Abstract
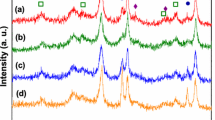
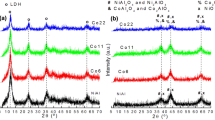
A series of Co–Ni catalysts, prepared from hydrotalcite (HT)-like materials by co-precipitation, has been studied for the hydrogen production by ethanol steam reforming. The total metal loading was fixed at 40% and the Co–Ni composition was varied (40–0, 30–10, 20–20, 10–30 and 0–40). The catalysts were characterized using X-ray diffraction, N2 physisorption, H2 chemisorption, temperature-programmed reduction, scanning transmission electron microscope and energy dispersive spectroscopy. The results demonstrated that the particle size and reducibility of the Co–Ni catalysts are influenced by the degree of formation of a HT-like structure, increasing with Co content. All the catalysts were active and stable at 575 °C during the course of ethanol steam reforming with a molar ratio of H2O:ethanol = 3:1. The activity decreased in the order 30Co–10Ni > 40Co ~ 20Ni–20Co ~ 10Co–30Ni > 40Ni. The 40Ni catalyst displayed the strongest resistance to deactivation, while all the Co-containing catalysts exhibited much higher activity than the 40Ni catalyst. The hydrogen selectivities were high and similar among the catalysts, the highest yield of hydrogen was found over the 30Co–10Ni catalyst. In general, the best catalytic performance is obtained with the 30Co–10Ni catalyst, in which Co and Ni are intimately mixed and dispersed in the HT-derived support, as indicated by the STEM micrograph and complementary mapping of Co, Ni, Al, Mg and O.






Similar content being viewed by others
References
Haryanto A, Fernando S, Murali N, Adhikari S (2005) Energy Fuel 19:2098
Vaidya PD, Rodrigues AE (2006) Chem Eng J 117:39
Ryden M, Lyngfelt A (2006) Int J Hydrogen Energy 31:1271
Ochoa-Fernandez E, Haugen G, Zhao T, Ronning M, Aartun I, Borresen B, Rytter E, Ronnekleiv M, Chen D (2007) Green Chem 9:654
Hossain MM, de Lasa HI (2007) AIChE J 53:1817
Ryden M, Lyngfelt A, Mattisson T (2006) Fuel 85:1631
Hossain MM, Sedor KE, de Lasa HI (2007) Chem Eng Sci 62:5464
Ni M, Leung DYC, Leung MKH (2007) Int J Hydrogen Energy 32:3238
Onsan ZI (2007) Turk J Chem 31:531
Bichon P, Haugom G, Venvik HJ, Holmen A, Blekkan EA (2008) Top Catal 49:38
Batista MS, Santos RKS, Assaf EM, Assaf JM, Ticianelli EA (2003) J Power Sour 124:99
Frusteri F, Freni S, Spadaro L, Chiodo V, Bonura G, Donato S, Cavallaro S (2004) Catal Commun 5:611
Montini T, De Rogatis L, Gombac V, Fornasiero P, Graziani M (2007) Appl Catal A 71:125
Kugai J, Velu S, Song CS (2005) Catal Lett 101:255
Haga F, Nakajima T, Yamashita K, Mishima S, Suzuki S (1997) Nippon Kagaku Kaishi 33
Hu X, Lu GX (2007) J Mol Catal A Chem 261:43
Choudhary VR, Mamman AS (1998) J Chem Technol Biotechnol 73:345
Hardiman KA, Hsu CH, Ying TT, Adesina AA (2005) J Mol Catal A Chem 239:41
Biswas P, Kunzru D (2007) Catal Lett 118:36
Youn MH, Seo JG, Kim P, Kim JJ, Lee HI, Song IK (2006) J Power Sour 162:1270
Svoboda K, Siewiorek A, Baxter D, Rogut J, Pohorely M (2008) Energy Convers Manage 49:221
Chen D, Bjorgum E, Lodeng R, Christensen KO, Holmen (2004) In: Bao X, Xu Y (eds) Studies in surface science and catalysis, vol 147. Elsevier Science, Amsterdam, 139 p
Besenbacher F, Chorkendorff I, Clausen BS, Hammer B, Molenbroek AM, Norskov JK, Stensgaard I (1998) Science 279:1913
Chen D, Christensen KO, Ochoa-Fernandez E, Yu ZX, Totdal B, Latorre N, Monzon A, Holmen A (2005) J Catal 229:82
Christensen KO, Chen D, Lodeng R, Holmen A (2006) Appl Catal A 314:9
Frusteri F, Freni S, Chiodo V, Donato S, Bonura G, Cavallaro S (2006) Int J Hydrog Energy 31:2193
Homs N, Llorca J, de la Piscina PR (2006) Catal Today 116:361
Wang H, Liu PX, Liu Y, Qin YN (2006) Chin. J. Catal 27:976
Zhang BC, Li Y, Cai WJ, Tang XL, Xu YD, Shen WJ (2006) Chin J Catal 27:567
Cavani F, Trifiro F, Vaccari A (1991) Catal Today 11:173
Ochoa-Fernandez E, Lacalle-Vila C, Christensen KO, Walmsley JC, Ronning M, Holmen A, Chen D (2007) Top Catal 45:3
PDF-2 (1994) Joint Committee on Powder Diffraction Standards, International Center of Diffraction Data, Park Lane Swarthmore, Pennsylvania, USA
Yu JJ, Jiang Z, Zhu L, Hao ZP, Xu ZP (2006) J. Phys. Chem. B 110:4291
Kawabata T, Shinozuka Y, Ohishi Y, Shishido T, Takaki K, Takehira K (2005) J Mol Catal A Chem 236:206
Bellotto M, Rebours B, Clause O, Lynch J, Bazin D, Elkaim E (1996) J Phys Chem 100:8535
Bartholomew CH (1990) Catal Lett 7:27
Chmielarz L, Kustrowski P, Rafalska-Lasocha A, Dziembaj R (2003) Thermochim Acta 395:225
Unnikrishnan R, Narayanan S (1999) J Mol Catal A Chem 144:173
Melo F, Morlanés N (2008) Catal Today 133–135:374
Benito M, Sanz JL, Isabel R, Padilla R, Arjona R, Daza L (2005) J Power Sour 151:11
Song H, Zhang LZ, Watson RB, Braden D, Ozkan US (2007) Catal Today 129:346
Resini C, Cavallaro S, Frusteri F, Freni S, Busca G (2007) React Kinet Catal Lett 90:117
Shustorovich E, Sellers H (1998) Surf Sci Rep 31:5
Acknowledgement
The Norwegian Research Council (NFR) is acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, L., Berntsen, H., Ochoa-Fernández, E. et al. Co–Ni Catalysts Derived from Hydrotalcite-Like Materials for Hydrogen Production by Ethanol Steam Reforming. Top Catal 52, 206–217 (2009). https://doi.org/10.1007/s11244-008-9157-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-008-9157-1