I, Tsirigotakis N, Dokianakis E, and Antoniou M (2015). Detection of Leishmania Infantum in red foxes (Vulpes vulpes) in Central Greece. Parasitology, 142(13):1574–1578. https://doi.org/10.1017/S0031182015001158
Kassahun A, Sadlova J, Dvorak V, Kostalova T, Rohousova I, Frynta D, Aghova T, Yasur-Landau D, Lemma W, Hailu A, Baneth G, Warburg A, Volf and Votypka j (2015). Detection of leishmania donovani and L. tropica in Ethiopian wild rodents.Acta Tropica, 145: 39–44. https://doi.org/10.1016/j.actatropica.2015.02.006
Killick-Kendrick R (1990). Phlebotomine vectors of the leishmaniases: a review. Medical and Veterinary Entomology, 4(1): 1–24. https://doi.org/10.1111/j.1365-2915.1990.tb00255.x
Killick-Kendrick R (1999). The biology and control of Phlebotomine sand flies.Clinics in Dermatology, 17: 279–289. https://doi.org/10.1016/S0738-081X(99)00046-2
Koehler K, Stechele M, Hetzel U, Domingo M, Schönian G, Zahner H, et al. (2002). Cutaneous leishmaniosis in a horse in southern Germany caused by Leishmania infantum. Veterinary Parasitology, 109(1–2): 9–17. https://doi.org/10.1016/S0304-4017(02)00246-7
Kouam MK, Diakou A, Kantzoura V, Papadopoulos E, Gajadhar AA, and Theodoropoulos G (2010). A seroepidemiological study of exposure to Toxoplasma, Leishmania, Echinococcus and Trichinella in equids in Greece and analysis of risk factors. Veterinary Parasitology, 170(1–2):. 170–175. https://doi.org/10.1016/j.vetpar.2010.02.004
Lara-Silva FDO, Barata RA, Michalsky ÉM, Ferreira EDC, Lopes MOG, Pinheiro ADC, et al. (2014). Rattus norvegicus (rodentia: Muridae) infected by Leishmania (Leishmania) infantum (syn. Le. chagasi) in Brazil. BioMed Research International, 2014:592986. https://doi.org/10.1155/2014/592986
Leontides LS, Saridomichelakis MN, Billinis C, Kontos V, Koutinas AF, Galatos AD, et al. (2002). A cross-sectional study of Leishmania spp. infection in clinically healthy dogs with polymerase chain reaction and serology in Greece.Veterinary Parasitology, 109(1–2): 19–27 https://doi.org/10.1016/S0304-4017(02)00201-7
Libert C, Ravel C, Pratlong F, Lami P, Dereure J, and Keck N (2012). Leishmania infantum infection in two captive barbary lions (Panthera leo leo). Journal of Zoo and Wildlife Medicine, 43(3):685–688. https://doi.org/10.1638/2012-0056.1
Madeira MF, Schubach A, Schubach TMP, Pacheco RS, Oliveira FS, Pereira SA, et al. (2006). Mixed infection with Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi in a naturally infected dog from Rio de Janeiro, Brazil.Transactions of the Royal Society of Tropical Medicine and Hygiene, 100(5):442–445. https://doi.org/10.1016/j.trstmh.2005.07.011
Maia C, and Campino L (2011). Can domestic cats be considered reservoir hosts of zoonotic leishmaniasis? Trends in Parasitology. 27: 341–344. https://doi.org/10.1016/j.pt.2011.03.008
Maia C, Nunes M and Campino L (2008). Importance of cats in zoonotic leishmaniasis in Portugal. Vector-Borne and Zoonotic Diseases, 8(4):555–559. https://doi.org/10.1089/vbz.2007.0247
Maia C, Gomes J, Cristóvão J, Nunes M, Martins A, Rebêlo E, et al. (2010). Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Veterinary Parasitology, 174(3–4): 336–340. https://doi.org/10.1016/j.vetpar.2010.08.030
Maia C, Almeida B, Coimbra M, Fernandes MC, Cristóvão JM, Ramos C, et al. (2015). Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasites and Vectors, 8(1):138 https://doi.org/10.1186/s13071-015-0759-8
Mancianti F (2004). Feline leishmaniasis: what’s the epidemiological role of the cat? Parassitologia, 46(1–2): 203–206. Available at:https://api.semanticscholar.org/CorpusID:7982906
Marcelino AP, Ferreira EC, Avendanha JS, Costa CF, Chiarelli D, Almeida G, et al. (2011). Molecular detection of Leishmania braziliensis in Rattus norvegicus in an area endemic for cutaneous leishmaniasis in Brazil. Veterinary Parasitology, 183(1–2): 54–58. https://doi.org/10.1016/j.vetpar.2011.06.019
Maroli M, Pennisi MG, Di Muccio T, Khoury C, Gradoni L, Gramiccia M (2007). Infection of sandflies by a cat naturally infected with Leishmania infantum. Veterinary Parasitology, 145(3–4):357–60. https://doi.org/10.1016/j.vetpar.2006.11.009
Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, and Gradoni L (2013). Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Medical and Veterinary Entomology. 27(2):123–147. https://doi.org/10.1111/j.1365-2915.2012.01034.x
Martín-Sánchez J, Acedo C, Muñoz-Pérez M, Pesson B, Marchal O, and Morillas-Márquez F (2007). Infection by Leishmania infantum in cats: Epidemiological study in Spain. Veterinary Parasitology, 145(3–4): 267–273. https://doi.org/10.1016/j.vetpar.2006.11.005
Mendoza-Roldan JA, Latrofa MS, Tarallo VD, Manoj RRS, Bezerra-Santos MA, Annoscia G, et al. (2022). Leishmania spp. in Squamata reptiles from the Mediterranean basin. Transboundary and Emerging Diseases, 69(5): 2856–2866. https://doi.org/10.1111/tbed.14438
Michel G, Pomares C, Ferrua B, and Marty P (2011). Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Tropica., 119(2–3): 69–75. https://doi.org/10.1016/j.actatropica.2011.05.012
Millán J, Zanet S, Gomis M, Trisciuoglio A, Negre N, and Ferroglio E (2011). An Investigation into Alternative Reservoirs of Canine Leishmaniasis on the Endemic Island of Mallorca (Spain). Transboundary and Emerging Diseases, 58(4): 352–357. https://doi.org/10.1111/j.1865-1682.2011.01212.x
Millán J, Ferroglio E, and Solano-Gallego L (2014). Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitology Research, 113(6):2005–2014 https://doi.org/10.1007/s00436-014-3929-2
Miró G, Rupérez C, Checa R, Gálvez R, Hernández L, García M, et al. (2014). Current status of L. infantum infection in stray cats in the Madrid region (Spain): Implications for the recent outbreak of human leishmaniosis? Parasites and Vectors, 7(1): 112. https://doi.org/10.1186/1756-3305-7-112
Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, Zarei Z, et al. (2005). Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran.Veterinary Parasitology, 129(3–4): 243–251. https://doi.org/10.1016/j.vetpar.2005.01.010
Molina R, Jiménez MI, Cruz I, Iriso A, Martín-Martín I, Sevillano O, et al. (2012). The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Veterinary Parasitology, 190(1–2): 268–271. https://doi.org/10.1016/j.vetpar.2012.05.006
Moreno J, and Alvar J (2002). Canine leishmaniasis: epidemiological risk and the experimental model. Trends in parasitology, 18(9): 399–405. https://doi.org/10.1016/S1471-4922(02)02347-4
Moreno I, Álvarez J, García N, de la Fuente S, Martínez I, Marino E, et al. (2014). Detection of anti-Leishmania infantum antibodies in sylvatic lagomorphs from an epidemic area of Madrid using the indirect immunofluorescence antibody test. Veterinary Parasitology, 199(3–4): 264–267. https://doi.org/10.1016/j.vetpar.2013.10.010
Morse SS (1995). Factors in the emergence of infectious diseases. Emerging Infectious Diseases, 1(1):7–15. https://doi.org/10.3201/eid0101.950102
Mukhtar MM, Sharief AH, El Saffi SH, Harith AE, Higazzi TB, Adam AM, et al. (2000). Detection of antibodies to Leishmania donovani in animals in a kala-azar endemic region in eastern Sudan: A preliminary report.Transactions of the Royal Society of Tropical Medicine and Hygiene, 94(1): 33–36. https://doi.org/10.1016/S0035-9203(00)90429-2
Navea-Pérez HM, Díaz-Sáez V, Corpas-López V, Merino-Espinosa G, Morillas-Márquez F, and Martín-Sánchez J (2015). Leishmania infantum in wild rodents: reservoirs or just irrelevant incidental hosts? Parasitology Research, 114(6): 2363–2370. https://doi.org/10.1007/s00436-015-4434-y
Oliveira FS, Pirmez C, Pires MQ, Brazil RP, and Pacheco RS (2005). PCR-based diagnosis for detection of Leishmania in skin and blood of rodents from an endemic area of cutaneous and visceral leishmaniasis in Brazil. Veterinary Parasitology, 129(3–4): 219–227. https://doi.org/10.1016/j.vetpar.2005.01.005
Ortuño M, Latrofa MS, Iborra MA, Pérez-Cutillas P, Bernal LJ, Risueño J, et al. (2019). Genetic diversity and phylogenetic relationships between Leishmania infantum from dogs, humans and wildlife in south-east Spain. Zoonoses and Public Health, 66(2): 132–140. https://doi.org/10.1111/zph.12646
Ozon C, Marty P, Pratlong F, Breton C, Blein M, Lelièvre A, et al. (1998). Disseminated feline leishmaniosis due to Leishmania infantum in Southern France. Veterinary Parasitology, 75(2–3): 273–277. https://doi.org/10.1016/S0304-4017(97)00174-X
Pagliano P, Carannante N, Rossi M, Gramiccia M, Gradoni L, Faella FS, et al. (2005). Visceral leishmaniasis in pregnancy: A case series and a systematic review of the literature.Journal of Antimicrobial Chemotherapy, 55(2): 229–233. https://doi.org/10.1093/jac/dkh538
Papadogiannakis E, Spanakos G, Kontos V, Menounos PG, Tegos N, and Vakalis N (2010). Molecular detection of Leishmania infantum in wild rodents (Rattus norvegicus) in Greece.Zoonoses and Public Health, 57(7–8): e23-e25. https://doi.org/10.1111/j.1863-2378.2009.01264.x
Pennisi MG (2015). Leishmaniosis of companion animals in Europe: An update. Veterinary Parasitology, 208(1–2): 35–47. https://doi.org/10.1016/j.vetpar.2014.12.023
Pennisi MG, and Persichetti MF (2018). Feline leishmaniosis: Is the cat a small dog? Veterinary Parasitology, 251:131–137. https://doi.org/10.1016/j.vetpar.2018.01.012
Pennisi MG, Cardoso L, Baneth G, Bourdeau P, Koutinas A, Miró G, et al. (2015). LeishVet update and recommendations on feline leishmaniosis. Parasites and Vectors, 8:302 https://doi.org/10.1186/s13071-015-0909-z
Piantedosi D, Veneziano V, Di Muccio T, Manzillo VF, Fiorentino E, Scalone A, et al. (2016). Epidemiological survey on Leishmania infection in red foxes (Vulpes vulpes) and hunting dogs sharing the same rural area in Southern Italy. Acta Parasitologica, 61(4): 769–775. https://doi.org/10.1515/ap-2016-0106
Poli A, Abramo F, Barsotti P, Leva S, Gramiccia M, Ludovisi A, et al. (2002). Feline leishmaniosis due to Leishmania infantum in Italy. Veterinary Parasitology, 106(3): 181–191. https://doi.org/10.1016/S0304-4017(02)00081-X
Psaroulaki A, Antoniou M, Toumazos P, Mazeris A, Ioannou I, Chochlakis D, et al. (2010). Rats as indicators of the presence and dispersal of six zoonotic microbial agents in Cyprus, an island ecosystem: A seroepidemiological study.Transactions of the Royal Society of Tropical Medicine and Hygiene, 104(11): 733–739. https://doi.org/10.1016/j.trstmh.2010.08.005
Quinnell RJ and Courtenay O (2009). Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology, 136: 1915–1934. https://doi.org/10.1017/S0031182009991156
Ready PD (2010). Leishmaniasis emergence in Europe. Eurosurveillance, 15(10):19505. https://doi.org/10.2807/ese.15.10.19505-en
Ready PD (2014). Epidemiology of visceral leishmaniasis. Clinical Epidemiology, 6: 147–154. https://doi.org/10.2147/CLEP.S44267
Rezvan Η (2014). Evaluation of different approaches in Leishmania diagnosis. International Journal of Advanced Biological and Biomedical Research, 2(2): 238–261. Available at: https://api.semanticscholar.org/CorpusID:35367443
Rocchigiani G, Ebani VV, Nardoni S, Bertelloni F, Bascherini A, Leoni A, et al. (2018). Molecular survey on the occurrence of arthropod-borne pathogens in wild brown hares (Lepus europaeus) from Central Italy. Infection, Genetics and Evolution, 59: 142–147. https://doi.org/10.1016/j.meegid.2018.02.005
Rolào N, Martins MJ, Joào A, and Campino L (2005). Equine infection with Leishmania in Portugal. Parasite, 12(2): 183–186. https://doi.org/10.1051/parasite/2005122183
Roque ALR, and Jansen AM (2014). Wild and synanthropic reservoirs of Leishmania species in the Americas.International Journal for Parasitology: Parasites and Wildlife, 3(3): 251–262. https://doi.org/10.1016/j.ijppaw.2014.08.004
Rosypal AC, Troy GC, Zajac AM, Frank G, and Lindsay DS (2005). Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle.Journal of Parasitology, 91(4): 970–972. https://doi.org/10.1645/GE-483R.1
Rotureau B (2006). Ecology of the Leishmania species in the Guianan ecoregion complex.American Journal of Tropical Medicine and Hygiene, 74(1): 81–96. https://doi.org/10.4269/ajtmh.2006.74.81
Ruiz-Fons F, Ferroglio E, and Gortázar C (2013). Leishmania infantum in free-ranging hares, Spain, 2004–2010. Eurosurveillance, 18(30). https://doi.org/10.2807/1560-7917.ES2013.18.30.20541
Sastre N, Francino O, Ramírez O, Enseñat C, Sánchez A, and Altet L (2008). Detection of Leishmania infantum in captive wolves from Southwestern Europe. Veterinary Parasitology, 158(1–2): 117–120. https://doi.org/10.1016/j.vetpar.2008.08.008
Semião-Santos SJ, Abranches P, Silva-Pereira MCD, Santos-Gomes GM, Fernandes JP, and Vetter JCM (1996). Reliability of serological methods for detection of leishmaniasis in Portuguese domestic and wild reservoirs. Memorias do Instituto Oswaldo Cruz, 91(6): 747–750. https://doi.org/10.1590/S0074-02761996000600018
Sgorbini M, Bonelli F, Pizzolli I, Tognetti R, and Corazza M (2014). Seroprevalence of leishmania sp. infection in healthy horses housed in endemic areas in Tuscany. Journal of Equine Veterinary Science, 34(4): 572–574. https://doi.org/10.1016/j.jevs.2013.09.009
Shaw J (1988). Animal reservoirs of Leishmania in different ecological situations and their importance in the epidemiology of the disease. Memórias do Instituto Oswaldo Cruz, 83(Suppl 1): 486–490. https://doi.org/10.1590/S0074-02761988000500054
Shaw J (1997). Ecological and evolutionary pressures on leishmanial parasites. Brazilian Journal of Genetics, 20(1): 123–128. https://doi.org/10.1590/S0100-84551997000100021
Shehata MG, Samy AM, Doha SA, Fahmy AR, Kaldas RM, Furman BD, et al. (2009). First report of Leishmania tropica from a classical focus of L. major in North-Sinai, Egypt.American Journal of Tropical Medicine and Hygiene, 81(2): 213–218. https://doi.org/10.4269/ajtmh.2009.81.213
Silva FL, Oliveira RG, Silva TMA, Xavier MN, Nascimento EF, and Santos RL (2009). Venereal transmission of canine visceral leishmaniasis. Veterinary Parasitology, 160(1–2): 55–59. https://doi.org/10.1016/j.vetpar.2008.10.079
Sobrino R, Ferroglio E, Oleaga A, Romano A, Millan J, Revilla M, et al. (2008). Characterization of widespread canine leishmaniasis among wild carnivores from Spain. Veterinary Parasitology, 155(3–4): 198–203. https://doi.org/10.1016/j.vetpar.2008.05.003
Solano-Gallego L, Morell P, Arboix M, Alberola J, and Ferrer L (2001). Prevalence of Leishmania infantum infection in dogs living in an area of canine Leishmaniasis endemicity using PCR on several tissues and serology. Journal of Clinical Microbiology, 39(2): 560–563 https://doi.org/10.1128/JCM.39.2.560-563.2001
Solano-Gallego L, Fernández‐Bellon H, Serra P, Gállego M, Ramis A, Fondevila D, et al. (2003). Cutaneous leishmaniosis in three horses in Spain. Equine veterinary journal, 35(3): 320–323. https://doi.org/10.2746/042516403776148336
Solano-Gallego L, Koutinas A, Miró G, Cardoso L, Pennisi MG, Ferrer L, et al. (2009). Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Veterinary Parasitology, 165(1–2):1–18. https://doi.org/10.1016/j.vetpar.2009.05.022
Solano-Gallego L, Mirá G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. (2011). LeishVet guidelines for the practical management of canine leishmaniosis. Parasites and Vectors. 4:86. https://doi.org/10.1186/1756-3305-4-86
Souza TD, Turchetti AP, Fujiwara RT, Paixão TA, and Santos RL (2014). Visceral leishmaniasis in zoo and wildlife. Veterinary Parasitology, 200: 233–241. https://doi.org/10.1016/j.vetpar.2013.12.025
Spada E, Perego R, Vitale F, Bruno F, Castelli G, Tarantola G, et al. (2020). Feline leishmania spp. Infection in a non-endemic area of Northern Italy. Animals, 10(5): 817. https://doi.org/10.3390/ani10050817
Tabar MD, Altet L, Francino O, Sánchez A, Ferrer L, and Roura X (2008). Vector-borne infections in cats: Molecular study in Barcelona area (Spain). Veterinary Parasitology, 151(2–4):332–336. https://doi.org/10.1016/j.vetpar.2007.10.019
Taylor LH, Latham SM, and Woolhouse MEJ (2001). Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B: Biological Sciences, 356(1411): 983–989. https://doi.org/10.1098/rstb.2001.0888
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J and Arenas R (2017). Leishmaniasis: a review. F1000 Research, 6: 750. https://doi.org/10.12688/f1000research.11120.1
Tsakmakidis Ι, Angelopoulou K, Dovas CI, Dokianakis Ε, Tamvakis Α, Symeonidou I, Antoniou Μ, Diakou Α (2017). Leishmania infection in rodents in Greece. Trop Med Int Health, (12):1523–1532. https://doi.org/10.1111/tmi.12982
Tsakmakidis Ι, Pavlou C, Tamvakis Α, Papadopoulos T, Christodoulou V, Angelopoulou K, et al. (2019). Leishmania infection in lagomorphs and minks in Greece. Veterinary Parasitology: Regional Studies and Reports, 16: 100279. https://doi.org/10.1016/j.vprsr.2019.100279
Tsokana CN, Sokos C, Giannakopoulos A, Mamuris Z, Birtsas P, Papaspyropoulos K, et al. (2015). First evidence of Leishmania infection in European brown hare (Lepus europaeus) in Greece: GIS analysis and phylogenetic position within the Leishmania spp. Parasitology Research, 115(1):313–21. https://doi.org/10.1007/s00436-015-4749-8
Van der Lugt J. J., Carlyon, J. F., & de Waal, D. T. (1992). Cutaneous leishmaniosis in a sheep. Journal of the South African Veterinary Association, 63(2): 74–77. Available at: https://hdl.handle.net/10520/AJA00382809_2953
Verin R, Poli A, Ariti G, Nardoni S, Fanucchi MB, and Mancianti F (2010). Detection of leishmania infantum DNA in tissues of free-ranging red foxes (vulpes vulpes) in Central Italy. European Journal of Wildlife Research, 56(4): 689–692. https://doi.org/10.1007/s10344-010-0395-8
Veronesi F, Deak G, and Diakou A (2023). Wild Mesocarnivores as Reservoirs of Endoparasites Causing Important Zoonoses and Emerging Bridging Infections across Europe. Pathogens12(2):178. https://doi.org/10.3390/pathogens12020178
Vilhena H, Martinez-Díaz VL, Cardoso L, Vieira L, Altet L, Francino O, et al. (2013). Feline vector-borne pathogens in the north and centre of Portugal. Parasites and Vectors, 6(1): 99. https://doi.org/10.1186/1756-3305-6-99
Williams AO, Mutinga J, and Rodgers M (1991). Leishmaniasis in a domestic goat in Kenya. Molecular and Cellular Probes, 5(5): 319–325. https://doi.org/10.1016/S0890-8508(06)80002-2
World Health Organisation (WHO). (1984). The Leishmaniases. Report of a WHO Expert Committee. WHO technical report series; no. 701, Geneva. Available at: https://www.who.int/publications/i/item/WHO-TRS-701
World Health Organization (WHO). (1990). The leishmaniasis. Technical Report Series, 793, 27. Available at: https://www.who.int/publications/i/item/WHO-TRS-793
World Health Organization (WHO). (2000). Report on Global Surveillance of Epidemic-prone Infectious Diseases. Available at: https://www.who.int/publications/i/item/WHO-CDS-CSR-ISR-2000.1
World Health Organization (2003). WHO | WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases – Chorela WHO. Available at: https://www.who.int/publications/i/item/WHO-CDS-CSR-ISR-2000.1
World Health Organisation (WHO). (2010). Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26. (WHO technical report series; no. 949). Available at: https://www.who.int/publications/i/item/WHO-TRS-949
Zanet S, Sposimo P, Trisciuoglio A, Giannini F, Strumia F, and Ferroglio E (2014). Epidemiology of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Rattus rattus in absence of domestic reservoir and definitive hosts. Veterinary Parasitology, 199(3–4): 247–249. https://doi.org/10.1016/j.vetpar.2013.10.023
Zavitsanou A, Koutis C, and Babatsikou F (2008). Leishmaniasis: an overlooked public health concern. Health Science Journal, 2(4): 196–205. Available at: https://api.semanticscholar.org/CorpusID:10997945
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Ioannis Tsakmakidis: Conceptualization, Methodology, Validation Review of the literature, Investigation, Writing original draft, Writing review and editing. Menelaos Lefkaditis: Conceptualization, Methodology, Investigation, Contributing to original draft. Konstantinos Zaralis: Methodology, Investigation, Review of the literature, Writing original draft, Writing review and editing. Georgios Arsenos: Investigation, Methodology, Validation, Contributing to original draft.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article. Konstantinos Zaralis serves as an associate editor for Tropical Animal Health and Production Journal, but did not participate in the editorial decision of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsakmakidis, I., Lefkaditis, M., Zaralis, K. et al. Alternative hosts of Leishmania infantum: a neglected parasite in Europe. Trop Anim Health Prod 56, 128 (2024). https://doi.org/10.1007/s11250-024-03978-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-024-03978-0