Abstract
The photocatalytic activity of AgBr has been investigated. AgBr(N2) was prepared by solid(AgNO3)–solid(KBr) reaction at different temperatures in a stream of N2. AgBr(N2) prepared at 250 °C showed the highest H2 generation activity although the larger crystallites of Ag were observed. When the preparation was carried out under air [AgBr(air)] at 250 °C, the photoactivity and the crystallization of Ag were lowered by the formation of silver oxides species in AgBr(air) probably during the natural cooling under air. It is pointed out however that the amount of hydrogen of both AgBr(N2) and AgBr(air) increased linearly increasing with reaction time regardless of the formation of large Ag crystallites even after UV irradiation for 50 h. This suggests that the behavior of Ag formed might be different from that of the latent image in the photographic process.
Similar content being viewed by others
1 Introduction
Photosensitive property of silver bromide (AgBr) is used in photographic film in the past but many efforts have been made to develop the photosensitive silver halides systems for the creation of the clear latent image formed as Ag metals [1]. It was believed that when AgBr was used as a photocatalyst, the formation of the latent image decreased the photocatalytic activity of AgBr. We have previously evaluated the photocatalytic activity of AgBr from the hydrogen evolution. Although Ag metals were formed in AgBr, the hydrogen evolution was continuously observed from CH3OH/H2O aqueous solution under UV irradiation for 200 h [2]. In addition, the photo-evolution of oxygen was observed using AgBr dispersed in silver nitrate aqueous solution. This means that the potentials of the conduction band (CB) and the valence band (VB) are allowable for the photo-water splitting reaction. This was supported by potential studies concerning both CB and VB of AgBr [3–5]. On the other hand, the VB position of AgCl promotes the photo-generation of oxygen but no photo-generation hydrogen occurs [6, 7], which indicated that the position of CB is not enough.
Recently, photocatalytic studies on silver halides have been reported in the various fields due to the lower band gap energies of 2.6 eV for AgBr and 3.0 eV for AgCl, respectively [8]. Hu et al. [9] reported the photoactivities of Ag/AgBr/TiO2 and Ag/AgBr/Al2O3 [10] prepared by the unique preparation of Ag/AgBr, which were used cetyltrimethylammonium bromide (CTAB) or polyethylene glycol–block–polyethylene glycol (P123). The catalysts showed high activities for the degradation and mineralization of toxic persistent organic pollutants by the effective electron-transfer processes on AgBr. The roles of silver metal species were explained by the hole scavenger [9] and the plasmon-induced Ag nanoparticles [10, 11].
Porous AgCl/Ag derived from Ag20Al80 alloy foil showed a good performance in the degradation of methylic orange dye as a visible-light catalyst reported by Li and Ding [12]. They explained that the nanocomposite and the nanoporous silver with original structure caused the photoactivity in the visible-light region. Core shell Ag@AgBr has been prepared by the ion-exchange reaction between Ag2MoO4 and HBr, and Ag@AgBr showed an efficient visible-light photocatalytic activity, too [13]. The photoactivities of silver halides supported on various materials (BiOBr [3, 4], Bi2WO6 [14, 15], TiO2 [9, 16], Al2O3 [10], H2WO4 [17], AlMCM-41 [18], ZnO [19] and Zeolite [20, 21]) were investigated. Iijima and coauthors [22] reported that the inner space of single-wall carbon nanotubes acted effectively for the photoreactivity preservation of AgBr nanowires.
The photochromic effect is also known as another property of AgBr. A commercial sunglasses was manufactured by Corning Incorporated in past. The color of glass was changed reversibly between clear at dark places and opaque at bright places [23]. The sunglasses were prepared by a solid–solid reaction (melting method), which is an interesting method to avoid carbon residues in AgBr by the emulsion method using a gelatin. In this study, AgBr was prepared by the melting method in order to compare the photoactivity of AgBr prepared by the conventional precipitation method. The activity of AgBr was evaluated through H2 evolution under UV light irradiation. The effect of the preparation condition (N2 and air) on photoactivity of AgBr was also examined.
2 Experimental
Silver bromide (AgBr) was prepared by the melting method under deferent environments (N2 and air). All chemicals were purchased and used without further purifications. In brief, physically mixed KBr–AgNO3 powder, with equimolar composition, was heated using quartz tube at different temperatures (250, 350 and 650 °C) for 2 h. After the reaction, products were naturally cooled in the stream of N2 or air down to room temperature. Products were washed to remove KNO3, and then the samples were dried at room temperature. AgBr by the precipitation method was also prepared. The AgNO3 solution was poured into the KBr solution at 60 °C. The temperature of mixed solution was kept at 60 °C. In order to assist the crystallization of AgBr, the precipitate samples was further aged at 100 °C for 2 h in boiling water, and then finally the precipitate samples was dried under vacuum.
The photoactivity under UV light irradiation (100 W High pressure Hg Lamp: Ushio) was evaluated using an inner-irradiated photoreaction cell and generation of H2 was recorded by using an on-line gas chromatograph(GC-8A, Shimazu) equipped with a thermal conductivity detector (TCD). X-ray diffractometer (XRD, RINTO 2000, Rigaku) was operated at 30 kV and 20 mA for the determination of crystallite structures before and after photoreactions.
3 Results and Discussion
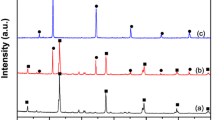
Figure 1 exhibits XRD patterns of AgBr prepared by the melting method with different reaction temperatures and the precipitation method. No clear differences in XRD profiles were observed. The crystallite size of AgBr was calculated by Scherrer equation from XRD (200) peak position. The crystallites sizes prepared by the melting method were found to be more than 50 nm, while the crystallite size by the precipitation method was somewhat small (46 nm). The larger crystallite sizes indicated the formation of crystallized AgBr.
The hydrogen evolution activity of AgBr is exhibited in Fig. 2. Na2S/Na2SO3 aqueous solution was used as a sacrificial reagent. AgBr prepared by the melting method at 250 °C showed the highest H2 production activity for 5 h in comparison with the activities of other silver bromides. The H2/Ag molar ratio exceeded 1.0, indicating that photoelectrons excited to the CB were not completely trapped by the electron traps for the formation of latent image but a part of photogenerated electrons of CB were consumed for the formation of H2 molecules. Presumably, the silver atoms act also as the electron trapping sites and promote the formation of H2 molecules such as Pt on TiO2.
In order to investigate the effect of the preparation condition (N2 and air) on the hydrogen evolution activity, AgBr(N2) and AgBr(air) were prepared by the melting method at 250 °C. The result is shown in Fig. 3. The photoactivities were clearly different, where AgBr(N2) showed higher H2 evolution activity as compared to AgBr(air). The induction period was observed on AgBr(air) but not on AgBr(N2). It is reported that silver oxide (Ag2O) was decomposed by the exposure to light even at 25 °C [24]. It is expected that the concentration of oxidized silver species in AgBr(air) is higher than that of AgBr(N2). Those oxidized silver species in AgBr(air) inhibited the H2 evolution at the beginning of the photoreaction. The hydrogen evolution rate was not so improved after the induction period as expected (Fig. 3). This seems that the active sites (Ag metals) created during the induction period, which the concentration was dependent on the light penetration depth, do not facilitate effectively for the hydrogen generation due to the lower concentration of AgBr species which promotes the absorption of UV-light in the neighborhood of active sites. On the other hands, when the preparation was carried out in a N2 stream, the induction period caused by oxidized silver species was not measured on AgBr(N2). Figure 4 shows the H2 evolution activities of the prolonged photoreaction for 50 h. The hydrogen evolution on both AgBr(N2) and AgBr(air) increased linearly with irradiation time, although the evolution activity of AgBr(air) was still low. The low activity of AgBr(air) suggests that the amount of oxide silver species acted negatively to the photoproperty of AgBr although no XRD peaks assigned to silver oxides were detected as shown in Fig. 1. We presume that oxide silver species are amorphous phase or unstabilized form. However, the formation of surface oxide or oxygen-adsorbed species on AgBr(N2), which were probably formed by the exposure to air at room temperature, did not affect the H2 evolution efficiency. XRD results after the photoreaction for 50 h are shown in Fig. 5. XRD peaks corresponding to Ag were clearly observed. The peak intensity ratios of Ag(111)/AgBr(200) were 0.13 for AgBr(air) and 0.20 for AgBr(N2), respectively. This means that the crystallization of Ag species is assisted by the photoreaction. The AgBr property might be influenced by the presence and the concentration of oxidized silver species in the preparation of AgBr in air at 250 °C. As the decomposition of silver oxides begins around 160 °C [24], most of silver species might be not oxidized at 250 °C (reaction temperature). However, it is possible that silver oxides species might be re-formed during the natural cooling down to room temperature in the furnace. The location and concentration of silver oxides species in AgBr might be changeable by the cooling down condition. The formation of Ag atoms derived from those species is one of the reasons for the lower photoactivity of AgBr(air). In addition, the species caused by oxygen act negatively the aggregation of Ag species formed during the photoreaction from peak intensity ratios of Ag/AgBr between AgBr(air) and AgBr(N2).
We know that when AgBr is exposed to light, the color is changed due to the formation of Ag metals. This means that the location of Ag metals is expected to be distributable on the AgBr surface, indicating the formation of thin Ag metal islands. Therefore, the photoreaction is deactivated by Ag metal islands covered on AgBr surface. When AgBr was prepared under the N2 stream, the XRD results after the photoreaction confirmed the formation of Ag metals with larger crystallite size. However, the H2 evolution activity of AgBr(N2) increased linearly with increasing irradiation time. This means that Ag metals, the size is surprisingly larger, do not fully cover with AgBr surface to inhibit the absorption of UV light, as reported previously [4]. It is believed that Ag metals as latent image formed on AgBr are located within the specific sites (such as Frenkel defect sites and electron trapping sites); the locations are limited due to a very small number of those sites [1]. Presumably, the mechanism of the crystal growth of Ag metals induced by the photoreaction in the liquid phase seems to be the the different from that of the latent image formation of photographic films in air. Futher investigation is necessary to evaluate the behavior of Ag formed, resulting in the formation of larger Ag crystallites in AgBr, and development of a new preparation method for the retarding the formation of Ag crystallite during the photoreaction.
In addition, it is known that Ag species play an important role to inhibit bacterial growth. We examined the antibacterial effect of AgBr and Ag/AgBr. As shown in Fig. 6, the inhibition of colony growth was also observed on AgBr with Ag metals. The occurrences of antibacterial activity were observed mainly the interface of AgBr regardless of the presence of Ag atoms, which indicated that AgBr is useful also for the sterilization such as Ag species.
4 Conclusion
AgBr was an effective photocatalyst although the aggregation of Ag metals occurred during the photoreaction. The photoactivities of AgBr(N2) and AgBr(air) increased linearly with increasing irradiation time and the activities were not deactivated by the formation of larger crystallites of Ag even after UV irradiation for 50 h. The presence of silver oxide formed during the cooling process [AgBr(air)] gave the induction period for hydrogen generation but the aggregation of Ag metals was retarded somewhat. It is pointed out that the hydrogen generation activity of AgBr(air) was low but the amount of hydrogen generation increased linearly with increasing irradiation time as mentioned above. The photoactivity suggests that the behavior of Ag formed seems to be different from the mechanism of the latent image formation of photographic field. As AgBr is an effective photocatalyst for the hydrogen generation, it is necessary to evaluate the behavior of Ag formed during the photoreaction. This leads to development of a new AgBr with new electron trap elements, resulting in the retarding the aggregation of Ag metals. In addition, AgBr was also effective for the sterilization such as Ag species.
References
Tani T (1995) Photographic sensitivity: theory and mechanisms. Oxford series in optical and imaging sciences. Oxford University press, New York
Kakuta N, Goto N, Ohkita H, Mizushima T (1999) J Phys Chem B 103:5917–5919
Cheng H, Huang B, Wang P, Wang Z, Lou Z, Wang J, Qin X, Zhang X, Dai Y (2011) Chem Commun 47:7054–7056
Kong L, Jiang Z, Lai HH, Nicholls RJ, Xiao T, Jones MO, Edwards PP (2012) J Catal 293:116–125
Belloni J, Treguer M, Remita H, De Keyzer R (1999) Nature 402:865–867
Calzaferri G (1997) Catal Today 39:145–157
Currao A, Reddy VR, van Veen MK, Schropp REI, Calzafferri G (2004) Photochem Photobiol Sci 3:1017–1025
Seki K, Yanagai H, Kobayashi Y, Ohta T, Tani T (1994) Phys Rev B 49:2760–2767
Hu C, Lan Y, Qui J, Hu X, Wang A (2006) J Phys Chem B 110:4066–4072
Zhou X, Hu C, Hu X, Peng T, Qu J (2010) J Phys Chem C 114:2746–2750
Kuai L, Geng B, Chen X, Zhao Y, Luo Y (2010) Langmuir 26:18723–18727
Li Y, Ding Y (2010) J Phys Chem C 114:3175–3179
Wang P, Huang B, Zhang X, Qin X, Jin H, Dai Y, Wang Z, Wei J, Zhan J, Wang S, Wang J, Whangbo M-H (2009) Chem Eur J 15:1821–1824
Zhang L, Wong K-H, Chen Z, Yu JC, Zhao J, Hu C, Chan C-Y, Wong P-K (2009) Appl Catal A 363:221–229
Zhang L-S, Wong K-H, Yip H-Y, Hu C, Yu JC, Chan C-Y, Wong P-K (2010) Environ Sci Technol 44:1392–1398
Cao J, Xu B, Luo B, Lin H, Chen S (2011) Appl Surf Sci 257:7083–7089
Cao J, Luo B, Lin H, Chen S (2011) J Mol Catal A 344:138–144
Pourahmad A, Sohrabnezhad Sh, Kashefian E (2010) Specrochimica Acta Part A 77:1108–1114
Krishnalumar B, Subash B, Swaminathan M (2012) Sep Purif Technol 85:35–44
Zang Y, Farnood R, Currie J (2009) Chem Eng Sci 64:2881–2886
Yamashita Y, Gao Y, Yoshida K, Kakuta N, Petrykin V, Kakihana M (2005) J Ceram Soc Jpn 113:509–512
Kobayashi K, Suenaga K, Saito T, Shinohara H, Iijima S (2010) Adv Mater 22:3156–3160
Tomonaga H, Morimoto T (2000) J Sol–Gel Sci Technol 19:681–685
Bailar JC, Emeleus HJ, Nyholm R, Trotman-Dicerson AF (1973) Comprehensive inorganic chemistry, vol 3. Pergamon, Oxford, pp 97–98
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, B., Yamamoto, R., Ohkita, H. et al. Photocatalytic Activity of AgBr as an Environmental Catalyst. Top Catal 56, 618–622 (2013). https://doi.org/10.1007/s11244-013-0020-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0020-7