Abstract
This pilot study used an alternative and economically efficient technique, the Kompetitive Allele-Specific Polymerase Chain Reaction (KASP-PCR) to examine 48 SNPs from 11 parasite-resistance genes found on 8 chromosomes in 110 animals from five sheep breeds reared in Hungary; Hungarian Tsigai, White Dorper, Dorper, Ile de France, and Hungarian Merino. Allele and genotype frequencies, fixation index, observed heterozygosity, expected heterozygosity, F statistic, and their relationship with the Hardy–Weinberg equilibrium (WHE) and the polymorphic information content (PIC) were determined, followed by principal component analysis (PCA). As much as 32 SNPs out of the 48 initially studied were successfully genotyped. A total of 9 SNPs, 4 SNPs in TLR5, 1 SNP in TLR8, and 4 SNPs in TLR2 genes, were polymorphic. The variable genotype and allele frequency of the TLRs gene indicated genetic variability among the studied sheep breeds, with the Hungarian Merino exhibiting the most polymorphisms, while Dorper was the population with the most SNPs departing from the HWE. According to the PIC value, the rs430457884-TLR2, rs55631273-TLR2, and rs416833129-TLR5 were found to be informative in detecting polymorphisms among individuals within the populations, whereas the rs429546187-TLR5 and rs424975389-TLR5 were found to have a significant influence in clustering the population studied. This study reported a moderate level of genetic variability and that a low to moderate within-breed diversity was maintained in the studied populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small ruminants account for a substantial percentage of the worldwide livestock sector, with estimates of 1173 million heads for sheep and 1003 million heads for goats, respectively (FAO 2018). Sheep in Hungary is the third largest livestock population species, after poultry and pigs (Hungarian Central Statistical Office 2020). Hungary’s vast pastureland and temperate climate are excellent for sheep farming, making this country a significant sheep producer in the past, during nineteenth-century Europe. However, the changing political situations and agricultural activities in 1989 had a massive impact on Hungarian sheep farming, and the lowest point in sheep farming was recorded in 1996 (Nagy et al. 2011; Beke-Lisányi 2018). According to EUROSTAT (2022), Hungary’s sheep population was 1,061 thousand heads in 2019, with more than 90% of the breed structure being represented by Merino or Merino-derived breeds (Fésüs et al. 2008), with the remainder filled by several other imported European and African breeds such as Dorper, White Dorper, and Ile de France, as well the indigenous Hungarian Tsigai and Racka are also commonly reared in Hungary (Kukovics and Jávor 1999). In recent years, numerous efforts have been undertaken to revitalize sheep production using several approaches, one of which is through increasing production levels through better animal health and pasture management.
One of the main causes of production losses in small ruminants worldwide is represented by parasitic infections in grazing animals. For instance, Charlier et al. (2020) estimated that helminth infections cost € 151 million/year in dairy sheep and € 206 million/year in meat sheep farming in the European countries. Furthermore, Mavrot (2016) previously estimated higher costs of € 157–477 million/year in sheep meat farming, based on individual European country reports. These findings evidenced a significant economic loss caused by parasite infection in sheep.
Gastrointestinal parasitism’s impact on the health and production levels of grazing ruminants are well documented and includes significant weight and body condition losses and decreased milk yields (Carta et al. 2009; van der Voort et al. 2014; Ertaş et al. 2022), diarrhea (Jacobson et al. 2020), anorexia (al Kalaldeh et al. 2019), anemia (Mongruel et al. 2020), and higher levels of mortality Underwood et al. 2015). Furthermore, with the threat of climate change, the prevalence of parasite infection in sheep is expected to increase, even in regions where such aspects were not significantly problematic up to date. For instance, the seasonality of helminth parasites is expected to change as a result of warmer temperatures, and while the parasite survival rates during wintertime in temperate climates are expected to improve (Short et al. 2017). Moreover, increased rainfall levels are predicted to increase the prevalence of the liver fluke, Fasciola hepatica (Shrestha et al. 2020), Leishmania (Short et al. 2017), and several tick-borne Hemiparasitic illnesses such as Anaplasma spp., Babesia spp., and Theileria spp., which have been demonstrated to be influenced by changes in temperature and humidity (Abdullah et al. 2019).
Limited studies have been made focusing on gastrointestinal parasite infections in sheep reared in Hungary. Tóth et al. (2019) reported the most prevalent parasitic nematodes burden in Hungary to consist mainly out of Protostrongylus sp. and Strongylus sp., tapeworm Moniezia sp. and the Coccidian eimeria sp. Understanding genetic diversity and susceptibility for gastrointestinal parasitism resistance would be an excellent starting point, given that the variability in genetics within a population is fundamental to the immune response during infectious diseases. Up-to-date, emphasis has been made on the significance and potential benefits of heterogeneous genetic populations, notably in terms of the complex reactions they confer to epidemics, their longevity, and general resilience (Springbett et al. 2003; Kristensen et al. 2015). The preservation of genetic heterogeneity in livestock populations is critical to maintaining healthy livestock practices and for the preservation of biodiversity, considering the genetic advantages that locally adapted breeds have in reacting to epidemiological outbreaks.
The current pilot study in gastrointestinal parasite infection related genes in African and European sheep breeds reared in Hungary investigated the polymorphism of 48 SNPs belonging to 11 genes related to genetic gastrointestinal parasite resistance in five European and African sheep breeds which currently are concerned as part of Hungary’s sheep genetic selection and improvement program: Hungarian Tsigai, White Dorper, Dorper, Ile de France, and Hungarian Merino. The Hungarian Merino and the Hungarian Tsigai are two indigenous breeds of sheep vital to the Hungary’s efforts to preserve its genetic resources in the face of rising global demand for sheep genetics due to climate change. The Hungarian Merino sheep breed accounts for more than 80% of Hungary’s total sheep population. There were less than 5000 Hungarian Merinos left in 2014, rendering them an endangered species. Although not as numerous as Hungarian Merino, Hungarian Tsigai has remained a consistent component of the local livestock (< 10%) over the last two centuries.
In this study, the Kompetitive Allele Specific Polymerase Chain Reaction (KASP-PCR) was used. Fluorescence resonance energy transfer (FRET) generates signals in KASP-PCR. Two luminous cassettes detect bi-allelic SNP allele-specific amplification (Suo et al. 2020). Biallelic characterization of SNPs, insertions, and deletions in specific loci is easy, fast, and inexpensive (Alvarez-Fernandez et al. 2021). In the first round of PCR, allele-specific primers match the target SNP, and the common reverse primer amplifies the target area. The fluor-labeled oligos keep connected to their quencher-labeled complementary oligos, preventing fluorescence. The allele-specific primer is integrated into the template. As the PCR progresses, a fluor-labeled oligo corresponding to the amplified allele is incorporated into the template and no longer linked to its quencher-labeled complement, forming an adequate fluorescent signal (He et al. 2014).
Understanding the genetic diversity of sheep breeds will aid in understanding the significance of genetic variants in parasite resistance. In addition, the findings of the current study might be used for future sheep genetic improvement programs in Europe and Africa, as well as for future conservation initiatives, in order to improve sheep productivity, health, and animal welfare.
Materials and methods
Genomic DNA extraction
Blood samples were collected from 110 indigenous and non-indigenous sheep, as follows: Hungarian Tsigai = 10 and Hungarian Merino = 50 as the indigenous breed, and White Dorper = 10, Dorper = 10, and Ile de France = 30 as the non-indigenous breeds. Although the sample population was relatively low because this is a pilot project, all of the animals selected were genetically unrelated, and sampling was conducted in several different farms to better represent the breed. The method of Zsolnai and Orbán (1999) for isolating genomic DNA from blood was utilized. The DNA was stored at − 20 °C until it was analyzed. A NanoDrop Spectrophotometer was used to assess the concentration and quality of DNA (Thermo Scientific, Waltham, MA, USA). All samples were diluted to a uniform concentration before genotyping, which was done with 50 ng of DNA per sample.
Selection of SNPs
A panel of 48 SNPs from 11 parasite-resistance genes located on 8 chromosome was chosen based on previous genome-wide association studies (GWAS) and marker-assisted selection studies (Carracelas et al. 2022; Oget et al. 2019; Archibald et al. 2010) across the sheep genome (Table 1). The SNP data for Ovis were obtained from the NCBI’s Single Nucleotide Polymorphism Database (dbSNP http://www.ncbi.nlm.nih.gov) or Ensembl (http://www.ensembl.org). In the end, only 32 SNPs out of the 48 initially studied were successfully genotyped.
Genotyping and quality control
KASP PCR (KASPTM, LGC Genomics, Teddington, Middlesex, UK) was used to perform bi-allelic discrimination of the selected 48 SNPs. The data were visualized using SNP Viewer software version 1.99 (Hoddesdon, UK). All genotype data were exported for statistical analysis. Only SNPs that were found in at least 90% of the breeds were included. Quality control of genotyped data comprised of eliminating a number of animals with more than 10% missing SNP calls and a number of SNPs with call rates less than 90%, resulting in disparities for the number of individuals among SNPs.
Data analysis
The raw allele calls provided from LGC Genomics were examined using LGC Genomics’ KlusterCaller program. POPGENE software version 1.31 (Yeh et al. 1999) was used to calculate allele and genotype frequencies, fixation index (Fis), observed heterozygosity (Ho), expected heterozygosity (He), F statistic (Fst), and their accordance with or deviation from the Hardy–Weinberg equilibrium(HWE). Polymorphic information content (PIC) was determined online at https://gene-calc.pl/pic.
To visualize the genetic divergences between sheep breeds, the principal component analysis (PCA) was performed using FactoMineR (Lê et al. 2008) and ggplot2 (Wickham 2016) tools from the R Program (R Core Team 2020).
Results
Genetic diversity
This work studied 48 SNPs in 11 genes related to gastrointestinal parasite resistance in five European and African sheep breeds using the KASP genotyping technique (Table 1). A number of 32 SNPs out of the 48 initially studied were successfully genotyped (66.67%), while 16 others failed. As many as 9 markers (18.75%) of the successfully genotyped were found to be polymorphic (Table 2). The monomorphic markers were removed from further investigation.
The identified allele calls from the nine polymorphic markers with a 99.80% allele call rate. Polymorphic markers were rs429546187-TLR5, rs403288183-TLR8, rs401390846-TLR2, rs424975389-TLR5, rs55631273-TLR2, rs430457884-TLR2, rs160821602-TLR2, rs412232316-TLR5, and rs416833129-TLR5. The polymorphic markers were discovered in TLR5, TLR8, and TLR2 genes on chromosomes 12, 27, and 17, respectively. Varies genotype and allele frequency were observed (Table 3).
The genotype of homozygous GG in rs429546187-TLR5 was found dominantly in all five populations studied, with the range of G allele frequency varying between 0.80 to 1.00, followed by the heterozygote GA, which was only found in Dorper and Hungarian Merino breeds. The same pattern was observed with the homozygote AA for rs424975389-TLR5, with A allele frequency of 0.80 to 1.00. For rs416833129-TLR5, the G allele was more frequent in all five populations, with an allele frequency ranging between 0.50 and 1.00, except for the Dorper, where the heterozygote GA was the most frequent. While for rs412232316-TLR5, the C allele was the most dominant in all populations, except for the Dorper breed, for which the genotypes CA and AA were more frequent, with an A allele frequency of 0.70.
For rs403288183-TLR8, the homozygote TT was found in most Hungarian Tsigai, White Dorper, and Merino individuals, with the allele frequency ranging between 0.92 and 1.00, while in Dorper and Ile de France breeds, the heterozygote TC was the most prevalent, with the homozygote CC being observed as well.
For the TLR2 locus, there were four polymorphic markers observed. For the rs401390846, both alleles A and C were moderately found in Hungarian Tsigai (A = 0.550 and C = 0.450), White Dorper (A = 0.650 and C = 0.350), and Ile de France (A = 0.533 and C = 0.467), even though the A allele was dominant in Dorper and Merino breeds, with a frequency of 1.00 and 0.79, respectively. For the rs55631273, both alleles C and T were found moderately in each of the sampled populations, except for the White Dorper and Merino. In rs430457884, the genotype GG was highly observed in all populations, although the heterozygote GA was dominantly observed in the White Dorper population, while the G allele dominant for rs160821602 with the allele frequency varied between 0.85 and 1.00.
The Ho, He, PIC, and Fst values for the various breeds studied were determined and are listed in Table 4. The most polymorphic markers were found in rs55631273-TLR2 (0.269–0.375), rs430457884-TLR2 (0–0.375), and rs416833129-TLR5 (0–0.375). In general, the obtained results showed that the PIC values were smaller than 0.5, with the minimum and maximum values of 0.044 and 0.361, suggesting that all investigated markers are considered moderately informative.
The highest observed heterozygosity, which indicated a high within-population diversity, was obtained for rs403288183-TLR8, rs412232316-TLR5, and rs416833129-TLR5 in the Dorper population, with a value of 0.700, 0.600, and 0.600, respectively. With a similar pattern for rs424975389-TLR5 and rs55631273-TLR2 in the Ile de France population, with the value of 0.600 for both SNPs. The Fst value ranged from 0.104 to 0.462, indicating a moderate relationship among the observed breeds.
The HWE test (x2) is also shown in Table 3. Some SNPs have deviated from the HWE, such as the SNP rs416833129-TLR5 in Hungarian Tsigai, rs430457884-TLR2 in White Dorper, rs55631273 and rs430457884 of TLR2 in Dorper, and rs55631273-TLR2 in the Hungarian Merino population. The South African Dorper population was found to be a population with most SNPs deviating from the HWE. It was found that the Hungarian Merino population had the highest proportion of polymorphic markers (100%), and Ile de France had the lowest one (33.33%), with a fixed allele in 6 SNPs, except for rs403288183-TLR8, rs401390846-TLR2, and rs55631273-TLR2.
Principal component analysis
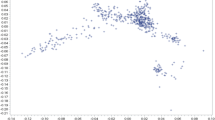
The result of the PCA is shown in Figs. 1 and 2. As displayed in Fig. 1, PC1 and PC2 account for 26.62% and 20.35% of the total variation in the five breeds, respectively, with a cumulative variance of 46.97%. The PCA was unsuccessful in separating breeds based on the genetic data. Based on the PCA loadings value (Fig. 2), the rs429546187-TLR5 (SNP1) and rs424975389-TLR5 (SNP4) had maximum values in PC1, as they showed maximum variance.
Loadings biplot of principal component analysis (PCA) of 9 SNPs of 110 sheep. SNP1 = rs429546187-TLR5; SNP2 = rs403288183-TLR8; SNP3 = rs401390846-TLR2; SNP4 = rs424975389-TLR5; SNP18 = rs55631273-TLR2; SNP19 = rs430457884-TLR2; SNP20 = rs160821602-TLR2; SNP27 = rs412232316-TLR5; and SNP28 = rs416833129-TLR5
Discussion
This study aimed to examine the genetic polymorphism of 48 SNPs in five European and African sheep breeds reared in Hungary, with an emphasis on gastrointestinal parasite resistance, using the KASP-PCR technique. As many as 16 (33.33%) of 48 SNPs were failed for genotyping, resulting in the success rate of the KASP technique in this study (66.67%) being lower than in previous studies on similar genes in goats (Ilie et al. 2018) and genes involved in milk composition in goats (Kusza et al. 2018) Due to the fact that monomorphic markers were excluded, only 9 SNPs (18.75%) were included for further analysis.
In this study, 9 polymorphic markers located in 3 genes associated with the parasite resistance were observed, namely the TLR5 (4 SNPs; rs429546187, rs424975389, rs412232316, and rs416833129), TLR8 (1 SNPs; rs403288183), and TLR2 (4 SNPs; rs401390846, rs430457884, rs55631273 and rs160821602).
When studying disease resistance in livestock, toll-like receptors (TLRs) are frequently investigated, considering that they are proteins pattern-recognition receptors (PRRs) that initiate the inflammatory processes (Ruiz-Larrañaga et al. 2011; Nie et al. 2018) and induce innate immune responses by identifying pathogen-associated molecular patterns (PAMPs) produced by pathogens such as bacteria, viruses, fungi, and parasites (Ma et al. 2011; Vijay et al. 2018). Mammalian TLRs are a large family with at least 13 members (Roach et al. 2005); however, only 10 members have been identified in sheep, even though all TLR genes in the species are highly comparable to caprine TLR, also showing over 95% similarity to bovine orthologs (Jungi et al. 2011). TLRs are present in a variety of cellular locations; for instance, TLR2 and TLR5 are found on the cell surface, which acts as bacterial and fungal sensors, whereas TLR8 acts as a sensor for intercellular pathogens (e.g., viruses), being found on the membranes of intracellular vesicles such as endosomes (Takeda 2004; Kawai and Akira 2006; Schumann and Tapping 2007).
Numerous studies have implicated TLR genes in natural genetic resistance to a variety of diseases in sheep, including ovine paratuberculosis (Yaman 2020), Mycoplasma pneumonia infections (Du et al. 2020), brucellosis (Li et al. 2021), and Haemonchus contortus infections (Toscano et al. 2019), outlining the importance of these genes in sheep health and immune system functioning. Several polymorphisms of the TLR genes have also been investigated and proven to be associated with sheep health and immunity (Taylor et al. 2008; Mikula et al. 2010; Olech et al. 2021). In this study, the PIC value of rs430457884-TLR2, rs55631273-TLR2, and rs416833129-TLR5 indicated these SNPs to some degree, informative in detecting the polymorphism among individuals of the five populations investigated. Aside from that, the loading value of PCA showed that rs429546187-TLR5 and rs424975389-TLR5 have a substantial influence on clustering for the Hungarian populations (Tsigai and Merino).
The different genotype and allele frequency of TLRs genes in this study demonstrated genetic diversity amongst Hungarian sheep breeds. These results are encouraging for future development of selection and genetic improvement programs for disease and environmental adaptation, as well as utilization for conservation efforts of Hungarian local sheep breeds. The Dorper and Hungarian Merino showed the most distinctive genotype and allele frequency for the studied SNPs compared to the other three breeds. An indigenous breed such as the Hungarian Tsigai is assumed to maintain high levels of genetic diversity, compared to commercial breeds, as indigenous breeds are usually under less selection pressure; however, in this study, a high proportion of monomorphic loci were found in the Hungarian Tsigai (n = 10) as well as in the Ile de France population (n = 30). In some instances, monomorphic loci associated with disease resistance are observed, such as Tendinopathies in the Greek native horse breed (Giantsis et al. 2020) and Mycobacterium bovis infection in Hostein Friesien cattle (Richardson et al. 2016). This is likely because native breeds are highly tolerant of and adaptable to a wide range of environmental circumstances has led to the evolution of disease-resistant phenotype. Although the moderate Fst value and the PCA indicated a moderate relationship among the observed breeds, no noteworthy clustering was formed from the PCA score value biplot (Fig. 1), as it was also in line with the previous finding by Loukovitis et al. (2022). This failed grouping in PCA was also affected by the small sample size and the large variation in sample size across populations.
The Hungarian Merino observed in this study showed the highest polymorphism levels among all. The breed is a commercial breed and has been developed through crossbreeding with a variety of Spanish Merino and Merino-derived breeds over years of history, which has resulted in extensive genetic admixture, results in line with those reported previously by Ciani et al. (2015), which stated that the Hungarian Merino sheep dates back over 250 years and evolved from the original fine-wool Spanish Merino rams and local semi-fine wool populations. Over the years, numerous different Merino and indigenous Hungarian breeds have contributed to the breed’s evolution. Moreover, the Hungarian Merino has been through a dynamic population expansion for the past years. To support this, a study by Loukovitis et al. (2022) has confirmed the high levels of genetic variation within the population of the Hungarian Merino.
The South African Dorper is also a widely spread commercial breed, being a hardy composite breed established by crossing the Black-headed Persian and the Dorset Horn. Due to its outstanding characteristics, such as the thermotolerance ability (Joy et al. 2020), the Dorper has gained popularity in certain European regions, including Hungary (Gavojdian et al. 2013). Since its introduction in 2007, the Dorper has been one of the most prolific breeds found in Hungary (Budai et al. 2013), although the number of purebreds in Hungary is still limited, and the ram is mainly used as terminal sires for crossbreeding with indigenous maternal breeds to increase production traits (Gavojdian et al. 2015a, b). According to some authors, the drawback of this breed to European rearing conditions would be the lower resistance to diseases, as evidenced by previous findings by Guo et al. (2016) and Estrada-Reyes et al. (2019). However, according to reports by Gavojdian et al. (2015a, b), the Dorper and White Dorper breeds have similar disease resistance and health indicators as the local Hungarian Tsigai, when reared under identical conditions.
Our current findings serve as a starting point for the characterization of European and African sheep breeds based on gastrointestinal parasitism resistance genes. They can be utilized to establish precise conservation measures aimed at improving disease resistance and monitoring the genetic variability of sheep breeds, especially those reared in Central Europe. However, populations with a larger sample size would be beneficial to obtain a better figure of the genetic diversity of the European sheep breeds.
Conclusion
This study describes polymorphisms in parasite resistance genes in European and African sheep breeds reared in Hungary. Our study revealed nine polymorphic markers in TLR5, TLR8, and TLR2 genes associated with parasite resistance in Hungarian Tsigai, White Dorper, Dorper, and Hungarian Merino sheep using the KASP assay, giving room for genetics selection for parasite resistance traits in Hungarian sheep population. In general, the study reported a moderate level of genetic variability and that a low to moderate within-breed diversity was maintained in the studied populations. Although these SNPs require additional research into marker associations and their marker-quantitative trait locus phase relationships in each population to precisely define each SNP effect, and the number of samples is limited in this pilot study, the results obtained may prove valuable and contribute to the future molecular marker studies on disease resistance in sheep.
Data availability
All data generated or analyzed during this study are included in this article.
References
Abdullah D, Ali M, Omer S, Fadunsin S, Ali F, Gimba F. Prevalence and climatic influence on hemoparasites of cattle and sheep in Mosul, Iraq J Adv Vet Anim Res 2019; 6(4), 492. https://doi.org/10.5455/javar.2019.f373
al Kalaldeh M, Gibson J, Lee SH, Gondro C, van der Werf JHJ. Detection of genomic regions underlying resistance to gastrointestinal parasites in Australian sheep. Genet Sel Evol 2019; 51(1), 37. https://doi.org/10.1186/s12711-019-0479-1
Alvarez-Fernandez, A., Bernal MJ., Fradejas I., Ramirez A.M., Yusuf N. A. M., Lanza M., Hisam S., de Ayala A. P., and Rubio J.M. KASP: a genotyping method to rapid identification of resistance in Plasmodium falciparum. Malaria J 2021; 20(16). https://doi.org/10.1186/s12936-020-03544-7
Archibald, A. L., Cockett, N. E., Dalrymple, B. P., Faraut, T., Kijas, J. W., Maddox, J. F., McEwan, J. C., Hutton Oddy, V., Raadsma, H. W., Wade, C., Wang, J., Wang, W., & Xun, X. The sheep genome reference sequence: a work in progress. Anim Genet 2010; 41(5), 449–453. https://doi.org/10.1111/j.1365-2052.2010.02100.x
Beke-Lisányi J. Integration Efforts in Agriculture. Appl. Stud. Agribus. and Commer 2018; 12(1–2), 91–96. https://doi.org/10.19041/APSTRACT/2018/1-2/12
Budai C, Oláh J, Egerszegi I, Jávor A, Kovács A. Seasonal variations in some reproductive parameters of Dorper rams in Hungary. Agrártudományi Közlemények 2013; 53, 17–20.
Carta A, Casu S, Salaris S. Invited review: Current state of genetic improvement in dairy sheep. J Dairy Sci 2009; 92(12), 5814–5833. https://doi.org/10.3168/jds.2009-2479
Carracelas, B., Navajas, E. A., Vera, B., & Ciappesoni, G. Genome-Wide Association Study of Parasite Resistance to Gastrointestinal Nematodes in Corriedale Sheep. Genes 2022; 13(9), 1548. https://doi.org/10.3390/genes13091548
Charlier J, Rinaldi L, Musella V, Ploeger HW, Chartier C, Vineer HR, Hinney B, von Samson-Himmelstjerna G, Băcescu B, Mickiewicz M, Mateus TL, Martinez-Valladares M, Quealy S, Azaizeh H, Sekovska B, Akkari H, Petkevicius S, Hektoen L, Höglund J, … Claerebout E. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe Prev Ve. Med 2020; 182, 105103. https://doi.org/10.1016/j.prevetmed.2020.105103
Ciani E, Lasagna E, D’Andrea M, Alloggio I, Marroni F, Ceccobelli S, Delgado Bermejo Jv, Sarti FM, Kijas J, Lenstra JA, Pilla F. Merino and Merino-derived sheep breeds: a genome-wide intercontinental study. Genet Sel Evol 2015; 47(1), 64. https://doi.org/10.1186/s12711-015-0139-z
Du Z, Sun Y, Wang J, Liu H, Yang Y, Zhao N. Comprehensive RNA-Seq profiling of the lung transcriptome of Bashbay sheep in response to experimental Mycoplasma ovipneumoniae infection. PLOS ONE 2020; 15(7), e0214497. https://doi.org/10.1371/journal.pone.0214497
Ertaş F, Sona Karakuş A, Ayan A. The Prevalence of Commonly Encountered Parasites in Sheep in Iğdır Province, Turkey. Turk J Agric- Food Sci Tech 2022; 10(2), 260–262. https://doi.org/10.24925/turjaf.v10i2.260-262.4662
Estrada-Reyes Z M, Tsukahara Y, Amadeu RR, Goetsch AL, Gipson TA, Sahlu T, Puchala R, Wang Z, Hart SP, , Mateescu RG, Signatures of selection for resistance to Haemonchus contortus in sheep and goats. BMC Genomics 2019; 20(1), 735. https://doi.org/10.1186/s12864-019-6150-y
EUROSTAT. Number of sheep. Sheep population in Hungary. Latest update: 01/03/2022 23.00. Accessed 08/03/2022. https://ec.europa.eu/eurostat/databrowser/view/tag00017/default/table?lang=en , 2022.
FAO. Crops and livestock products. License: CC BY-NC-SA 3.0 IGO. Extracted from: https://www.fao.org/faostat/en/#data/QCL. Accessed 25 May 2022
Fésüs L, Zsolnai A, Anton I, Sáfár L. Breeding for scrapie resistance in the Hungarian sheep population. Acta Veterinaria Hungarica 2008; 56(2), 173–180. https://doi.org/10.1556/avet.56.2008.2.4
Gavojdian D, Budai C, Cziszter LT, Csizmar N, Javor A, Kusza S. Reproduction Efficiency and Health Traits in Dorper, White Dorper, and Tsigai Sheep Breeds under Temperate European Conditions. Asian-Australas J Anim Sci 2015; 28(4), 599–603. https://doi.org/10.5713/ajas.14.0659
Gavojdian D, Cziszter L, Pacala N, Sauer M. Productive and reproductive performance of Dorper and its crossbreds under a Romanian semi-intensive management system. S Afr J Anim Sci 2013; 43(2), 2019–228. https://doi.org/10.4314/sajas.v43i2.12
Gavojdian D, Cziszter LT, Budai C, Kusza S. Effects of behavioral reactivity on production and reproduction traits in Dorper sheep breed. J Vet Behav 2015; 10(4), 365–368. https://doi.org/10.1016/j.jveb.2015.03.012
Giantsis, I. A., Diakakis, N. E., & Avdi, M. High Frequencies of TNC and COL5A1 Genotypes Associated With Low Risk for Superficial Digital Flexor Tendinopathy in Greek Indigenous Horse Breeds Compared With Warmblood Horses. J Equine Vet Sci 2020; 92, 103173. https://doi.org/10.1016/j.jevs.2020.103173
Guo Z, González JF, Hernandez JN, McNeilly TN, Corripio-Miyar Y, Frew D, Morrison T, Yu P, Li RW. Possible mechanisms of host resistance to Haemonchus contortus infection in sheep breeds native to the Canary Islands. Sci Rep 2016; 6(1), 26200. https://doi.org/10.1038/srep26200
He C., Holme J., and Anthony J. SNP genotyping: the KASP assay. Methods in Molecular Biology 2014; 1145:75–86.
Hungarian Central Statistical Office. Agricultural Census. https://www.ksh.hu/docs/eng/agrar/html/tabl1_5_1_1.html. Accessed 12 April 2022, 2020.
Ilie DE, Kusza S, Sauer M, Gavojdian D. Genetic characterization of indigenous goat breeds in Romania and Hungary with a special focus on genetic resistance to mastitis and gastrointestinal parasitism based on 40 SNPs. PLOS ONE 2018; 13(5), e0197051. https://doi.org/10.1371/journal.pone.0197051
Jacobson C, Larsen JW, Besier RB, Lloyd JB, Kahn LP. Diarrhoea associated with gastrointestinal parasites in grazing sheep. Vet Parasitol 2020; 282, 109139. https://doi.org/10.1016/j.vetpar.2020.109139
Joy A, Dunshea FR, Leury BJ, DiGiacomo K, Clarke IJ, Zhang MH, Abhijith A, Osei-Amponsah R, Chauhan SS, Comparative Assessment of Thermotolerance in Dorper and Second-Cross (Poll Dorset/Merino × Border Leicester) Lambs. Animals 2020; 10(12), 2441. https://doi.org/10.3390/ani10122441
Jungi TW, Farhat K, Burgener IA. Werling, D. Toll-like receptors in domestic animals. Cell Tissue Re 2011; 343(1), 107–120. https://doi.org/10.1007/s00441-010-1047-8
Kawai T, Akira S. TLR signaling. Cell Death Differ 2006; 13(5), 816–825. https://doi.org/10.1038/sj.cdd.4401850
Kristensen TN, Hoffmann AA, Pertoldi C, Stronen AV. What can livestock breeders learn from conservation genetics and vice versa? Front Genet 2015; 6(38), 1–12. https://doi.org/10.3389/fgene.2015.00038
Kukovics S, Jávor A. Sheep and Goat Production Systems in Hungary. In : Rubino R. (ed.), Morand-Fehr P. (ed.). Systems of sheep and goat production: Organization of husbandry and role of extension services . Zaragoza : CIHEAM 1999; p. 231–237
Kusza S, Cziszter LT, Ilie DE, Sauer M, Padeanu I, Gavojdian D. Kompetitive Allele Specific PCR (KASPTM) genotyping of 48 polymorphisms at different caprine loci in French Alpine and Saanen goat breeds and their association with milk composition. PeerJ 2018; 6, e4416. https://doi.org/10.7717/peerj.4416
Lê S, Josse J, Husson F. “FactoMineR: A Package for Multivariate Analysis.” J Stat Software 2008; 25(1), 1–18. https://doi.org/10.18637/jss.v025.i01
Li G, Lv D, Yao Y, Wu H, Wang J, Deng S, Song Y, Guan S, Wang L, Ma W, Yang H, Yan L, Zhang J, Ji P, Zhang L, Lian Z, Liu G. Overexpression of ASMT likely enhances the resistance of transgenic sheep to brucellosis by influencing immune-related signaling pathways and gut microbiota. The FASEB J 2021; 35(9). https://doi.org/10.1096/fj.202100651R
Loukovitis D, Szabó M, Chatziplis D, Monori I, Kusza S. Genetic diversity and substructuring of the Hungarian merino sheep breed using microsatellite markers. Anim Biotechnol 2022; 9, 1–9. https://doi.org/10.1080/10495398.2022.2042307
Ma JL, Zhu YH, Zhang L, Zhuge ZY, Liu PQ, Yan XD, Gao HS, Wang JF. Serum concentration and mRNA expression in milk somatic cells of toll-like receptor 2, toll-like receptor 4, and cytokines in dairy cows following intramammary inoculation with Escherichia coli. J Dairy Sci 2011; 94(12), 5903–5912. https://doi.org/10.3168/jds.2011-4167
Mavrot, F. Livestock Nematode Infection in a Changing World: Investigating the European Situation. PhD thesis. Universität Zürich, 2016; pp. p70–130.
Mikula I, Bhide M, Pastorekova S, Mikula I. Characterization of ovine TLR7 and TLR8 protein coding regions, detection of mutations and Maedi Visna virus infection. Vet Immunol Immunopathol 2010; 138(1–2), 51–59. https://doi.org/10.1016/j.vetimm.2010.06.015
Mongruel ACB, Spanhol VC, Valente JDM, Porto PP, Ogawa L, Otomura FH, Marquez E de S, André MR, Vieira TSWJ, Vieira RF da C. Survey of vector-borne and nematode parasites involved in the etiology of anemic syndrome in sheep from Southern Brazil. Rev Bras Parasitol Vet 2020; 29(3), e007320. https://doi.org/10.1590/s1984-29612020062
Nagy S, Németh A, Mihályfi S, Toldi G, Gergátz E, Holló I. The short history of Hungarian sheep breeding and Hungarian merino breed. Acta Agraria Kaposváriensis 2011; 15(1), 19–26.
Nie L, Cai SY, Shao JZ, Chen J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front Immunol 2018; 9, 1523. https://doi.org/10.3389/fimmu.2018.01523
Oget, C., Allain, C., Portes, D., Foucras, G., Stella, A., Astruc, J.-M., Sarry, J., Tosser-Klopp, G., & Rupp, R. A validation study of loci associated with mastitis resistance in two French dairy sheep breeds. Genet Sel Evol 2019; 51(1), 5. https://doi.org/10.1186/s12711-019-0448-8
Olech M, Ropka-Molik K, Szmatoła T, Piórkowska K, Kuźmak J. Single Nucleotide Polymorphisms in Genes Encoding Toll-Like Receptors 7 and 8 and Their Association with Proviral Load of SRLVs in Goats of Polish Carpathian Breed. Animals 2021; 11(7), 1908. https://doi.org/10.3390/ani11071908
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/. Accessed 16 May 2022
Richardson, I. W., Berry, D. P., Wiencko, H. L., Higgins, I. M., More, S. J., McClure, J., Lynn, D. J., & Bradley, D. G. A genome-wide association study for genetic susceptibility to Mycobacterium bovis infection in dairy cattle identifies a susceptibility QTL on chromosome 23. Genet Sel Evol 2016; 48(1), 19. https://doi.org/10.1186/s12711-016-0197-x
Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proceedings of the National Academy of Sciences 2005; 102(27), 9577–9582. https://doi.org/10.1073/pnas.0502272102
Ruiz-Larrañaga O, Manzano C, Iriondo M, Garrido JM, Molina E, Vazquez P, Juste RA, Estonba A. Genetic variation of toll-like receptor genes and infection by Mycobacterium avium ssp. paratuberculosis in Holstein-Friesian cattle. J Dairy Sci 2011; 94(7), 3635–3641. https://doi.org/10.3168/jds.2010-3788
Schumann RR, Tapping RI. Genomic variants of TLR1 – It takes (TLR-) two to tango. Eur J Immunol 2007; 37(8), 2059–2062. https://doi.org/10.1002/eji.200737604
Short EE, Caminade C, Thomas, BN. Climate Change Contribution to the Emergence or Re-Emergence of Parasitic Diseases. Infect Dis Res Treat 2017; 10, 117863361773229. https://doi.org/10.1177/1178633617732296
Shrestha S, Barratt A, Fox NJ, Vosough Ahmadi B, Hutchings MR. Financial Impacts of Liver Fluke on Livestock Farms Under Climate Change–A Farm Level Assessment. Front Vet Sci 2020; 7, 564795. https://doi.org/10.3389/fvets.2020.564795.
Springbett AJ, MacKenzie K, Woolliams JA, Bishop SC. The Contribution of Genetic Diversity to the Spread of Infectious Diseases in Livestock Populations. Genetics 2003; 165(3), 1465–1474. https://doi.org/10.1093/genetics/165.3.1465
Suo W., Shi X., Xu S., Li X., and Lin Y. Towards low cost, multiplex clinical genotyping: 4-fluorescent Kompetitive Allele-Specific PCR and its application on pharmacogenetics. PLoS ONE. 2020;15:e0230445.
Takeda K. Toll-like receptors in innate immunity. Int Immunol 2004; 17(1), 1–14. https://doi.org/10.1093/intimm/dxh186
Taylor DL, Zhong L, Begg DJ, de Silva K, Whittington RJ. Toll-like receptor genes are differentially expressed at the sites of infection during the progression of Johne’s disease in outbred sheep. Vet Immunol Immunopathol 2008; 124(1–2), 132–151. https://doi.org/10.1016/j.vetimm.2008.02.021
Toscano JHB, Okino CH, dos Santos IB, Giraldelo LA, von Haehling MB, Esteves SN, de Souza Chagas AC. Innate Immune Responses Associated with Resistance against Haemonchus contortus in Morada Nova Sheep. J Immunol Res. 2019; 1–10. https://doi.org/10.1155/2019/3562672
Tóth M, Khangembam R, Farkas R, Oláh J, Vass N, Monori I. A case report: sheep endoparasitism dynamics under semi-dry continental climate of Karcag, Hungary. Anim Biol 2019; 21(2), 66–69. https://doi.org/10.15407/animbiol21.02.066
Underwood WJ, Blauwiekel R, Delano ML, Gillesby R, Mischler SA, Schoell A. Biology and Diseases of Ruminants (Sheep, Goats, and Cattle). In Laboratory Animal Medicine 2015; pp. 623–694). Elsevier. https://doi.org/10.1016/B978-0-12-409527-4.00015-8
van der Voort M, van Meensel J, Lauwers L, Vercruysse J, van Huylenbroeck G, Charlier J. A stochastic frontier approach to study the relationship between gastrointestinal nematode infections and technical efficiency of dairy farms. J Dairy Sci 2014; 97(6), 3498–3508. https://doi.org/10.3168/jds.2013-7444
Vijay K, Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int Immunopharmacol 2018; 59, 391–412. https://doi.org/10.1016/j.intimp.2018.03.002
Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. 2016. https://ggplot2.tidyverse.org. Accessed 16 May 2022
Yaman Y., Association of toll-like receptor 4 (TLR 4) gene exon 3 variants with serostatus of the ovine Johne’s disease (paratuberculosis) in Turkish sheep. Turkish J Vet Anim Sci 2020; 44(3), 542–547. https://doi.org/10.3906/vet-1909-98
Yeh F, Yang R, Boyle T. The User Friendly Shareware for Population Genetic Analysis. Molecular Biology and Biotechnology Center. University of Alberta, Alberta, 1999.
Zsolnai A, Orbán L. Accelerated separation of random complex DNA patterns in gels: Comparing the performance of discontinuous and continuous buffers. Electrophoresis 1999; 20(7), 1462–1468. https://doi.org/10.1002/(SICI)1522-2683(19990601)20:7<1462::AID-ELPS1462>3.0.CO;2-0
Acknowledgements
The authors are grateful to János Oláh, Attila Zsemkó and Attila Harcsa in sample collecting. P.K.A. and G.W were supported by Tempus Public Foundation within the Stipendium Hungaricum Programme.
Funding
Open access funding provided by University of Debrecen. The work was supported by the EFOP-3.6.2–16-2017–00001 project, entitled “Complex rural economic development and sustainability research, development of the service network in the Carpathian Basin”. This study has also received financial support by VP3-16.1.1–4.1.5–4.2.1–4.2.2–8.1.1–8.2.1–8.3.1–8.5.1–8.5.2–8.6.1–17 programme, and a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS – UEFISCDI (PN-II-RU-TE-2014–4-0023).
Author information
Authors and Affiliations
Contributions
Conceptualization Szilvia Kusza and Dinu Gavojdian; methodology, Daniela Elena Ilie and Szilvia Kusza; resources, Dinu Gavojdian, Daniela Elena Ilie, István Monori, and Szilvia Kusza; data analysis, Putri Kusuma Astuti; visualization, Putri Kusuma Astuti; writing-original draft, Putri Kusuma Astuti; writing-review and editing, Dinu Gavojdian, Daniela Elena Ilie, George Wanjala, István Monori, Zoltán Bagi, and Szilvia Kusza; funding, Dinu Gavojdian, István Monori, and Szilvia Kusza; supervision, Szilvia Kusza; acquisition Szilvia Kusza. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethic approval
All methods were carried out in accordance with the European Union’s Animal Experimentation Directive (Directive 2010/63/EU) and ARRIVE guidelines.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Astuti, P.K., Gavojdian, D., Ilie, D.E. et al. Genetic polymorphism in European and African sheep breeds reared in Hungary based on 48 SNPs associated with resistance to gastrointestinal parasite infection using KASP-PCR technique. Trop Anim Health Prod 55, 197 (2023). https://doi.org/10.1007/s11250-023-03609-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03609-0