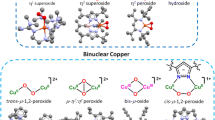
Abstract
Dioxygen (O2) is a key component of the earth’s atmosphere and is essential for life activities on the planet. Upon O2 activation, metalloenzymes are able to mediate various oxidative transformations related to many biosynthesis and metabolism processes. In this mini-review, we focus on the recent computational insights into the oxygen activation by several copper-dependent enzymes, including mono-copper enzyme lytic polysaccharide monooxygenase (LPMO), particulate methane monooxygenase (pMMO), as well as the binuclear copper enzymes peptidylglycine α-hydroxylating monooxygenase (PHM) and dopamine beta-monooxygenase (DβM). Understanding the structure–function relationships of these enzymes is of fundamental importance in chemistry and biology, and is also beneficial to engineering of these metalloenzymes for new functions.
Similar content being viewed by others
References
Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L (2014) Copper active sites in biology. Chem Rev 114(7):3659–3853
Meier KK, Jones SM, Kaper T, Hansson H, Koetsier MJ, Karkehabadi S, Solomon EI, Sandgren M, Kelemen B (2018) Oxygen activation by Cu LPMOs in recalcitrant carbohydrate polysaccharide conversion to monomer sugars. Chem Rev 118(5):2593–2635
Wang VCC, Maji S, Chen PRY, Lee HK, Yu SSF, Chan SI (2017) Alkane oxidation: methane monooxygenases, related enzymes, and their biomimetics. Chem Rev 117(13):8574–8621
Mano N, de Poulpiquet A (2018) O2 reduction in enzymatic biofuel cells. Chem Rev 118(5):2392–2468
Koo CW, Rosenzweig AC (2021) Biochemistry of aerobic biological methane oxidation. Chem Soc Rev 50:3424–3426
Hedegard ED, Ryde U (2018) Molecular mechanism of lytic polysaccharide monooxygenases. Chem Sci 9(15):3866–3880
Wang BJ, Johnston EM, Li PF, Shaik S, Davies GJ, Walton PH, Rovira C (2018) QM/MM studies into the H2O2-dependent activity of lytic polysaccharide monooxygenases: evidence for the formation of a caged hydroxyl radical intermediate. Acs Catal 8(2):1346–1351
Wang BJ, Wang ZF, Davies GJ, Walton PH, Rovira C (2020) Activation of O2 and H2O2 by lytic polysaccharide monooxygenases. Acs Catal 10(21):12760–12769
Kim S, Stahlberg J, Sandgren M, Paton RS, Beckham GT (2014) Quantum mechanical calculations suggest that lytic polysaccharide monooxygenases use a copper-oxyl, oxygen-rebound mechanism. Proc Natl Acad Sci USA 111(1):149–154
Cowley RE, Tian L, Solomon EI (2016) Mechanism of O2 activation and substrate hydroxylation in noncoupled binuclear copper monooxygenases. Proc Natl Acad Sci USA 113(43):12035–12040
Chylenski P, Bissaro B, Sorlie M, Rohr AK, Varnai A, Horn SJ, Eijsink VGH (2019) Lytic polysaccharide monooxygenases in enzymatic processing of lignocellulosic biomass. Acs Catal 9(6):4970–4991
Ciano L, Davies GJ, Tolman WB, Walton PH (2018) Bracing copper for the catalytic oxidation of C-H bonds. Nat Catal 1(8):571–577
Vaaje-Kolstad G, Westereng B, Horn SJ, Liu ZL, Zhai H, Sorlie M, Eijsink VGH (2010) An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330(6001):219–222
Kjaergaard CH, Qayyum MF, Wong SD, Xu F, Hemsworth GR, Walton DJ, Young NA, Davies GJ, Walton PH, Johansen KS, Hodgson KO, Hedman B, Solomon EI (2014) Spectroscopic and computational insight into the activation of O2 by the mononuclear Cu center in polysaccharide monooxygenases. Proc Natl Acad Sci USA 111(24):8797–8802
Beeson WT, Phillips CM, Cate JHD, Marletta MA (2012) Oxidative cleavage of cellulose by fungal copper-dependent polysaccharide monooxygenases. J Am Chem Soc 134(2):890–892
Courtade G, Ciano L, Paradisi A, Lindley PJ, Forsberg Z, Sorlie M, Wimmer R, Davies GJ, Eijsink VGH, Walton PH, Aachmann FL (2020) Mechanistic basis of substrate-O2 coupling within a chitin-active lytic polysaccharide monooxygenase: an integrated NMR/EPR study. Proc Natl Acad Sci USA 117:19178–19189
Bissaro B, Rohr AK, Muller G, Chylenski P, Skaugen M, Forsberg Z, Horn SJ, Vaaje-Kolstad G, Eijsink VGH (2017) Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat Chem Biol 13(10):1123–1128
Kuusk S, Bissaro B, Kuusk P, Forsberg Z, Eijsink VGH, Sorlie M, Valjamae P (2018) Kinetics of H2O2-driven degradation of chitin by a bacterial lytic polysaccharide monooxygenase. J Biol Chem 293(2):523–531
Filandr F, Man P, Halada P, Chang HH, Ludwig R, Kracher D (2020) The H2O2-dependent activity of a fungal lytic polysaccharide monooxygenase investigated with a turbidimetric assay. Biotechn Biofuels 13(1):37
Kracher D, Forsberg Z, Bissaro B, Gangl S, Preims M, Sygmund C, Eijsink VGH, Ludwig R (2020) Polysaccharide oxidation by lytic polysaccharide monooxygenase is enhanced by engineered cellobiose dehydrogenase. FEBS J 287(5):897–908
Zhou HC, Zhang YB, Li T, Tan HD, Li GH, Yin H (2020) Distinct interaction of lytic polysaccharide monooxygenase with cellulose revealed by computational and biochemical studies. J Phys Chem Lett 11(10):3987–3992
Kittl R, Kracher D, Burgstaller D, Haltrich D, Ludwig R (2012) Production of four Neurospora crassa lytic polysaccharide monooxygenases in Pichia pastoris monitored by a fluorimetric assay. Biotechn Biofuels 5:79
Kracher D, Scheiblbrandner S, Felice AKG, Breslmayr E, Preims M, Ludwicka K, Haltrich D, Eijsink VGH, Ludwig R (2016) Extracellular electron transfer systems fuel cellulose oxidative degradation. Science 352(6289):1098–1101
Wang BJ, Walton PH, Rovira C (2019) Molecular mechanisms of oxygen activation and hydrogen peroxide formation in lytic polysaccharide monooxygenases. Acs Catal 9(6):4958–4969
Phillips CM, Beeson WT, Cate JH, Marletta MA (2011) Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. Acs Chem Biol 6(12):1399–1406
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VGH (2012) Novel enzymes for the degradation of cellulose. Biotechn Biofuels 5:45
Bertini L, Breglia R, Lambrughi M, Fantucci P, De Gioia L, Borsari M, Sola M, Bortolotti CA, Bruschi M (2018) Catalytic mechanism of fungal lytic polysaccharide monooxygenases investigated by first-principles calculations. Inorg Chem 57(1):86–97
Warren JJ, Mayer JM (2010) Tuning of the thermochemical and kinetic properties of ascorbate by its local environment: solution chemistry and biochemical implications. J Am Chem Soc 132(22):7784–7793
Hedegard ED, Ryde U (2017) Targeting the reactive intermediate in polysaccharide monooxygenases. J Biolog Inorg Chem 22(7):1029–1037
Jones SM, Transue WJ, Meier KK, Kelemen B, Solomon EI (2020) Kinetic analysis of amino acid radicals formed in H2O2-driven Cu-I LPMO reoxidation implicates dominant homolytic reactivity. Proc Natl Acad Sci USA 117(22):11916–11922
Loose JSM, Forsberg Z, Kracher D, Scheiblbrandner S, Ludwig R, Eijsink VGH, Vaaje-Kolstad G (2016) Activation of bacterial lytic polysaccharide monooxygenases with cellobiose dehydrogenase. Protein Sci 25(12):2175–2186
Tan TC, Kracher D, Gandini R, Sygmund C, Kittl R, Haltrich D, Hallberg BM, Ludwig R, Divne C (2015) Structural basis for cellobiose dehydrogenase action during oxidative cellulose degradation. Nat Commun 6:7542
Courtade G, Wimmer R, Rohr AK, Preims M, Felice AKG, Dimarogona M, Vaaje-Kolstad G, Sorlie M, Sandgren M, Ludwig R, Eijsink VGH, Aachmann FL (2016) Interactions of a fungal lytic polysaccharide monooxygenase with beta-glucan substrates and cellobiose dehydrogenase. Proc Natl Acad Sci USA 113(21):5922–5927
Bodenheimer AM, Odell WB, Oliver RC, Qian S, Stanley CB, Meilleur F (2018) Structural investigation of cellobiose dehydrogenase IIA: Insights from small angle scattering into intra- and intermolecular electron transfer mechanisms. Biochim Biophys Acta Gen Subj 1862(4):1031–1039
Laurent CVFP, Breslmayr E, Tunega D, Ludwig R, Oostenbrink C (2019) Interaction between cellobiose dehydrogenase and lytic polysaccharide monooxygenase. Biochemistry 58(9):1226–1235
Wang ZF, Feng SS, Rovira C, Wang BJ (2021) How Oxygen binding enhances long-range electron transfer: lessons from reduction of lytic polysaccharide monooxygenases by cellobiose dehydrogenase. Angew Chem Int Ed 60(5):2385–2392
Wang ZF, Shaik S, Wang BJ (2021) Conformational motion of ferredoxin enables efficient electron transfer to heme in the full-length P450(TT). J Am Chem Soc 143(2):1005–1016
Singh RK, Blossom BM, Russo DA, Singh R, Weihe H, Andersen NH, Tiwari MK, Jensen PE, Felby C, Bjerrum MJ (2020) Detection and characterization of a novel copper-dependent intermediate in a lytic polysaccharide monooxygenase. Chem Eur J 26(2):454–463
Paradisi A, Johnston EM, Tovborg M, Nicoll CR, Ciano L, Dowle A, McMaster J, Hancock Y, Davies GJ, Walton PH (2019) Formation of a copper(II)-Tyrosyl complex at the active site of lytic polysaccharide monooxygenases following oxidation by H2O2. J Am Chem Soc 141(46):18585–18599
McEvoy A, Creutzberg J, Singh RK, Bjerrum MJ, Hedegard ED (2021) The role of the active site tyrosine in the mechanism of lytic polysaccharide monooxygenase. Chem Sci 12(1):352–362
Ross MO, Rosenzweig AC (2017) A tale of two methane monooxygenases. J Biol Inorg Chem 22(2–3):307–319
Jin Z, Wang L, Zuidema E, Mondal K, Zhang M, Zhang J, Wang C, Meng X, Yang H, Mesters C, Xiao F-S (2020) Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367:193–197
Newton M, Knorpp AJ, Sushkevich V, Palagin D, van Bokhoven J (2020) Active sites and mechanisms in the direct conversion of methane to methanol using Cu in zeolitic hosts: a critical examination. Chem Soc Rev 49:1449–1486
Schwarz H, Shaik S, Li J (2017) Electronic effect on room-temperature, gas-phase C-H activation by cluster oxides and metal carbides: the methane challenge. J Am Chem Soc 139:17201–17212
Hakemian AS, Rosenzweig AC (2007) The biochemistry of methane oxidation. Annu Rev Biochem 76:223–241
Sirajuddin S, Rosenzweig AC (2015) Enzymatic oxidation of methane. Biochemistry 54:2283–2294
Sazinsky MH, Lippard SJ (2015) Methane monooxygenase: functionalizing methane at iron and copper. Met Ions Life Sci 15:205–256
Tol RS, Heintz RJ, Lammers PEM (2003) Methane emission reduction: an application of FUND. Clim Change 57:71–98
Chan SI, Yu SSF (2019) Copper protein constructs for methane oxidation. Nat Catal 2:286–287
Kim HJ, Huh J, Kwon YW, Park D, Yu Y, Jang YE, Lee B-R, Jo E, Lee EJ, Heo Y, Lee W, Lee J (2019) Biological conversion of methane to methanol through genetic reassembly of native catalytic domains. Nat Catal 2:342–353
Murrell JC, Gilbert B, McDonald IR (2000) Molecular biology and regulation of methane monooxygenase. Arch Microbiol 173:325–332
Banerjee R, Jones JC, Lipscomb JD (2019) Soluble methane monooxygenase. Annu Rev Biochem 88:409–431
Baik MH, Newcomb M, Friesner RA, Lippard SJ (2003) Mechanistic studies on the hydroxylation of methane by methane monooxygenase. Chem Rev 103:2385–2419
Banerjee R, Proshlyakov Y, Lipscomb JD, Proshlyakov DA (2015) Structure of the key species in the enzymatic oxidation of methane to methanol. Nature 518:431–434
Tinberg CE, Lippard SJ (2011) Dioxygen activation in soluble methane monooxygenase. Acc Chem Res 44:280–288
Sirajuddin S, Barupala D, Helling S, Marcus K, Stemmler TL, Rosenzweig AC (2014) Effects of zinc on particulate methane monooxygenase activity and structure. J Biol Chem 289:21782–21794
Lieberman RL, Rosenzweig AC (2005) Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434:177–182
Smith SM, Rawat S, Telser J, Hoffman BM, Stemmler TL, Rosenzweig AC (2011) Crystal structure and characterization of particulate methane monooxygenase from Methylocystis species strain M. Biochemistry 50:10231–10240
Ro SY, Ross MO, Deng YW, Batelu S, Lawton TJ, Hurley JD, Stemmler TL, Hoffman BM, Rosenzweig AC (2018) From micelles to bicelles: effect of the membrane on particulate methane monooxygenase activity. J Biol Chem 293:10457–10465
Cao L, Caldararu O, Rosenzweig AC, Ryde U (2018) Quantum refinement does not support dinuclear copper sites in crystal structures of particulate methane monooxygenase. Agnew Chem Int Ed 57:162–166
Ross MO, MacMillan F, Wang J, Nisthal A, Lawto TJ, Olafson BD, Mayo SL, Rosenzweig AC, Hoffman BM (2019) Particulate methane monooxygenase contains only mononuclear copper centers. Science 364(6440):566–570
Labourel A, Frandsen KEH, Zhang F, Brouilly N, Grisel S, Haon M, Ciano L, Ropartz D, Fanuel M, Martin F, Navarro D, Rosso M-N, Tandrup T, Bissaro B, Johansen KS, Zerva A, Walton PH, Henrissat B, Leggio LL, Berrin J-G (2020) A fungal family of lytic polysaccharide monooxygenase-like copper proteins. Nat Chem Biol 16(3):345–350
Liew EF, Tong D, Coleman NV, Holmes AJ (2014) Mutagenesis of the hydrocarbon monooxygenase indicates a metal centre in subunit-C, and not subunit-B, is essential for copper-containing membrane monooxygenase activity. Microbiology 160:1267–1277
Ro SY, Schachner LF, Koo CW, Purohit R, Remis JP, Kenney GE, Liauw BW, Thomas PM, Patrie SM, Kelleher NL, Rosenzweig AC (2019) Native top-down mass spectrometry provides insights into the copper centers of membrane bound methane monooxygenase. Nat Commun 2019:2675–2686
Opden Cam HJM, Islam T, Stott MB, Harhangi HR, Hynes A, Schouten S, Jetten MSM, Birkeland N-K, Pol A, Dunfield PF (2009) Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ Microbiol Rep 1(5):293–306
Peng W, Qu X, Shaik S, Wang B (2021) Deciphering the oxygen activation mechanism at the CuC site of particulate methane monooxygenase. Nat Catal 4:266–273
Klinman JP (1996) Mechanisms whereby mononuclear copper proteins functionalize organic substrates. Chem Rev 96(7):2541–2562
Prigge ST, Eipper BA, Mains RE, Amzel LM (2004) Dioxygen binds end-on to mononuclear copper in a precatalytic enzyme complex. Science 304(5672):864–867
Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM (1997) Amidation of bioactive peptides: the structure of peptidylglycine alpha-hydroxylating monooxygenase. Science 278(5341):1300–1305
Thomas SA, Matsumoto AM, Palmiter RD (1995) Noradrenaline is essential for mouse fetal development. Nature 374:643–646
Hidaka H (1971) Fusaric (5-butylpicolinic) acid, an inhibitor of dopamine beta-hydroxylase, affects serotonin and noradrenaline. Nature 231(5297):54–55
Vendelboe TV, Harris P, Zhao Y, Walter TS, Harlos K, El Omari K, Christensen HE (2016) The crystal structure of human dopamine beta-hydroxylase at 2.9 Å resolution. Sci Adv 2(4):e1500980
de la Lande A, Marti S, Parisel O, Moliner V (2007) Long distance electron-transfer mechanism in peptidylglycine alpha-hydroxylating monooxygenase: a perfect fitting for a water bridge. J Am Chem Soc 129(38):11700–11707
Blackburn NJ, Rhames FC, Ralle M, Jaron S (2000) Major changes in copper coordination accompany reduction of peptidylglycine monooxygenase: implications for electron transfer and the catalytic mechanism. J Biol Inorg Chem 5(3):341–353
Jaron S, Blackburn NJ (2001) Characterization of a half-apo derivative of peptidylglycine monooxygenase. Insight into the reactivity of each active site copper. Biochemistry 40(23):6867–6875
Siebert X, Eipper BA, Mains RE, Prigge ST, Blackburn NJ, Amzel LM (2005) The catalytic copper of peptidylglycine alpha-hydroxylating monooxygenase also plays a critical structural role. Biophys J 89(5):3312–3319
Chufan EE, Prigge ST, Siebert X, Eipper BA, Mains RE, Amzel LM (2010) Differential reactivity between two copper sites in peptidylglycine alpha-hydroxylating monooxygenase. J Am Chem Soc 132(44):15565–15572
Evans JP, Ahn K, Klinman JP (2003) Evidence that dioxygen and substrate activation are tightly coupled in dopamine beta-monooxygenase Implications for the reactive oxygen species. J Biolog Chem 278(50):49691–49698
Klinman JP (2006) The copper-enzyme family of dopamine beta-monooxygenase and peptidylglycine alpha-hydroxylating monooxygenase: resolving the chemical pathway for substrate hydroxylation. J Biol Chem 281(6):3013–3016
Itoh S (2015) Developing mononuclear copper-active-oxygen complexes relevant to reactive intermediates of biological oxidation reactions. Acc Chem Res 48(7):2066–2074
Elwell CE, Gagnon NL, Neisen BD, Dhar D, Spaeth AD, Yee GM, Tolman WB (2017) Copper-oxygen complexes revisited: structures, spectroscopy, and reactivity. Chem Rev 117(3):2059–2107
Kunishita A, Ertem MZ, Okubo Y, Tano T, Sugimoto H, Ohkubo K, Fujieda N, Fukuzumi S, Cramer CJ, Itoh S (2012) Active site models for the CuA site of peptidylglycine α-hydroxylating monooxygenase and dopamine β-monooxygenase. Inorg Chem 51:9465–9480
Sanchez-Eguia BN, Flores-Alamo M, Orio M, Castillo I (2015) Side-on cupric-superoxo triplet complexes as competent agents for H-abstraction relevant to the active site of PHM. Chem Commun 51(55):11134–11137
Chen P, Solomon EI (2004) Oxygen activation by the noncoupled binuclear copper site in peptidylglycine alpha-hydroxylating monooxygenase. Reaction mechanism and role of the noncoupled nature of the active site. J Am Chem Soc 126(15):4991–5000
Crespo A, Marti MA, Roitberg AE, Amzel LM, Estrin DA (2006) The catalytic mechanism of peptidylglycine alpha-hydroxylating monooxygenase investigated by computer simulation. J Am Chem Soc 128(39):12817–12828
Wu P, Fan F, Song J, Peng W, Liu J, Li C, Cao Z, Wang B (2019) Theory demonstrated a “coupled” mechanism for O2 activation and substrate hydroxylation by binuclear copper monooxygenases. J Am Chem Soc 141(50):19776–19789
Gherman BF, Cramer CJ (2009) Quantum chemical studies of molecules incorporating a Cu2O22+ core. Coord Chem Rev 253:723–753
Acknowledgements
We thank the financial support from NSFC (Nos. 21933009 and 22073077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Fang, W., Peng, W. et al. Recent Computational Insights into the Oxygen Activation by Copper-Dependent Metalloenzymes. Top Catal 65, 187–195 (2022). https://doi.org/10.1007/s11244-021-01444-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01444-x