Abstarct
Despite a broad application of ureolytic bacteria in many bioremediation and biocementation processes, very limited studies have reported their transport and retention behaviors under various physical–chemical–biological conditions. In this study, we report transport and retention of Sporosarcina pasteurii in saturated sand, based on a series of column breakthrough experiments under different conditions including ionic strengths (ISs: 0.5 mM–1 M), flow velocity (50, 100, 200 cm/h), bacteria optical density (OD600 = 1.0, 0.48), column length (280 mm, 150 mm), and changes in IS conditions (0.5 M CaCl2 or deionised water). We use a two-site kinetic model, representing (1) attachment on grain surfaces, and (2) straining at crevices and constrictions, to quantify and predict the bacterial attachment and straining. Model parameters were calibrated by tracer (NaCl) breakthrough curves (BTCs) and bacteria BTCs at different IS/velocity conditions. The model was then applied to successfully predict the bacteria BTCs at lower initial bacteria density (OD600 = 0.48) and for shorter column lengths (150 mm). We demonstrated that higher ionic strength (from 0.5 to 1000 mM) dramatically enhanced the retention efficiency of S. pasteurii through an enhancement of attachment (from 9.4 to 69.6%) and straining (from 8.1 to 34.2%), whilst the bacterial survival and the urease activity were unaffected at high IS conditions (500 and 1000 mM NaCl) within 5 h. Increasing flow velocity (from 50 to 200 cm/h) caused a decrease in attachment (from 39.5 to 22.4%) and decrease in straining (from 40.5 to 19.3%) as a result of the increased hydrodynamic shear forces, which tends to reduce the attachment at the secondary minimum and decrease the extent of flow stagnation regions for straining. Lower initial bacteria OD600 (from 1.0 to 0.48) enhanced the attachment (from 31.8 to 40.9%) and the straining (from 22.9 to 42.2%) as a result of reducing the site-blockage effect. In addition, 0.5 M CaCl2 with a stronger IS increased the retention of in the column, whilst deionised water with a lower IS caused bacterial release. These findings provide useful information for a better understanding of the transport and fate of Sporosarcina pasteurii in saturated soil, and can be used to optimise bioaugmentation strategy and cementation efficiency for soil improvement.
Article Highlights
-
Transport of S. pasteurii in sands is highly affected by ionic strength, flow velocity, bacteria density, and even column size
-
Straining was enhanced (from 8.1% to 34.2%) if increasing IS (from 0.5 to 500 mM) without affecting bacterial survival
-
Bacteria coagulation among 2–3 bacterial cells occurs under ISs of 500 and 1000 mM without forming large flocculation
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microbially induced calcium carbonate precipitation (MICP) is an environmentally sustainable bio-remediation process that has been widely studied for a range of geo-environmental and geotechnical applications including co-precipitation of metal contaminants or radionuclides (Fujita et al. 2008; Lauchnor et al. 2013), carbon capture and storage (Minto et al. 2017; Mitchell et al. 2010), rock fracture sealing/strengthening (Minto et al. 2016; Phillips et al. 2016; Tobler et al. 2018) and soil improvement (DeJong et al. 2010; Gomez et al. 2015; El Mountassir et al. 2018; Whiffin et al. 2007). One of the most extensively utilised microorganisms for MICP is Sporoscarcina pasteurii (S. pasteurii) which, via the production of the urease enzyme, catalyses the hydrolysis of urea to carbonate and ammonia, resulting in an increase in pH and precipitation of calcium carbonate if calcium is available (Stocks-Fischer et al. 1999). The precipitation of calcium carbonate can act to immobilise contaminants, reduce permeability of porous/fractured media, and improve the hydromechanical properties of soils and/or fractured rocks by bonding solid grains and bridging across surfaces (e.g. increasing shear strength, stiffness, elastic velocity) (Cuthbert et al. 2013; Minto et al. 2016, 2018; El Mountassir et al. 2014).
Bioremediation processes of MICP for soil improvement involves either a bioaugmentation or a biostimulation scheme, or a combination of both (Burbank et al. 2013; Gomez et al. 2017; Jiang et al. 2022). Major advantages of bioaugmentation schemes include faster and higher treatment efficiency. Despite the viability of bioaugmentation having been demonstrated at both laboratory and field scale (Gomez et al. 2015; Martinez et al. 2013; Nassar et al. 2018; van Paassen et al. 2010; Phillips et al. 2016; Salifu et al. 2016), it remains challenging to optimise treatment strategies so as to achieve an effective and even distribution of MICP along the flow direction. Field bioaugmentation treatment strategies involve the injection of multiple fluids: bacterial suspension (e.g. S. pasteurii), cementing solution (composed of urea and calcium chloride as a minimum), and injection of water pulses to minimise precipitation within injection lines and clogging around the well (Minto et al. 2019). Indeed, bioaugmentation may involve many repeated cycles of injection of some, or all, of these fluids. As such, a variety of chemical and physical perturbations are introduced after bacterial injection, such as changes in pore solution chemistry due to injection of the cementing solution (calcium chloride and urea), and due to injection of water pulses to prevent clogging. In addition, the injection of bacteria in porous/fractured media involves a non-uniform distribution of flow velocity, potentially causing spatial differences in attachment/detachment behavior due to variations in the hydrodynamic forces (Camesano and Logan 1998; Hendry et al. 1999). A fundamental understanding of the transport and fate of S. pasteurii in porous/fractured media under various controlling factors (e.g. ionic strength, chemical perturbation, flow velocity) is key to achieving an effective delivery of bacteria in bioaugmentation schemes and hence to achieving more efficient and economical MICP applications (Hommel et al. 2016; Kwak et al. 2023).
Two main bacteria retention phenomena are known to occur; physicochemical deposition (attachment) and straining. Colloid filtration theory (Yao et al. 1971) is widely used to predict retention of particles in suspension, but may not be adequate for predicting bacterial attachment and straining (Bradford et al. 2006; Foppen et al. 2007) due to the bacteria-specific cell characteristics (Liu et al. 2011; Stumpp et al. 2011). Predicting bacterial attachment and straining in natural porous media is considered challenging (Hommel et al. 2016) as both may be influenced by factors such as fluid chemistry (Harvey et al. 2010; Kinoshita et al. 1993; Redman et al. 2004; Torkzaban et al. 2008b), pore velocity (Camesano and Logan 1998; Choi et al. 2007), temperature (McCaulou et al. 1995), surface roughness (Shellenberger and Logan 2002), and grain size (Bai et al. 2016; Gargiulo et al. 2007). Bradford et al. (2013) reviewed the transport and fate of microbial pathogens in agricultural settings and summarised the literature on one- or two-site kinetic models for quantifying attachment and straining of bacteria or colloids based on the HYDRUS-1D software package (Bradford et al. 2006, 2003; Foppen et al. 2007; Simunek et al. 2005; Šimůnek et al. 1999, 2006; Torkzaban et al. 2008a). They noted that each study advanced the understanding of attachment and pore straining phenomena under relevant physical and chemical conditions. However, bacterial transport and fate is known to vary with bacterial strain and is highly dependent on cell surface composition (Rijnaarts et al. 1996; Walker et al. 2004), cell motility (Creppy et al. 2019; Liu et al. 2011), cell size and shape (Li and Ma 2018; Seymour et al. 2013; Weiss et al. 1995), and even growth stage (Walker et al. 2005). To date, very limited studies have been systematically conducted on investigation of the transport behavior of S. pasteurii, which may behave differently to other bacteria and colloids. Tobler et al. (2014) investigated S. pasteurii transport within sandstone cores for two flow rates, two different bacteria densities and three rock core lengths. Jain and Arnepalli (2020) studied attachment and detachment of ureolytic bacteria on sands under a range of ionic strengths (0–100 mM). However, to underpin the optimization of bioaugmentation schemes for engineering soil improvement, there remains a need to understand and predict the transport and fate of S. pasteurii under a broad range of ionic strengths, with varying flow velocities and bacterial concentrations (Minto et al. 2019; van Wijngaarden et al. 2016, 2011). In particular, research has shown that the increase in soil strength via calcite precipitation is mainly determined by the formation of calcite bridges between grain contacts, rather than by the coating of grain surfaces with calcite (Ismail et al. 2002; Tang et al. 2020; Xiao et al. 2019), To this end, maximising straining of S. pasteurii via pore straining or wedging near grain-to-grain contact points, is critical for optimizing soil strength. To-date, there have been no reports on quantifying straining of S. pasteurii in saturated porous media.
The main purpose of this work is to explore the transport and fate of S. pasteurii in saturated sands under a wide range of ionic strengths (ISs from 0.5 to 1000 mM), flow velocities (50, 100, 200 cm/h), initial bacteria optical densities (1.0 and 0.48 OD600), column lengths (280 and 150 mm), and under changes in IS conditions (i.e. 0.5 M CaCl2 and DI water) in the context of bioaugmentation schemes for soil improvement via MICP. The straining profiles of bacteria under the tested IS conditions were obtained based on extruded sand segments after conducting breakthrough tests. We also tested the survival of S. pasteurii and its urease activity under a series of IS conditions (DI water, tap water, 500 and 1000 mM NaCl) to evaluate its adaptability under such low and high salinities. Finally, a two-site kinetic model, representing both attachment and straining of S. pasteurii, was calibrated and then applied to predict tracer and bacteria breakthrough curves using different IS conditions and bacteria concentration. This research can used to provide model parameters for design of MICP-based bioaugmentation strategies for soil improvement.
2 Materials and Methods
2.1 Bacterial Culture
The ureolytically active bacterial strain S. pasteurii (DSMZ, DSM No. 33) were cultured in yeast extract media supplemented with urea (20 g/L) over a period of 24 h (at 30 °C and 115 rpm shaker). The cells were harvested by centrifugation of several 40-ml inoculated solutions in 50-ml tubes at a speed of 6000 g for 8 min. Then the centrifuged bacteria in each tube were re-suspended into 40-ml deionised (DI) water and centrifuged again to further remove any residual media that may cause continued bacterial growth. The desired optical density (1 OD600 or 0.48 OD600) of the bacteria was then achieved by diluting in either tap water or NaCl solution at various IS and was determined by a UV–VIS spectrophotometer at a wavelength of 600 nm. Figure 1a shows the OD600 of S. pasteurii and the corresponding cell concentration (Ccells/ml) as measured at the University of Strathclyde (Steinacher 2021) on the same bacteria strain (DSM No. 33). Ccells/ml at a given OD600 is well-fitted by the empirical relationship by Ramachandran et al. (2001): \({C}_{cells/ml}=8.59\times {10}^{7}\times {\left({\mathrm{OD}}_{600}\right)}^{1.3627}\). Zeta potential values of S. pasteurii, under varying IS, were measured (Fig. 1b) and used to evaluate the extended DLVO interaction energies.
a Empirical relation between cell concentration and bacteria OD600 of S. pasteurii. The experimental data were cited from (Steinacher 2021) whilst the fitting relation was adopted from (Ramachandran et al. 2001). b zeta potentials of S. pasteurii and sands measured under ionic strengths of 0.5, 30, 500, 1000 mM NaCl
2.2 Sand Characterization
Brown gravelly quartz sands (BAM Ritchies, UK) were selected for the column breakthrough tests. Particle-size distribution (SI Figure S1 in the Supporting Information) was determined by using US standard sieves according to ASTM D422. The median grain size (d50) was determined as 490 µm. The uniformity index (d60/d10) was measured as 2.98. The sand samples were washed using DI water several times to remove impurities and fines that may affect the measurement of bacteria optical density. Prior to the column breakthrough tests, the oven-dried sand samples were autoclaved for 3 h to sterilise the sand. The zeta potential values of the sands, under varying IS, were measured and are plotted in Fig. 1b.
2.3 Column Breakthrough Experiments
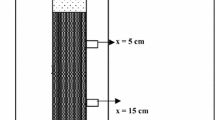
Figure 2 shows the schematic diagram of the set-up used in the column breakthrough experiments. Sand was packed into polycarbonate columns with an inner diameter of 28 mm and an effective length of 280 mm. Screens were mounted at both inlet and outlet ends to evenly distribute 1-dimensional flow in the column. Prior to the bacterial breakthrough test, the sand columns were flushed with DI water for 10 min at a relatively high Darcy velocity (~ 400 cm/hour) to achieve a fully-saturated state and to further remove impurities and fines that may influence the measurement of bacteria density of effluent samples. A pore volume (PV) was defined as the change in mass (in grams) of the sand column before and after flushing with DI water (the small amount of water in the column head space was excluded). One PV of each 28-cm column was estimated to be ~ 50 cm3, assuming a density of water of 1 g/cm3. Note that this method may not precisely quantify the PV of each sand column, hence, a slight correction to PV was applied after fitting the tracer breakthrough curves (BTCs). The packing density of the sands in each column was calculated to be 1.75 ± 0.02 g/cm3, with a porosity of 0.29 ± 0.01. The column breakthrough tests, under each condition as described below, were conducted in duplicate, triplicate or quadruplicate.
We set up the following four groups of experiments to study the effects of IS, flow velocity, initial bacteria density, and column size on the transport and fate of S. pasteurii in saturated sand columns. Details of the experimental conditions for the column breakthrough test are summarised in Table 1. Highlighted in bold are the controlled variables. For example, Experiment group 0 describes tracer breakthrough tests under three different flow velocities (50, 100, 200 cm/h), and Experiment group 1 describes bacteria breakthrough tests under an IS of 0.5, 30, 100, 500, 1000 mM.
Experiment 0 Tracer breakthrough test.
A tracer (NaCl) breakthrough test was conducted at the three flow (Darcy) velocities (50 cm/h, 100 cm/h, 200 cm/h) for the determination of the dispersion parameters. 3 PVs of 100 mM NaCl solution were injected at each velocity, followed by the injection of 3 PVs of DI water at the same velocity. The electrical conductivities of effluent samples were measured and the normalised breakthrough curves of tracer (NaCl) were obtained based on the linear relationship between electrical conductivity and ionic concentration.
Experiment 1 Effect of ionic strength (tap water, 30 mM, 100 mM, 500 mM, and 1000 mM NaCl).
In the first stage, 3 PVs of bacterial suspension with OD600 = 1 and prepared at each IS condition, i.e., tap water (electrical conductivity equivalent to that of 0.5 mM NaCl), 30 mM, 100 mM, 500 mM and 1 M NaCl solutions, were injected at a Darcy velocity of 100 cm/h. In the second stage, the 3-way valve was switched to continuously inject 3 PVs of bacteria-free background (NaCl or tap water) solution with the same IS and same flow velocity as applied in the first stage. The total 6 PVs of effluent samples were evenly distributed into 50 collection tubes with each one containing ~ 0.12 PV (~ 6 ml) solution. Electrical conductivity and OD600 of the effluent samples were determined using an electrical conductivity meter and a spectrophotometer at 600 nm, respectively. Normalised breakthrough curves of bacteria and tracer (NaCl) were obtained based on the linear relationship between electrical conductivity and ionic concentration, while normalised breakthrough curves of bacteria were obtained based on a relationship between OD600 and cell concentration for S. pasteurii (refer to Fig. 1a): \({C}_{cells/ml}=8.59\times {10}^{7}\times {\left({\mathrm{OD}}_{600}\right)}^{1.3627}\). After the bacteria breakthrough experiment at each IS condition, the spatial distribution of the strained bacteria retained in each sand column was also obtained based on similar experimental protocols from previous studies (Bradford et al. 2002; Sang et al. 2013). Specifically, the sand samples were extracted from the column and segmented into fourteen 2-cm sections. Each section was gently immersed into 20 ml background NaCl (or tap water) solution with the same IS applied in the breakthrough experiment to release the bacteria retained exclusively by pore straining. Since there were no changes in ionic strength and negligible physical perturbation (gentle shaking), one can reasonably assume that there was a negligible release of the bacteria that were deposited on the grain surfaces due to attachment. These samples were left for 5 h to allow settlement of any fines that may interrupt the measurement of OD600. Then the supernatant, i.e. the suspension containing the released bacteria was removed via a pippette and the OD600 was determined using a spectrophotometer.
Experiment 2 Effects of flow velocity (50 cm/h, 100 cm/h, 200 cm/h) and 0.5 M CaCl2 solution.
At the first stage, 3 PVs of S. pasteurii (OD600 = 1) at an IS of 100 mM NaCl solution were injected under each flow (Darcy) velocity (50 cm/h, 100 cm/h, 200 cm/h). At the second stage, instead of injecting 3 PVs of background NaCl solution, 3 PVs of 0.5 M bacteria-free CaCl2 solution (with a much stronger IS than that of a 100 mM NaCl solution) were injected under the same flow velocity to reproduce the injection of cementing solution during MICP treatment. This process caused an enhancement of the IS (from 100 to 1500 mM); The three velocities (50 cm/h, 100 cm/h, 200 cm/h) were selected to approximately cover the velocity range of radial flow (influential area: diameter = 1 m) around an injection well, based on an injection rate of ~ 20 L/minute and an injection depth of 1 m.
Experiment 3 effect of bacteria density (0.48 OD600).
Similar to Experiment 2, 3 PVs of S. pasteurii (OD600 = 0.48) at an IS of 100 mM NaCl solution and a flow velocity of 100 cm/h were flushed in the first stage, followed by an injection of 3 PVs of 0.5 M CaCl2 solution at a flow rate of 100 cm/h in the second stage. The final bacterial breakthrough curve was normalised by the initial bacterial concentration at an OD600 of 0.48.
Experiment 4 Effects of column size and DI water elution.
To study the effect of column size on bacterial transport, breakthrough experiments were conducted in 52-mm diameter and 150-mm length columns at a flow velocity of 100 cm/h. At the first stage, 3 PVs of S. pasteurii (OD600 = 1) at ISs of 100 mM or 500 mM (NaCl) were injected, followed by an injection of 3PVs of background NaCl solution at the second stage with the same IS and same flow velocity as applied in the first stage. At last, 5 PVs of DI water were flushed at the same velocity.
2.4 Bacterial Survival Under High IS
Bioaugmentation typically involves a two-phase grouting strategy i.e. Phase 1—augmenting/injecting bacteria solution, and Phase 2—Circulation of cementing solution CaCl2 & Urea. Between the two phases there may, or may not, exist a short static (no-flow) period for bacterial settlement. To explore the survival of S. pasteurii and its effectiveness for urea hydrolysis especially under high IS conditions (i.e. 500 and 1000 mM) within the timescales required for bioaugmentation, we conducted 5 sets of urease activity tests and OD600 tests (each set in duplicate). Specifically, after 24-h culturing in the yeast extract media the S. pasteurii were centrifuged and re-suspended either into DI water, tap water, 500 mM NaCl solution, 1000 mM NaCl solution or the originally centrifuged media. After 5-h static period, 2 ml re-suspended bacteria (OD600 = 0.75) in the above solutions were re-inoculated into 40 ml fresh media. After 24-h incubation at 30 °C and on a 115-rpm shaker, bacteria OD600 and specific urease activity, which quantifies the rate of urea hydrolysis (unit: mM/min/OD600) for MICP, were measured and compared to evaluate the influence of high NaCl concentration (500 and 1000 mM) on the effectiveness of S. pasteurii. The experimental results indicated that the effect of high salinity (up to 1000 mM) on bacteria survival and activity was negligible (refer to SI Figure S2 in the supporting information for more details).
2.5 Two-site Kinetic Modelling
The transport of bacteria and tracer in homogeneous and saturated porous sand columns can be described by the following two equations (Tan et al. 1994), respectively.
where ϕ [-] is water-filled porosity of the sand column; C [Nc/L3] is the bacterial concentration suspended in the solution (number of cells per unit volume of the aqueous solution) and c [M/L3] is the concentration of the tracer; t is time [T]; S [Nc/M] is the bacterial concentration deposited on the solid particles (number of cells per unit mass of solid particles); ρb [M/L3] is the bulk density of the dry sand column; Accordingly, (ρb·S/ϕ) is the deposited bacterial concentration defined as the number of bacterial cells attached on the solid particles per unit volume of pore-solution. u [L/T] is the Darcy velocity; Rd and Rg are the rates [Nc/L3/T] of bacterial decay and growth, respectively; D [L2/T] is the dispersion coefficient of the bacteria, which is a function of the hydrodynamic dispersivity α [L] and effective diffusion coefficient dc [L2/T] (Minto et al. 2019; Tobler et al. 2014).
Assuming that there is negligible bacterial decay and growth during the transport process, the bacterial concentration (S) deposited on the solid particles can be governed by a two-site kinetic model (Bradford et al. 2013; Foppen et al. 2007), which defines attachment (S1) on the surfaces of soil particles (site 1) and pore straining (S2) at crevices and constrictions (site 2). The total number of deposited bacteria equals the sum of bacterial retention in both sites as S = S1 + S2. The two-site deposition model is given by the following two equations.
where Kattach0 [1/T] is the initial first-order attachment rate; Kdetach0 [1/T] is the detachment rate due to hydrodynamic shear; Kstrain0 [1/T] is the initial first-order straining rate. Here, we assume that pore straining (S2) is an irreversible process and there is no term for bacteria release in Eq. (5).ψ1 and ψ2 in Eqs. (4) and (5) are dimensionless decay functions for site 1 (attach) and site 2 (straining) respectively. Decay of the attachment rate could be attributed to a decreasing number of collisions between suspended bacteria and grains according to colloid filtration theory. Whereas, decay of the straining rate is due to blocking or filling at the crevices and constrictions as straining proceeds. To model the decay of the attachment rate and straining rate due to a decrease in collisions between bacteria and grains or site blocking, both ψ1 and ψ2 decrease with increasing bacterial retention in each site (Gargiulo et al. 2008; Liao et al. 2021; Sasidharan et al. 2014). In this study, we adopt an exponential form (Xu et al. 2006; Xu and Saiers 2009) of the decaying functions, given by
where 1/λ1 and 1/λ2 are the decay constants of bacterial attachment (site 1) and straining (site 2), respectively. λ1 and λ2 have the same dimension as S [Nc/M] and quantifies the exponential decline in rates of attachment and pore straining.
The total mass recovery of the bacteria is defined as the total accumulation of effluent bacteria passing through the outlet divided by the total accumulation of bacteria injected at the inlet.
where L [L] and A [L2] are the length and cross-sectional area of the column respectively. C (0, t) and C (L, t) are bacterial concentrations at the inlet and outlet at time t, respectively. t0 [T] is the duration of bacterial injection, tend is the total duration from the beginning of bacterial injection to the end of the target stage.
The above transport model was solved by the finite-element-based COMSOL Multiphysics to fit and validate the 1-dimensional column test results. The geometry contained 280 domain elements with the mesh size being 1 mm. The initial condition, inlet and outlet boundary conditions are given by,
where x is the distance to inlet. The initial concentration of tracer and bacteria was set to 0 during the 1st Stage (i.e., the injection of bacteria solution). Then, the value of final step in the 1st Stage was used as the initial condition for the 2nd stage (i.e., flushing). The inlet boundary for bacteria transport was: Cinlet = 1 OD600 (or 0.48 OD600 in Experiment 3) during flushing with a bacteria solution (\({C}_{cells/ml}=8.59\times {10}^{7}\times {\left({\mathrm{OD}}_{600}\right)}^{1.3627}\)); Cinlet = 0 OD600 during flushing with a bacteria-free solution. The inlet boundary for tracer transport was given by the realistic ionic strengths, i.e. cinlet = 0.5, 30, 100, 500, or 1000 mM for Experiment 1, cinlet = 1500 mM for flushing 0.5 M CaCl2 in Experiments 2&3 during the 2nd stage, and cinlet = 0 mM for flushing DI water in Experiment 4 during the 3rd stage.
The attachment/straining rates under the injection of 0.5 M CaCl2 solution are defined as follows.
where IS1 and IS2 are the realistic ionic strengths of the 100 mM NaCl solution (IS1 = 100 mM) and 0.5 M CaCl2 solution (IS2 = 1500 mM), respectively. ΔKattach and ΔKstrain are the enhanced attachment rate and straining rate, respectively. \(\Delta {\lambda }_{1}\) and \(\Delta {\lambda }_{2}\) are the changes in decay constants under the injection of 0.5 M CaCl2. Equations (12) and (13) indicate that the retention parameters will be enhanced once the moving front of the CaCl2 solution ingresses (i.e. \({I}_{S}>{I}_{S1}=100 \mathrm{mM}\)), until it reaches a steady equilibrium value (i.e. \({I}_{S}={I}_{S2}=1500 \mathrm{mM}\)).
Similarly, for Experiment 4 during the 3rd Stage, the injection of 5 PVs of DI water also caused a gradual decrease in IS. Hence, the release of bacteria due to DI water elution is modelled by introducing an increase in the detachment rate kdetach as follows.
where \({\Delta K}_{detach}\) is the increment of the detachment rate under the elution of DI water.
3 Results and Discussion
3.1 Transport of a Conservative Tracer
Figure 3 shows the observed and modelled BTCs of the tracer NaCl (normalised) during injection of 3 PVs of 100 mM NaCl followed by injection of 3PVs of DI water. The hydrodynamic dispersivity (α) and the effective diffusion coefficient (dc) were determined as 3 × 10–3 m and 1 × 10–9 m2/s, respectively, which were independent of flow velocity. The fitted result indicates that that diffusion is negligible compared to hydrodynamic dispersion within the range of velocities tested.
Normalised BTCs of the conservative tracer for Experiments 1–4 were also determined (refer to SI Figure S3-S5). Based on the same hydrodynamic dispersivity (3 × 10–3 m) and effective diffusion coefficient (1 × 10–9 m2/s) as used for Experiment 0, the modelled tracer BTCs for Experiments 1–4 show a good match with the experimental results (SI Figure S3-S5). This validates that NaCl can serve as a non-reactive tracer, consistent with a previous study by Vasiliadou and Chrysikopoulos (2011). The good agreement between the experimental and modelled results also reflects well-packed sand columns with no notable signs of preferential flow paths or “wall effects”. For all Experiments 0–4, the tracer (NaCl) started to breakthrough at ~ 0.6 PV of the bacteria solution and reached equilibrium at ~ 1.6 PV. This indicates that the dispersion effect is non-negligible. Otherwise, both the breakthrough and the equilibrium would occur at 1PV.
3.2 Transport and Retention of Bacteria
3.2.1 Experiment 1: Effect of Ionic Strength
Figure 4 shows the observed and modelled bacterial BTCs (normalised) under different IS conditions (Experiment 1). Compared to the conservative tracer, as shown by the black dashed line in Fig. 4 (the experimental tracer BTCs are shown in SI Figure S3), breakthrough of the bacteria is more delayed and reaches a lower concentration, due to bacterial retention. This behavior becomes more obvious as the IS increases from 0.5 to 1000 mM. It is also evident in Fig. 4 that after injection of 3 PVs of bacterial solution, i.e. during injection of 3 PVs of the bacteria-free background solution, the bacteria concentration in the BTCs continues to increase for another ~ 0.6 PV, before sharply declining. The continuous increase of the BTCs is attributed to the displacement of bacteria in the pore solution (injected during Stage 1), whilst the sharp decline in bacterial breakthrough without notable “tailing” indicates negligible detachment.
Experimental and modelled bacterial BTCs (normalised) under different IS conditions (Experiment 1). Error bars represent standard deviations in results from replicate experiments. The modelled tracer BTC is also plotted (black dash line) for comparison. Black arrow marks where the injection of bacterial solution was changed to the background solution
The two-site kinetic model was calibrated using the bacterial BTCs (Fig. 4) and the straining profiles (Fig. 5) under ISs of 0.5–1000 mM. The increase of ionic strength from 0.5 to 1000 mM enhanced the attachment of S. pasteurii from 9.4 to 69.6% (model: 6.7% to 67.8%) as shown in Table 2. Figure 6 shows the interaction energies between S. pasteurii and quartz sands, and between S. pasteurii cells, as a function of separation distance, which were calculated based on the measured zeta potential values (Fig. 1b) according to the extended DLVO theory (Hahn et al. 2004; Hogg et al. 1966). As IS increased from 0.5 to 1000 mM, the interaction energy shifts from an energy barrier (unfavourable conditions for retention at 0.5 mM) to the appearance of secondary energy minima (30 and 100 mM) and primary energy minima (500 and 1000 mM), thus leading to an increase in the attachment on grain surfaces. This is consistent with a recent study by Jain and Arnepalli (2020) who also demonstrated an enhanced attachment of S. pasteurii (strain type: ATCC 11859) with increasing IS from 0.1 to 100 mM in saturated sand columns. Note that the bacteria breakthrough curves at 30 and 100 mM in this study show higher breakthrough bacteria concentrations than those by Jain and Arnepalli (2020). This occurs because the bacteria strains (DSM No. 33) and sands in our study had smaller zeta potential values (more negative) at the same IS conditions (i.e. 30 and 100 mM). This demonstrates that even for the same bacteria, calibration of model parameters is required, since there can be significant variation between different type strains.
As IS increased from 0.5 to 1000 mM (Fig. 5), straining of S. pasteurii was increased from 8.1 to 28.2% (model: 8.0 to 29.8%). Straining is well-known to be influenced by pore space geometry (McDowell‐Boyer et al. 1986), solution chemistry and hydrodynamics (Bradford et al. 2006, 2007; Foppen et al. 2007; Torkzaban et al. 2008b). (Bradford et al. 2002, 2007) reported that straining occurs in colloid deposition when the ratio of colloid diameter to median grain size (dp/dg) is as low as 0.2%. The S. pasteurii cells (DSM No. 33) in this study are rod shaped, with a length of ~ 2–8 µm and a diameter of ~ 0.5–0.8 µm (microscopic image in SI Figures S6 & S7), giving dp/dg values approximately 0.1% to 1.0% based on the average rod diameter and average length, respectively. Under chemically unfavorable condition (IS = 0.5 mM), straining only accounted for 8.1% of the total injected bacteria solution. With the increase of IS to 100 mM, bacterial straining increased to 22.9% (model: 25.8%), possibly due to the increased number of bacteria in the secondary energy minimum which are prone to funneling and retention in the small pores formed adjacent to grain–grain junctions (Bradford et al. 2007; Torkzaban et al. 2008a). With further IS increases to 500 and 1000 mM, straining increased to 34.2% and 28.2% respectively. Microscopic images of S. pasteurii cells under ISs of 0, 100, 500 and 1000 mM (refer to SI Figures S6 & S7) show that coagulation of 2–3 bacterial cells occurs under ISs of 500 and 1000 mM whilst almost no bacterial coagulation is observed under ISs of 0 and 100 mM. Hence, the increased straining at from 100-mM IS to 500- and 1000-mM IS could be a result of bacterial coagulation.
Figure 5 also shows an overall decrease in bacterial straining with increasing depth, and the decreasing trend is more obvious at higher IS (especially at 1000 mM). Bradford et al., (2003, 2005, 2006) applied a time- and depth- dependent straining function to describe this phenomenon. In this study, we use an exponential decay of strain rate due to site-blockage effects, as adopted from Xu et al. (2006) and Xu and Saiers (2009), to model the observation of decreasing straining with increased distance from the inlet (Fig. 5). Note that the straining profiles at depths of 0-50 mm (Fig. 5) deviate from this decreasing trend with increasing depth, possibly due to increased fluid velocities near the inlet where radial flow is approached at the point of injection, which results in increased hydrodynamic shear forces and hence the potential to release strained bacteria if the fluid velocity exceeds a critical value (Bradford et al. 2006). This hydrodynamic shearing of strained bacteria might also account for the higher percentage of straining observed at 500 mM IS (34.2%) than at 1000 mM (28.2%). The release of bacteria cells from straining was not captured in our model which assumed pore straining is an irreversible process.
3.2.2 Experiment 2: Effects of Flow Velocity and 0.5 M CaCl2 Solution
Figure 7 shows the observed and modelled bacterial BTCs (normalised) under different velocity conditions and with injection of 0.5 M CaCl2 in Stage 2 (Experiment 2). The total recovery of bacteria under velocities of 50, 100, and 200 cm/h was 18.3%, 40.7%, and 46.9% respectively, according to the experimental BTCs. The increasing bacterial recovery, or decreasing bacterial retention, with increasing flow velocity (from 50 to 200 cm/h) can be attributed to both a decrease in attachment (from 35.9 to 22.4%) and a decrease in straining (from 40.5 to 19.3%). Firstly, higher flow velocities create greater hydrodynamic shear forces, which act to offset the attractive forces between the bacteria and the grains and hence reduce the rate of attachment at secondary energy minima (Fig. 6a; IS = 100 mM). Note that Experiment 1 in Sect. 3.2.1 observes no obvious “tailing” at the same IS (100 mM) and at a velocity of 100 cm/h (Fig. 4). This indicates that the rate of detachment is negligible at IS = 100 mM with a velocity no greater than 100 cm/h (e.g. 50 and 100 cm/h). At a flow velocity of 200 cm/h and at IS = 100 mM, the detachment rate may not be negligible, and is a topic for future studies. Secondly, the increased hydrodynamic forces as a result of the increasing flow velocity also tends to (1) decrease the extent of flow stagnation regions; and (2) decrease the weakly-attached bacteria at the secondary energy minimum which can then be funneled into small regions of pore space formed adjacent to grain–grain junctions (Bradford et al. 2007). Both effects could potentially decrease bacterial straining (Bradford et al. 2007; Torkzaban et al. 2008a, 2008b). In this study, the deceasing straining with increasing flow velocity (50–200 cm/h) was modelled by an increase in the decay constant 1/λ2 at the straining site (i.e. λ2 decreased from 0.04 to 0.02 × 108 cells/g).
Experimental and modelled bacterial breakthrough curves under different flow velocity conditions (Experiment 2). Error bars represent standard deviations in results from replicate experiments. Due to the enhancement of the IS at Stage 2, the overall attaching/straining rates were enhanced accordingly. Black arrow marks where the injection of bacterial solution was changed to 0.5 M CaCl2 solution
Unlike in Experiment 1, which created no changes in ionic strength due to the injection of 3 PVs of background solution (at the same IS) in Stage 2, in Experiment 2 we injected 3 PVs of 0.5 M CaCl2 solution in Stage 2 to reproduce the commonly applied cementation fluids in MICP treatment strategies. This caused a discrepancy between the observed and modelled BTCs if changes to the retention parameters were not modelled (dashed lines in Fig. 7). By simulating the effect of the increased IS in the injection fluid on the disturbed retention parameters, as shown in the annotation in Table 2 and according to Eqs. (12) and (13), the two-site kinetic model was able to accurately reproduce the experimental BTCs during Stage 2 (solid lines in Fig. 7). Comparing the bacterial BTCs of Experiment 1 (Fig. 4) with Experiment 2 (Fig. 7), the injection of the stronger IS (0.5 M CaCl2) solution tends to stabilise the pre-attached and pre-strained bacteria rather than remove or mobilise them, as indicated by the more steeply declining slope of the post-peak BTCs between 3.6 and 4 PVs.
3.2.3 Experiment 3: Effect of Initial Bacteria Density
Figure 8 shows the observed normalised bacterial BTCs (marked by black triangles and red dots) under the initial OD600 of 1 and 0.48 (flow velocity: 100 cm/h; Stage 1: 100 mM bacterial solution; Stage 2: 0.5 M CaCl2 solution). Based on the model parameters calibrated in Experiment 1 for an OD600 = 1, the modelled bacterial BTC (normalised) under the initial OD600 of 0.48, as marked by the red solid line in Fig. 8, also shows a good match with the experimental observation. This verifies that the model is capable of describing the bacteria transport and retention process, regardless of the initial bacteria concentration. As seen in Table 2, the total bacteria masses recovered at the outlet for OD600 values of 1 and 0.48, were 45.3% (model: 47.7%) and 15.8% (model: 16.9%), respectively, with the attachment enhanced from 31.8 (model: 26.5%) to 40.9% (model only), and straining enhanced from 22.9% (model: 25.8%) to 42.2% (model only). The lower mass recovery or higher bacterial retention at 0.48 OD600 is attributed to a lower site-blocking effect, which enhances the overall attachment and straining rates throughout the sand column. Specifically, according to Eqs. (4) & (5), the numbers of cells deposited at grain surfaces (attachment S1) and pore throats or grain contact points (straining S2) increases more slowly for a lower initial bacteria concentration (C0), which in turn causes a smaller decay (\({\psi }_{1}\) and \({\psi }_{2}\)) of the attachment/straining rate – as indicated by Eqs. (6) & (7). Therefore, the injection of a lower initial bacteria concentration causes less site-blocking and hence results in more efficient bacterial retention.
3.2.4 Experiment 4: Effect of Residence Time and DI Water Elution
The observed bacterial BTCs in short columns (length: 150 mm) under ISs of 100 mM and 500 mM for Stage 2 of Experiment 4 are shown in Fig. 9a and b respectively, plotted against the previous results for the longer column (length: 280 mm) in Experiment 1. Compared to the bacterial BTC for longer column experiments, shorter columns exhibited a higher bacterial breakthrough at the same “PV” value (i.e. x-coordinate; note that the PV is not an absolute value but a relative value based on each column). This occurs because, since the column length is almost halved and the fluid velocity remains the same, the time taken for fluid to pass from the inlet to the outlet of the shorter column is also almost halved. At the same PV, the shorter residence time for fluid in the short column implies that less time is available for attachment/straining and, hence, the bacteria breakthrough is higher. (Tobler et al. 2014) also showed a decrease in bacterial (S. pasteurii) mass recovery with increasing column length. Figure 9a and b shows the modelled bacterial BTCs based on the previously calibrated parameters from Experiment 1. The model results show a good fit to the experimental data, which again demonstrates that the proposed two-site kinetic model can describe the transport process, regardless of column geometry.
Experimental and modelled bacterial (and tracer) breakthrough curves in shorter columns (length: 150 mm) under two IS conditions: a 100 mM; b 500 mM (Experiment 4). Error bars represent standard deviations in results from replicate experiments. Flushing of 5 PVs of DI water leads to a partial bacterial detachment in stage 3
The flushing of 5 PVs DI water at Stage 3 was conducted on the 52-mm diameter columns only, and caused a bacterial release, as shown in Fig. 9a and b. (Hahn et al. 2004; Sang et al. 2013) attributed the release of particles/colloid under the elution of DI water to the reversible deposition at shallow secondary minima while deposition at deep primary minima is irreversible. (Choi et al. 2017), however, points out that part of the bacteria attached by primary minimum can be detached under the elution of lower IS solution and thus contribute to the reversible attachment. The appearance of the secondary minimum and energy repulsion under DI water elution leads to a partial bacterial detachment at shallow minimum (or at energy barrier). From Fig. 9a and b, the two-site kinetic model, provides a good match to bacterial release under the application of DI water during Stage 3. Modelling predictions estimate the total mass recovery at the end of Stage 3 under ISs of 100 mM and 500 mM were 74.4% and 42.4%, respectively, with the mass recovery during Stage 3 alone being 17.9% and 13.4% respectively.
4 Implications for Field Applications of MICP
Microbially induced calcite precipitation via ureolysis has been widely applied in energy research including removal of radionuclide contaminants in groundwater (Fujita et al. 2008), soil stabilization (DeJong et al. 2010; Whiffin et al. 2007), remediation of subsurface rocks (Cuthbert et al. 2013; Minto et al. 2016), wellbore sealing (Phillips et al. 2016) and CO2 capture and storage (Mitchell et al. 2010; Phillips et al. 2013) among others. Almost all of these bioremediation applications involve a transport and retention process to achieve an effective delivery of the ureolytic microbes into porous or fractured media. Our study shows that the transport and fate of ureolytic S. pasteurii in saturated sand columns is highly dependent on the aqueous chemistry, flow velocity, bacteria density, as well as the bacteria residence time in the soil. This causes the extreme complexity of bacterial transport and retention under real field conditions at temporal and spatial scales. However, the complexity and challenges associated with MICP treatment could be overcome if bacterial retention mechanisms are well understood and, hence, can be accurately modelled. Our study provides a method for quantification and prediction of bacterial transport and fate in 1-dimensional saturated sand columns, which may be upscaled to a 3-dimensional real field with different IS conditions and with a non-uniform velocity distribution. This modelling approach can then be incorporated into 3D reactive transport models of the MICP process (Minto et al. 2019). El Mountassir et al., (2018) outlined some of the challenges associated with MICP treatment. Based on the observations and results presented herein some strategies to overcome these challenges are presented.
Challenge 1 Clogging near injection well and uniformity
Many laboratory-scale experiments have shown increased carbonate precipitation near the inlet (Tobler et al. 2012; Whiffin et al. 2007). The results presented here demonstrate that the retention of S. pasteurii can be reduced by increasing the flow velocity. Hence, clogging at the injection point could be reduced by increasing the flow rate at the injection point (since under radial flow conditions velocity decreases exponentially with radial distance from the injection point so this will only remobilise bacteria close to the well), or by applying a small pulse of low IS water to mobilise S. pasteurii in the volume immediately surrounding the injection well.
In particular for applications where strength gain is the target outcome, uniformity of carbonate precipitation is desirable (Barkouki et al. 2011). A relatively homogeneous bacterial retention in porous media may be achieved by pumping of bacterial and cementing solutions under appropriate aqueous chemistries (Yang et al. 2022), thus promoting effective MICP soil stabilization. For example, our study demonstrates that the injection of 0.5 M CaCl2, as is commonly conducted in MICP injection strategies (i.e. as part of cementing solution), has been shown to stabilise pre-attached and pre-strained S. pasteurii, limiting cell re-entrainment; On the contrary, the injection of DI water with a much lower ionic strength leads to a partial detachment of S. pasteurii. Thus, between the injection of bacteria solution and the subsequent injection of cementing solution (i.e. as applied in the traditional two-phase MICP soil improvement), an injection of leaching solution with an ionic strength lower than the bacteria solution could cause partial bacteria detachment near the injection zone (Choi et al. 2017), thus potentially mitigating the non-uniformity of bacteria deposition (i.e. more deposition near injection zones).
Challenge 2 Optimization of treatment fluids
A critical aspect in the up-scaling of MICP treatment is the optimization of treatment fluid in order to minimise both cost and carbon footprint of treatment and to achieve a better cementation efficiency. An ability to quantify and predict the transport of S. pasteurii, under varying physical and chemical conditions, will enable optimization of the injection strategy. For example, we have shown that using 0.48 OD600 bacteria results in a higher retention efficiency of S. pasteurii compared to 1.0 OD600. The injection of bacteria solution with higher ionic strengths (such as 500 and 1000 mM NaCl) also contributes to higher retention efficiency, without inhibiting bacterium survival and effectiveness over the time scale required for a bioaugmentation scheme (refer to SI Figure S2). Lowering the bacterial density and injecting the bacterial solution with a higher IS (e.g. 100 or 500 mM) would enhance the straining efficiency of S. pasteurii in a bioaugmentation scheme. The strength of the treated soil depends strongly on the bacteria retained by straining which enhances precipitation at grain-to-grain contacts. Higher straining means a more efficient use of the calcium carbonate and the bacteria, thus potentially reducing the cost and carbon footprint.
Previous studies (Ismail et al. 2002; Tang et al. 2020; Xiao et al. 2019) have proven that the overall cementation efficiency via calcite precipitation is mainly determined by the formation of calcite-bridges between grain contacts. This is closely related to the spatial distribution of the straining cells near grain-to-grain contact points. Our study shows that straining was enhanced from 8.1 to 34.2% when the ionic strength was increased from 0.5 to 500 mM (Fig. 5 and Table 2). In addition, lowering the initial bacteria OD600 (from 1.0 to 0.48) caused the straining to increase (from 22.9 to 42.2%), which will also increase the overall cementation efficiency during bioaugmentation treatment.
5 Conclusion
We investigated some key factors influencing the transport and rate of S. pasteurii in saturated sand columns such as ionic strength, flow velocity, initial bacteria density, column length, and IS perturbations under injection of 0.5 M CaCl2 solution and deionised water. The main conclusions are summarised as follows.
Higher ionic strength (from 0.5 to 1000 mM) dramatically enhanced the retention efficiency of S. pasteurii through an enhancement of attachment (from 9.4 to 69.6%) and straining (from 8.1 to 34.2%). The enhanced attachment, with increasing IS, is caused by a shift in the interaction energy between bacteria cells and sands from the presence of an energy barrier (0.5 mM) to the appearance of a secondary energy minimum (at 30 and 100 mM) and a primary energy minimum (at 500 and 1000 mM). Increased straining likely results from (1) an increased number of bacteria in the secondary energy minimum (at 30 and 100 mM) prone to be funneled to and retained in small pores formed adjacent to grain–grain junctions, and (2) more straining as a result of the bacterial coagulation of a small number of cells (2–3 calls) that occurs at high ISs (i.e. 500 and 1000 mM). Moreover, bacterial survival and the effectiveness of urease activity were not influenced by high ionic strengths (up to 1000 mM NaCl) over the timescale required for bioaugmentation.
Experiments using lower flow velocity, lower bacteria density, and a longer column length all correspond to higher bacterial retention. Specifically, increasing the flow velocity (from 50 to 200 cm/h) caused decreasing attachment (from 39.5 to 22.4%) and decreasing straining (from 40.5 to 19.3%) as a result of the increased hydrodynamic shear forces, which tend to reduce the attachment at the secondary minimum and decrease the extent of flow stagnation regions for straining. Lower initial bacteria OD600 (from 1.0 to 0.48) enhanced the attachment (from 31.8 to 40.9%) and straining (from 22.9 to 42.2%), respectively, as a result of less site-blockage effects. Shorter column lengths corresponded to a lower retention efficiency, as a result of shorter bacteria residence time. The injection of 0.5 M CaCl2 solution stabilised the bacterial retention, while the injection with deionised water caused bacterial release.
This study provides clues to guarantee an effective and efficient bacterial delivery into porous media. The two-site kinetic model can reliably quantify and predict bacterial attachment and straining behaviors under the different ionic and hydrodynamic conditions investigated. Our findings can be incorporated into 3D reactive transport models to simulate the MICP process in the field and, ultimately, to optimise the efficiency of bioaugmentation in practical engineering applications.
Data Availability
The data supporting the analyses and results of this paper are publicly available for download from the following data repository (https://doi.org/10.15129/82878bbb-d8be-45f6-826e-246fcb1c3530).
References
Bai, H., Cochet, N., Pauss, A., Lamy, E.: Bacteria cell properties and grain size impact on bacteria transport and deposition in porous media. Coll. Surf. B Biointerfaces 139, 148–155 (2016). https://doi.org/10.1016/J.COLSURFB.2015.12.016
Barkouki, T.H., Martinez, B.C., Mortensen, B.M., Weathers, T.S., De Jong, J.D., Ginn, T.R., Spycher, N.F., Smith, R.W., Fujita, Y.: Forward and inverse bio-geochemical modeling of microbially induced calcite precipitation in half-meter column experiments. Transp. Porous Media. 90, 23–39 (2011)
Bradford, S.A., Simunek, J., Bettahar, M., Van Genuchten, M.T., Yates, S.R.: Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ. Sci. Technol. 37, 2242–2250 (2003)
Bradford, S.A., Simunek, J., Bettahar, M., Tadassa, Y.F., Van Genuchten, M.T., Yates, S.R.: Straining of colloids at textural interfaces. Water Resour. Res. 41, 10404 (2005). https://doi.org/10.1029/2004WR003675
Bradford, S.A., Torkzaban, S., Walker, S.L.: Coupling of physical and chemical mechanisms of colloid straining in saturated porous media. Water Res. 41, 3012–3024 (2007). https://doi.org/10.1016/J.WATRES.2007.03.030
Bradford, S.A., Morales, V.L., Zhang, W., Harvey, R.W., Packman, A.I., Mohanram, A., Welty, C.: Transport and fate of microbial pathogens in agricultural settings. Crit. Rev. Environ. Sci. Technol. 43, 775–893 (2013). https://doi.org/10.1080/10643389.2012.710449
Bradford, S.A., Yates, S.R., Bettahar, M., Simunek, J.: Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resour. Res. 38, 63-1–63-12 (2002). https://doi.org/10.1029/2002wr001340
Bradford, S.A., Simunek, J., Walker, S.L.: Transport and straining of E. coli O157:H7 in saturated porous media. Water Resour. Res. (2006). https://doi.org/10.1029/2005WR004805
Burbank, M., Weaver, T., Lewis, R., Williams, T., Williams, B., Crawford, R.: Geotechnical tests of sands following bioinduced calcite precipitation catalyzed by indigenous bacteria. J. Geotech. Geoenviron. Eng. 139, 928–936 (2013). https://doi.org/10.1061/(ASCE)GT.1943-5606.0000781
Camesano, T.A., Logan, B.E.: Influence of fluid velocity and cell concentration on the transport of motile and nonmotile bacteria in porous media. Environ. Sci. Technol. 32, 1699–1708 (1998). https://doi.org/10.1021/es970996m
Choi, N.C., Kim, D.J., Kim, S.B.: Quantification of bacterial mass recovery as a function of pore-water velocity and ionic strength. Res. Microbiol. 158, 70–78 (2007). https://doi.org/10.1016/j.resmic.2006.09.007
Choi, N.-C., Choi, J.-W., Kwon, K.-S., Lee, S.-G., Lee, S.: Quantifying bacterial attachment and detachment using leaching solutions of various ionic strengths after bacterial pulse. AMB Express 71(7), 1–9 (2017). https://doi.org/10.1186/S13568-017-0340-2
Creppy, A., Clément, E., Douarche, C., D’Angelo, M.V., Auradou, H.: Effect of motility on the transport of bacteria populations through a porous medium. Phys. Rev. Fluids. 4, 013102 (2019). https://doi.org/10.1103/PHYSREVFLUIDS.4.013102/FIGURES/10/MEDIUM
Cuthbert, M.O., McMillan, L.A., Handley-Sidhu, S., Riley, M.S., Tobler, D.J., Phoenix, V.R.: A field and modeling study of fractured rock permeability reduction using microbially induced calcite precipitation. Environ. Sci. Technol. 47, 13637–13643 (2013). https://doi.org/10.1021/es402601g
DeJong, J.T., Mortensen, B.M., Martinez, B.C., Nelson, D.C.: Bio-mediated soil improvement. Ecol. Eng. 36, 197–210 (2010). https://doi.org/10.1016/j.ecoleng.2008.12.029
El Mountassir, G., Lunn, R.J., Moir, H., MacLachlan, E.: Hydrodynamic coupling in microbially mediated fracture mineralization: Formation of self-organized groundwater flow channels. Water Resour. Res. 50, 1–16 (2014). https://doi.org/10.1002/2013WR013578
El Mountassir, G., Minto, J.M., van Paassen, L.A., Salifu, E., Lunn, R.J.: Applications of Microbial Processes in Geotechnical Engineering. Adv. Appl. Microbiol. 104, 39–91 (2018). https://doi.org/10.1016/BS.AAMBS.2018.05.001
Foppen, J.W., van Herwerden, M., Schijven, J.: Measuring and modelling straining of Escherichia coli in saturated porous media. J. Contam. Hydrol. 93, 236–254 (2007). https://doi.org/10.1016/J.JCONHYD.2007.03.001
Fujita, Y., Taylor, J.L., Gresham, T.L.T., Delwiche, M.E., Colwell, F.S., McLing, T.L., Petzke, L.M., Smith, R.W.: Stimulation of microbial urea hydrolysis in groundwater to enhance calcite precipitation. Environ. Sci. Technol. 42, 3025–3032 (2008). https://doi.org/10.1021/ES702643G
Gargiulo, G., Bradford, S., Šimůnek, J., Ustohal, P., Vereecken, H., Klumpp, E.: Bacteria transport and deposition under unsaturated conditions: the role of the matrix grain size and the bacteria surface protein. J. Contam. Hydrol. 92, 255–273 (2007). https://doi.org/10.1016/J.JCONHYD.2007.01.009
Gargiulo, G., Bradford, S.A., Simunek, J., Ustohal, P., Vereecken, H., Klumpp, E.: bacteria transport and deposition under unsaturated flow conditions: the role of water content and bacteria surface hydrophobicity. Vadose Zo. J. 7, 406–419 (2008). https://doi.org/10.2136/VZJ2007.0068
Gomez, M.G., Anderson, C.M., Graddy, C.M.R., DeJong, J.T., Nelson, D.C., Ginn, T.R.: Large-scale comparison of bioaugmentation and biostimulation approaches for biocementation of sands. J. Geotech. Geoenvironmental Eng. 143, 04016124 (2017). https://doi.org/10.1061/(ASCE)GT.1943-5606.0001640
Gomez, M.G., Martinez, B.C., DeJong, J.T., Hunt, C.E., deVlaming, L.A., Major, D.W., Dworatzek, S.M.: Field-scale bio-cementation tests to improve sands. Proc. Inst. Civ. Eng. - Gr. Improv. 168, 206–216 (2015). https://doi.org/10.1680/grim.13.00052
Hahn, M.W., Dean, A., O’Melia, C.R.: Aquasols: on the role of secondary minima†. Environ. Sci. Technol. 38, 5915–5924 (2004). https://doi.org/10.1021/ES049746D
Harvey, R.W., Metge, D.W., Barber, L.B., Aiken, G.R.: Effects of altered groundwater chemistry upon the pH-dependency and magnitude of bacterial attachment during transport within an organically contaminated sandy aquifer. Water Res. 44, 1062–1071 (2010). https://doi.org/10.1016/j.watres.2009.09.008
Hendry, M.J., Lawence, J.R., Maloszewski, P.: Effects of velocity on the transport of two bacteria through saturated sand. Ground Water 37, 103 (1999)
Hogg, R., Healy, T.W., Fuerstenau, D.: Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 62, 1638–1651 (1966). https://doi.org/10.1039/TF9666201638
Hommel, J., Lauchnor, E., Gerlach, R., Cunningham, A.B., Ebigbo, A., Helmig, R., Class, H.: Investigating the influence of the initial biomass distribution and injection strategies on biofilm-mediated calcite precipitation in porous media. Transp. Porous Media. 114, 557–579 (2016)
Ismail, M.A., Joer, H.A., Randolph, M.F., Meritt, A.: Cementation of porous materials using calcite. Geotechnique 52, 313–324 (2002). https://doi.org/10.1680/GEOT.2002.52.5.313/ASSET/IMAGES/SMALL/GEOT52-313-F20.GIF
Jain, S., Arnepalli, D.N.: Adhesion and deadhesion of ureolytic bacteria on sand under variable pore fluid chemistry. J. Environ. Eng. 146, 04020038 (2020). https://doi.org/10.1061/(ASCE)EE.1943-7870.0001708
Jiang, N.J., Wang, Y.J., Chu, J., Kawasaki, S., Tang, C.S., Cheng, L., Du, Y.J., Shashank, B.S., Singh, D.N., Han, X.L., Wang, Y.Z.: Bio-mediated soil improvement: an introspection into processes, materials, characterization and applications. Soil Use Manag. 38, 68–93 (2022). https://doi.org/10.1111/SUM.12736
Kinoshita, T., Bales, R.C., Yahya, M.T., Gerba, C.P.: Bacteria transport in a porous medium: retention of bacillus and pseudomonas on silica surfaces. Water Res. 27, 1295–1301 (1993). https://doi.org/10.1016/0043-1354(93)90216-5
Kwak, E., Kim, J.H., Choi, J.W., Lee, S.: Injection strategy for effective bacterial delivery in bioaugmentation scheme by controlling ionic strength and pore-water velocity. J. Environ. Manage. 328, 116971 (2023). https://doi.org/10.1016/J.JENVMAN.2022.116971
Lauchnor, E.G., Schultz, L.N., Bugni, S., Mitchell, A.C., Cunningham, A.B., Gerlach, R.: Bacterially induced calcium carbonate precipitation and strontium coprecipitation in a porous media flow system. Environ. Sci. Technol. 47, 1557–1564 (2013)
Li, K., Ma, H.: Deposition dynamics of rod-shaped colloids during transport in porous media under favorable conditions. Langmuir 34, 2967–2980 (2018). https://doi.org/10.1021/ACS.LANGMUIR.7B03983/ASSET/IMAGES/MEDIUM/LA-2017-03983A_M022.GIF
Liao, S., Ghosh, A., Becker, M.D., Abriola, L.M., Cápiro, N.L., Fortner, J.D., Pennell, K.D.: Effect of rhamnolipid biosurfactant on transport and retention of iron oxide nanoparticles in water-saturated quartz sand. Environ. Sci. Nano. 8, 311–327 (2021). https://doi.org/10.1039/D0EN01033B
Liu, J., Ford, R.M., Smith, J.A.: Idling time of motile bacteria contributes to retardation and dispersion in sand porous medium. Environ. Sci. Technol. 45, 3945–3951 (2011). https://doi.org/10.1021/es104041t
Martinez, B.C., DeJong, J.T., Ginn, T.R., Montoya, B.M., Barkouki, T.H., Hunt, C., Tanyu, B., Major, D.: Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenvironmental Eng. 139, 587–598 (2013). https://doi.org/10.1061/(ASCE)GT.1943-5606.0000787
McCaulou, D.R., Bales, R.C., Arnold, R.G.: Effect of temperature-controlled motility on transport of bacteria and microspheres through saturated sediment. Water Resour. Res. 31, 271–280 (1995). https://doi.org/10.1029/94WR02569
McDowell-Boyer, L.M., Hunt, J.R., Sitar, N.: Particle transport through porous media. Water Resour. Res. 22, 1901–1921 (1986). https://doi.org/10.1029/WR022i013p01901
Minto, J.M., MacLachlan, E., El Mountassir, G., Lunn, R.J.: Rock fracture grouting with microbially induced carbonate precipitation. Water Resour. Res. 52, 8827–8844 (2016). https://doi.org/10.1002/2016WR018884
Minto, J.M., Hingerl, F.F., Benson, S.M., Lunn, R.J.: X-ray CT and multiphase flow characterization of a ‘bio-grouted’ sandstone core: the effect of dissolution on seal longevity. Int. J. Greenh. Gas Control. 64, 152–162 (2017). https://doi.org/10.1016/j.ijggc.2017.07.007
Minto, J.M., Tan, Q., Lunn, R.J., El Mountassir, G., Guo, H., Cheng, X.: ‘Microbial mortar’-restoration of degraded marble structures with microbially induced carbonate precipitation. Constr. Build. Mater. 180, 44–54 (2018). https://doi.org/10.1016/j.conbuildmat.2018.05.200
Minto, J.M., Lunn, R.J., El Mountassir, G.: Development of a reactive transport model for field-scale simulation of microbially induced carbonate precipitation. Water Resour. Res. 55, 7229–7245 (2019)
Mitchell, A.C., Dideriksen, K., Spangler, L.H., Cunningham, A.B., Gerlach, R.: Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 44, 5270–5276 (2010). https://doi.org/10.1021/ES903270W
Nassar, M.K., Gurung, D., Bastani, M., Ginn, T.R., Shafei, B., Gomez, M.G., Graddy, C.M.R., Nelson, D.C., DeJong, J.T.: Large-scale experiments in microbially induced calcite precipitation (MICP): reactive transport model development and prediction. Water Resour. Res. 54, 480–500 (2018). https://doi.org/10.1002/2017WR021488
Phillips, A.J., Lauchnor, E., Eldring, J., Esposito, R., Mitchell, A.C., Gerlach, R., Cunningham, A.B., Spangler, L.H.: Potential CO2 leakage reduction through biofilm-induced calcium carbonate precipitation. Environ. Sci. Technol. 47, 142–149 (2013). https://doi.org/10.1021/es301294q
Phillips, A.J., Cunningham, A.B., Gerlach, R., Hiebert, R., Hwang, C., Lomans, B.P., Westrich, J., Mantilla, C., Kirksey, J., Esposito, R., Spangler, L.: Fracture sealing with microbially-induced calcium carbonate precipitation: a field study. Environ. Sci. Technol. 50, 4111–4117 (2016). https://doi.org/10.1021/acs.est.5b05559
Ramachandran, S.K., Ramakrishnan, V., Bang, S.S.: Remediation of concrete using micro-organisms. ACI Mater. Journal-American Concr. Inst. 98, 3–9 (2001)
Redman, J.A., Walker, S.L., Elimelech, M.: Bacterial adhesion and transport in porous media: role of the secondary energy minimum. Environ. Sci. Technol. 38, 1777–1785 (2004). https://doi.org/10.1021/es034887l
Rijnaarts, H.H.M., Norde, W., Bouwer, E.J., Lyklema, J., Zehnder, A.J.B.: Bacterial deposition in porous media: effects of cell-coating, substratum hydrophobicity, and electrolyte concentration. Environ. Sci. Technol. 30, 2877–2883 (1996). https://doi.org/10.1021/ES9605984/ASSET/IMAGES/LARGE/ES9605984F00008.JPEG
Salifu, E., MacLachlan, E., Iyer, K.R., Knapp, C.W., Tarantino, A.: Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: a preliminary investigation. Eng. Geol. 201, 96–105 (2016). https://doi.org/10.1016/J.ENGGEO.2015.12.027
Sang, W., Morales, V.L., Zhang, W., Stoof, C.R., Gao, B., Schatz, A.L., Zhang, Y., Steenhuis, T.S.: Quantification of colloid retention and release by straining and energy minima in variably saturated porous media. Environ. Sci. Technol. 47, 8256–8264 (2013). https://doi.org/10.1021/es400288c
Sasidharan, S., Torkzaban, S., Bradford, S.A., Dillon, P.J., Cook, P.G.: Coupled effects of hydrodynamic and solution chemistry on long-term nanoparticle transport and deposition in saturated porous media. Coll. Surf. A Physicochem. Eng. Asp. 457, 169–179 (2014). https://doi.org/10.1016/J.COLSURFA.2014.05.075
Seymour, M.B., Chen, G., Su, C., Li, Y.: Transport and retention of colloids in porous media: does shape really matter? Environ. Sci. Technol. 47, 8391–8398 (2013). https://doi.org/10.1021/ES4016124/SUPPL_FILE/ES4016124_SI_001.PDF
Shellenberger, K., Logan, B.E.: Effect of molecular scale roughness of glass beads on colloidal and bacterial deposition. Environ. Sci. Technol. 36, 184–189 (2002). https://doi.org/10.1021/ES015515K/ASSET/IMAGES/LARGE/ES015515KF00007.JPEG
Simunek, J., Van Genuchten, M.T., Sejna, M.: The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat, and multiple solutes in variably-saturated media. Univ. California-Riverside Res. Reports. 3, 1–240 (2005)
Šimůnek, J., Šejna, M., Van Genuchten, M.T.: The HYDRUS-2D software package for simulating the two-dimensional movement of water, heat, and multiple solutes in variably-saturated media: Version 2.0. US Salinity Laboratory, Agricultural Research Service, US Department of Agriculture (1999)
Šimůnek, J.S., He, C., Pang, L., Bradford, S.A.: Colloid-facilitated solute transport in variably saturated porous medianumerical model and experimental verification. Vadose Zo. J. 5, 1035–1047 (2006). https://doi.org/10.2136/VZJ2005.0151
Steinacher, F.: The Potential for Microbially Induced Carbonate Precipitation to Support Wellbore Sealing and Zonal Isolation in the Hydrocarbon Industry, (2021)
Stocks-Fischer, S., Galinat, J.K., Bang, S.S.: Microbiological precipitation of CaCO3. Soil Biol. Biochem. 31, 1563–1571 (1999). https://doi.org/10.1016/S0038-0717(99)00082-6
Stumpp, C., Lawrence, J.R., Hendry, M.J., Maloszewski, P.: Transport and bacterial interactions of three bacterial strains in saturated column experiments. Environ. Sci. Technol. 45, 2116–2123 (2011). https://doi.org/10.1021/es103569u
Tan, Y., Gannon, J.T., Baveye, P., Alexander, M.: Transport of bacteria in an aquifer sand: experiments and model simulations. Water Resour. Res. 30, 3243–3252 (1994). https://doi.org/10.1029/94WR02032
Tang, C.S., Yin, L.Y., Jiang, N.J., Zhu, C., Zeng, H., Li, H., Shi, B.: Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: a review. Environ. Earth Sci. 79, 1–23 (2020). https://doi.org/10.1007/S12665-020-8840-9/FIGURES/24
Tobler, D.J., Maclachlan, E., Phoenix, V.R.: Microbially mediated plugging of porous media and the impact of differing injection strategies. Ecol. Eng. 42, 270–278 (2012)
Tobler, D.J., Cuthbert, M.O., Phoenix, V.R.: Transport of Sporosarcina pasteurii in sandstone and its significance for subsurface engineering technologies. Appl. Geochemistry. 42, 38–44 (2014). https://doi.org/10.1016/j.apgeochem.2014.01.004
Tobler, D.J., Minto, J.M., El Mountassir, G., Lunn, R.J., Phoenix, V.R.: Microscale analysis of fractured rock sealed with microbially induced CaCO3 precipitation: influence on hydraulic and mechanical performance. Water Resour. Res. 54, 8295–8308 (2018)
Torkzaban, S., Bradford, S.A., van Genuchten, M.T., Walker, S.L.: Colloid transport in unsaturated porous media: the role of water content and ionic strength on particle straining. J. Contam. Hydrol. 96, 113–127 (2008a). https://doi.org/10.1016/J.JCONHYD.2007.10.006
Torkzaban, S., Tazehkand, S.S., Walker, S.L., Bradford, S.A.: Transport and fate of bacteria in porous media: coupled effects of chemical conditions and pore space geometry. Water Resour. Res. (2008b). https://doi.org/10.1029/2007WR006541
van Paassen, L.A., Ghose, R., van der Linden, T.J.M., van der Star, W.R.L., van Loosdrecht, M.C.M.: Quantifying biomediated ground improvement by ureolysis: large-scale biogrout experiment. J. Geotech. Geoenvironmental Eng. 136, 1721–1728 (2010). https://doi.org/10.1061/(ASCE)GT.1943-5606.0000382
van Wijngaarden, W.K., Vermolen, F.J., van Meurs, G.A.M., Vuik, C.: Modelling biogrout: a new ground improvement method based on microbial-induced carbonate precipitation. Transp. Porous Media. 87, 397–420 (2011)
van Wijngaarden, W.K., van Paassen, L.A., Vermolen, F.J., van Meurs, G.A.M., Vuik, C.: Simulation of front instabilities in density-driven flow, using a reactive transport model for biogrout combined with a randomly distributed permeability field. Transp. Porous Media. 112, 333–359 (2016)
Vasiliadou, I.A., Chrysikopoulos, C.V.: Cotransport of pseudomonas putida and kaolinite particles through water-saturated columns packed with glass beads. Water Resour. Res. 47, 2543 (2011). https://doi.org/10.1029/2010WR009560
Walker, S.L., Redman, J.A., Elimelech, M.: Role of cell surface lipopolysaccharides in escherichia coli K12 adhesion and transport. Langmuir 20, 7736–7746 (2004). https://doi.org/10.1021/LA049511F/ASSET/IMAGES/LARGE/LA049511FF00009.JPEG
Walker, S.L., Redman, J.A., Elimelech, M.: Influence of growth phase on bacterial deposition: Interaction mechanisms in packed-bed column and radial stagnation point flow systems. Environ. Sci. Technol. 39, 6405–6411 (2005). https://doi.org/10.1021/ES050077T/ASSET/IMAGES/LARGE/ES050077TF00004.JPEG
Weiss, T.H., Mills, A.L., Hornberger, G.M., Herman, J.S.: Effect of bacterial cell shape on transport of bacteria in porous media. Environ. Sci. Technol. 29, 1737–1740 (1995)
Whiffin, V.S., van Paassen, L.A., Harkes, M.P.: Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 24, 417–423 (2007). https://doi.org/10.1080/01490450701436505
Xiao, Y., Stuedlein, A.W., Ran, J., Evans, T.M., Cheng, L., Liu, H., van Paassen, L.A., Chu, J.: Effect of particle shape on strength and stiffness of biocemented glass beads. J. Geotech. Geoenvironmental Eng. 145, 06019016 (2019). https://doi.org/10.1061/(ASCE)GT.1943-5606.0002165
Xu, S., Saiers, J.E.: Colloid straining within water-saturated porous media: effects of colloid size nonuniformity. Water Resour. Res. 45, 5501 (2009). https://doi.org/10.1029/2008WR007258
Xu, S., Gao, B., Saiers, J.E.: Straining of colloidal particles in saturated porous media. Water Resour. Res. 42, 12–16 (2006). https://doi.org/10.1029/2006WR004948
Yang, Y., Chu, J., Liu, H., Cheng, L.: Improvement of uniformity of biocemented sand column using CH3COOH-buffered one-phase-low-pH injection method. Acta Geotech. 18, 413–428 (2022). https://doi.org/10.1007/S11440-022-01576-8/FIGURES/17
Yao, K.-M., Habibian, M.T., O’melia, C.R.: Water and waste water filtration: concepts and applications. Environ. Sci. Technol. 5, 1105–1112 (1971)
Acknowledgements
This research was supported by the BAM Nuttall/Royal Academy of Engineering Research Chair in Biomineral Technologies for Ground Engineering, Engineering and Physical Sciences Research Council (EPSRC) Capital Award: Emphasising Support for Early Career Researchers (Grant Reference EP/S017917/1). The authors would like to thank Mr. Derek McNee, from Department of Civil and Environmental Engineering—University of Strathclyde (Glasgow), for his contribution to the experimental setups. The authors would also like to thank three anonymous reviewers for giving many constructive comments and invaluable suggestions.
Funding
Royal Academic of Engineering Research Chair, Engineering and Physical Sciences Research Council (EPSRC) Capital Award, EP/S017917/1, James M. Minto
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GS. The first draft of the manuscript was written by GS and all authors commented and edited on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest relevant to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sang, G., Lunn, R.J., El Mountassir, G. et al. Transport and Fate of Ureolytic Sporosarcina pasteurii in Saturated Sand Columns: Experiments and Modelling. Transp Porous Med 149, 599–624 (2023). https://doi.org/10.1007/s11242-023-01973-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-023-01973-x