Abstract
This study focuses on the effects of organic (humic acid substances) and inorganic (bentonite clay, silicate, and kaolinite clay) colloids on the transport of bacteria under saturated flow conditions. The transport experiments of Escherichia coli were performed in 40-cm long column filled with river sand of diameter 400 μm. Batch sorption experiments were conducted to understand the attachment kinetics of bacteria in the presence of humic acid and clay colloids. Humic acid was found to enhance the transport of bacteria by 17.3% in the presence of NaCl and by 10.3% in the presence of CaCl2 while clay colloids were found to reduce the transport. The transport of bacteria decreased by 11.8, 33.8, and 67% (in the presence of NaCl) and by 58.8, 41.18, and 76.5% (in the presence of CaCl2) with bentonite clay, silicate, and kaolinite clay colloids, respectively. The addition of divalent ion enhanced the complexity leading to the formation of complexes thereby reducing the transport. The combined existence of these colloids, the water chemistry, and the chemical composition of the colloids could govern the interaction processes in the subsurface matrix and can hence influence the transport of biocolloids in the environment significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater, an important source of drinking water, is often preferred owing to its biological quality in its existing form. In most countries worldwide, it is used as the main source of drinking water [1]. Water from various sources passes through the different zones of soil system which act as effective mechanical and biological filters hence providing a natural clean-up of newly generated groundwater [1]. The biological components, in aquifers, primarily the microbes, had been delivering the valued ecosystem services of water purification at high quality for centuries [2]. However, the present scenario of groundwater faces increasing threats from anthropogenic activities [3], that comprises contamination with pathogenic microorganisms and viruses [4]. Hence, there is an increased risk for human health from pathogen-contaminated groundwater that makes it unfit and unsafe for consumption.
Packed column experiments have been traditionally used to assess the effect of physical, chemical, and biological factors on the transport process [5]. The prime factors determining the effect of retention and transport processes of microbial colloids in the aquifer are the aquifer porosity and flow velocity [6]. Further, physiological state (e.g., starvation) or growth conditions can significantly affect bacterial transport behavior [7]. Most earlier studies have focused and researched upon the effects of different physical, chemical, and biological factors of bacterial deposition and transport and its model in porous media. However, less focus has been directed towards the effect of natural colloidal particles on the transport process [8].
Many colloids apart from biocolloids occur widely in natural soil media. Their movement in the porous media is an important process in pedogenesis, soil erosion, and aquifer formation as it would facilitate the porous media to transmit fluids and contaminants with the movement of pore water. This transport phenomenon would thereby steer the interaction of the charged colloidal particles and the charged soil media. The interaction process includes the attachment of the colloidal particles on the soil media surface by means of the electrostatic force of attraction and repulsion or the straining process or the intersection of the double layers because of the chemical conditions. Regardless of the cause of the interaction process, the existence of the colloidal particles in soil media could change the electrochemical properties of the soil media and thereby the transport process of the contaminants reaching the environment. The other colloids in the soil system include clay minerals, oxides of Fe and Al, silica, and/or natural organic matter [9].
The role of natural organic matter and inorganic colloids on the transport of bacteria has received significant attention in the past few years [10]. Studies have revealed that the inorganic colloids are primarily responsible for the migration of contaminants in soils and aquifers [11]. Clay particles, a type of inorganic colloids are present in abundance in natural aquifer systems. Kaolinite, montmorillonite, illite, and chlorite are the most common clay minerals which are ubiquitously present in the subsurface. Other clay substances are those composed of a mixture of these four clays [12]. These clay particles have high surface area and surface charge heterogeneity and hence are considered to be best natural adsorbents [13]. Depending on the physicochemical conditions in the porous media, clay particles may facilitate or hinder the mobility of colloids [14]. There have been studies addressing the effect of clay colloids on the transport of bacteria ([14, 15]). However, they have been generally been limited to the study of effect of a single clay colloid. The present study examines the effect of three most commonly occurring clay particles on the bacterial transport processes and the study also investigates the effect of these colloids along with natural organic matter on the transport processes which have not been addressed at all. Further, most early studies have examined the role of clay particles in the transport of bacteria in mineral-coated media [16]. The effect of these on the commonly existing river sand has not been looked into and hence requires investigation.
Phosphate, sulfate, silicate, and many other ions are present in abundance in aquifer systems. They are present widespread in groundwater with a typical concentration range from 5 to 35 mg/L [17]. However, the effect of silicate on the transport of bacteria has been studied and it has been found that the bacterial transport reduced in the presence of silicate on quartz sand and increased in the presence of silicate in mineral quartz sand [14].
Natural organic matter (NOM), a complex mixture of organic materials originating from plants and animals, is ubiquitously present in the environment [13]. The significance of organic matter in subsurface media is extensively acknowledged as the adsorption of metals and hydrophobic organic compounds onto the NOM might considerably enhance their aqueous solubility [18]. NOM contains a variety of organic components such as hydrophilic acids, amino acids, lipids, proteins, and humic substances [13]. Of these, humic acid substances are the prime constituent of soil matter and are the most prevailing organic substances present in surface and groundwater systems. These molecules have a high affinity for metal ions, oxides, hydroxides, mineral, and dissolved hydrophobic organic molecules and can form water soluble and water insoluble complexes [19]. They are negatively charged in aqueous solutions due to the presence of carboxylic and phenolic groups which affect the transport behavior [19]. The key phenomenon of humic substances in soil media is its adsorption onto the porous surfaces. It was found that humic substances could alter the surface charge of collector and could sterically hinder E. coli causing a reduction in removal [20]. The influence of humic acid on transport of bacteria was studied and was found that the presence of humic acid enhanced the bacterial transport irrespective of cell types (Gram-negative or Gram-positive), motility (non-motile or motile), presence or absence of EPS (Extracellular Polymeric Substance) on cell surfaces, and solution chemistry (ionic strength and ion valence) [13]. The pathogen adhesion to the naturally existing colloidal material was studied and was found that the attachment is due to a combination of DLVO (Derjaguin–Landau–Verwey–Overbeek), charge heterogeneity, hydrophobic and polymer interactions as a function of solution chemistry [8]. Though, there have been many studies related to this, it should be noted a good number of them aimed to understand the effect of natural organic matter on the adhesion of bacteria to metal oxide-coated porous surfaces and that only very limited works ([13, 21, 22] have examined the importance of natural organic matter on the biocolloid transport in sand/soil media devoid of the coating by metal oxide. Further, in the earlier works, the porous media used remains synthetic like microsphere and silica and the latter one uses quartz, but the effect on naturally existing river sand remains unaddressed. Hence, the effect of humic substances on the transport of biocolloid in usually occurring plain uncoated river sand needs more understanding.
In natural environment, these humic substances, clay colloids, and biocolloids co-exist. They interact with one another through various chemical and geological processes. The interaction processes could be competition between them to attach on to surfaces, or enhancement or reduction in transport or there could even be formation of complexes. Thereby, the variegated and the connected nature of the interaction processes make them difficult to predict the transport of biocolloids. Changes in flow rate, water saturation, and solution chemistry (reduction in ionic strength, increase in pH or attachment to solutes) by any natural phenomenon could lead to the release of significant quantities of these colloids from the aquifer matrix. Therefore, the study of interactions between these natural colloids, bacteria, and the environment is needed so as to prevent contaminants from simply migrating through the groundwater. The present study is designed to examine and compare the role of organic colloid (humic acid) and the inorganic colloids (bentonite clay, silicate, and kaolinite) on the transport of bacteria at different physicochemical environments. Also, there are not many studies that look into the transport of bacteria in the presence of both organic and inorganic colloids.
The two main important constituents of natural soil are the humic acid substances and the clay colloids. So, here in this study, we look into the effect of bentonite clay and humic acid on the transport of bacteria in the presence of a divalent ion (Ca2+). The solution chemistry in most natural aquifers is dominated by calcium ion. Also, lime-treated manure is one of the most common treatment mechanism for inactivating the pathogens and the solution chemistry in the presence of calcium ions is highly relevant in such soil media. Hence, it is of significance to know and understand the effect of Ca2+ on the bacterial transport and deposition behavior. Also, this would be applicable in creating porous media systems to attenuate pathogens downgradient at the well or at the source of contamination. Hence, the study of the transport of bacteria in the presence of multiple colloids, clay (inorganic), and humic substances (organic substances) is looked into in this study as they appear in combination in natural systems.
Materials and Methods
Bacteria
The bacterial species used in this study is E. coli BL21, a gram-negative and facultatively anaerobic bacteria. The culture was obtained from the Department of Biotechnology, IIT Madras. The bacterial culture was grown by taking a needle scoop of E. coli and blending it with 100 mL of Luria Bertani broth, overnight at 37 °C while shaking it at 150 rpm on an orbital shaker. After incubation, the bacterial suspension was centrifuged and the growth medium was decanted. This experimental step was repeated twice to remove all traces of the growth medium. The pellet was resuspended in a freshly prepared electrolyte (10 mM NaCl solution) and 10 mL of this solution was added to per liter of minimal salt media (MSM) solution in which bacteria was suspended. The composition of the minimal salt media was as follows (the parentheses denote the measure of chemicals in g/L): Na2HPO4·2H2O (3.0), KH2PO4 (1.5), (NH4)2SO4 (0.5), MgCl2·6H2O (0.1), CaCl2 (0.02) and trace elements in distilled water. Minimal salt media was autoclaved at 121 °C for 15 min before use. The pH of the medium was maintained at 6.7.
Clay Colloids and Humic Acid Substances
Bentonite clay, silicate, and kaolinite were the clay colloids used in the column experiments to understand the effect of clay particles on the transport of bacteria. The stock solution for clay particles was prepared by taking 20 mg per 100 mL of clay particle. The resulting suspension was kept in a shaker for 24 h and was sonicated for 5 min before used in the column studies.
Humic acid was used in the form of sodium salt (Sigma-Aldrich). The stock solution was prepared by dissolving 20 mg/L of the salt and stirring the solution for 24 h. The solution was then filtered through a 0.2-μm filter paper. TOC content of the humic acid solutions was found to be 180 mg/L using TOC analyzer. The solution was stored in dark at 4 °C until use. All the humic acid solutions were sonicated before use in the column experiments. The optical density of the clay colloids was analyzed at a wavelength of 280 nm by a UV-vis spectrophotometer. The hydrodynamic diameter of the clay colloids was measured by a zetasizer and was found equal to be d p = 2096 nm for bentonite clay, d p = 2020 nm for silicate clay colloid, and d p = 1723 nm for kaolinite clay. The zeta potentials of the bacteria and clays were measured at pH 7 in Millipore water by the zetasizer analyzer.
Sand
The porous media used for bacteria transport experiments was river sand which was sieved and the fraction of the sand retained between 300 and 500 μm in size was collected. The median of the grain size number distribution was 400 μm. The sand was cleaned by soaking in concentrated HNO3, NaOH, and deionized water as per the procedure described by [23], to remove metal oxides and trace organics.
The procedure was followed by repeated rinsing with distilled water and then drying at 105 °C overnight. The cleaned sand was then sterilized by autoclaving prior to the use in column study. The organic matter content and specific gravity of the sand were determined by chromic acid method (IS 2720-22) and pyknometer method (IS 2720-3-1). The organic content was found to be below the detectable limit and the specific gravity was 2.650.
Batch Sorption Studies
Sorption equilibrium studies for microbes in the presence and absence of humic acid and in the presence and absence of clay colloids were performed. It was conducted to estimate the adsorption coefficient on sand as per the procedure described by [24]. Adsorption kinetic study was conducted in reaction bottles, using 5 g of sterilized sand and 50 mL of bacterial suspension. Reaction bottles were kept in a shaker at 140 rpm. Samples were withdrawn at 10, 30, 60, 120, 180, and 360 min on self-sacrificing mode. Samples were centrifuged and analyzed for residual bacterial concentration.
The equilibrium time obtained from the kinetic study was used for isotherm studies. For the isotherm studies, the amount of sand was fixed to be 5 g. Bacterial concentration was varied from 100 to 500 CFU/mL in the reaction bottles. Reaction bottles were kept in the shaker for 100 min (pseudo-equilibrium time) at 140 rpm. At the end of 100 min, the supernatant was separated and analyzed for residual bacterial concentration.
Column for Transport Experiments
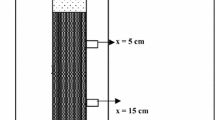
The river sand was wet-packed into the column (to get a uniform packing), by adding sand in small increments (about ~ 1–2 cm lifts) with a mild vibration of the column to minimize layering or entrapment of air. The dimensions of the plexiglass column used in the transport study are of 40 cm length and 3.45 cm diameter. On the top of the sand column, a water depth of approximately 5 cm was maintained as an overflow to ensure saturated conditions inside the column and to maintain a constant inlet pressure. The schematic of the experimental set-up is presented in Fig. 1.
A constant head was kept in the inlet chamber to acquire steady flow rate through the column. The flow rate was set by a control valve and was monitored by collecting the water at the outlet for a specific time interval. Once the constant flow condition was achieved, the colloidal suspension was introduced into the system from the inlet tank at a required flow rate. Liquid effluent samples were collected for about 5 h at specific time intervals from the collection tube placed at the outlet and the samples were then enumerated using plate count in TBX agar for bacteria. The list of experiments conducted is included in Table 1.
Result and Discussion
In the present study, saturated column experiments were performed to understand the effect of humic acid substances and clay colloids (bentonite clay, silicate, and kaolinite) on the transport of bacteria. It is important to understand that the bacterial transport processes depend on mechanical factors like flow rate and physicochemical processes like ionic strength, the valence of ions, pH, and the porous media surfaces. Further, the shape of the breakthrough curve of bacteria depends mainly on the attachment processes in the presence of other colloids in porous media. For this purpose, batch sorption studies were also performed to better understand the attachment kinetics. The constants obtained from the isotherm studies help us better comprehend the sorption onto the collector media. In case of the isotherms, the ordinate shows the logarithmic value of the equilibrium concentration (C e), and the abscissa portrays the logarithmic value of the maximum adsorption capacity (qe). In the case of the breakthrough curves, the ordinate displays the proportion of the outlet to the inlet concentration (C/C 0), and the abscissa portrays the time interval of the study at which samples were collected. The quicker the onset of the breakthrough curve, the faster is the transport process signaling bigger environmental hazards. The contaminant fronts were found to arrive rapidly with a sharp front stabilizing into a plateau with progress in time. However, differences were observed in terms of the arrival times and the peak C/C 0 values. For instance, in the case of transport of bacteria in the presence of humic acid (with NaCl), the transport was enhanced with a peak C/C 0 value of 0.8 as compared to 0.68 in the absence of humic acid. And due to enhanced sorption, the transport processes were significantly reduced in the presence of clay colloids. The peak C/C 0 values were observed to be 0.6, 0.45, and 0.22 in the case of bentonite clay, silicate, and kaolinite (with NaCl), respectively. Further, column experiments were performed to see the combined effect of clay colloids and humic substances on the transport of bacteria to mimic natural systems which have not been studied before. The transport appeared most reduced in this combined system with peak C/C 0 value of 0.2. It has to be noted that, all the experiments were conducted at a flow velocity of 0.107 cm/min. The results comprising the effect of colloids on the transport processes are presented below.
Effect of Humic Acid on the Transport of Bacteria
Batch studies for sorption of bacteria and humic acid onto porous media
Batch sorption studies were performed to examine the sorption of bacteria in the presence and absence of humic acid to sand media. The results are presented in Fig. 2. It was observed that the bacterial sorption reduced in the presence of humic acid. Similar observations have been stated in earlier works [25]. The attachment of humic compounds onto the porous media is primarily governed by the pH of the humic acid solution [25]. And furthermore, with a decrease in pH, the reduced sorption of bacteria in the presence of humic acid was found to be more significant and have been mentioned in earlier works [26]. The sorption is maximum at low pH values (pH 4) [26]. The adsorption is high at pH values wherein the humic acid compounds occur as polycationites that are thermodynamically less stable and hence have a tendency to precipitate. FTIR studies have confirmed the existence of humic acid as polycationites at high pH values. From the FTIR spectra of humic acid at different pH, it has been observed that there was a loss of –COOH (protonated carboxylic group) band at 1720 cm−1 with increasing pH. Thus, with the change in pH, the peaks differ indicating a degree of protonation in functional groups of humic acid. At low pH, the humic acid molecule is less negatively charged. Further, it is believed that the humic acid usually contains a wide variety of functional groups (–COOH, –OH, –NH2, etc.,), which are in the uncharged state at lower pH and hence tend to adsorb more. It implies that the adsorption is not dominated by the adsorption forces of humic matter onto a porous surface, but relatively by the intermolecular association forces between humic matter. The Freundlich model in studies of sorption of microbes onto organic matter, such as humic acid and fulvic acid was applied by, [8]. Table 2 displays the Freundlich coefficient (K f), exponent (1/n), and the corresponding correlation coefficients for all the equilibrium equations. It can be seen that sorption of bacteria is high in the absence of humic acid than in its presence. Similar observations have been reported by [8]. The Freundlich coefficient K f for sorption of bacteria to the sand in the absence of humic acid was higher than that for the sorption of cells in the presence of humic acid.
Transport of bacteria in presence of humic acid in saturated column study
There is a need to understand the humic acid-mediated transport of bacteria as they are present in significant amounts (few hundred micrograms to few hundred milligrams) in aquatic environments and is therefore essential for effective groundwater quality management. In this study, the column experiments were performed in the presence of humic acid (mono and divalent cation) and the absence of humic acid and with pre-treated humic acid in the column. To further understand the mechanism, the transport of humic acid itself was studied in the presence of mono and divalent cations. The results are shown in Fig. 3.
In this study, it was observed that the bacterial breakthrough decreased in the absence of humic acid compared to the transport in the presence of humic acid. The presence of humic acid was found to reduce cell deposition in the sand column. Similar results of reduced bacterial adhesion in the presence of organic matter has been reported previously by few researchers [8, 13, 20].
A multitude of mechanisms have been reported on the effect of humic substances on the transport of bacteria to collector media by prior research encompasses adhesion of bacteria onto surface (happens in metal oxide-coated media) [20], change in surface characteristics of porous media and adsorption of humic substances onto bacteria ([22], competition of deposition site by humic substances [20] and an increase in repulsive electrostatic force between bacteria and porous media [20]. Site competition by humic acid with bacteria and repulsion of bacteria by suspended humic acid have been reported as in the case of quartz sand. However, the mechanism of by which humic substances affect the transport of bacteria in plain river sand has not been done yet and needs further research.
The adsorption of humic acid to the bacteria leading to a substantial variation in surface charge has been reported by few researchers [21]. But, in some of the studies, changes in cell surface characteristics like zeta potential and hydrophobicity were not observed [13]. Likewise, in this work, it was found that the zeta potential values were analogous in the presence and absence of humic substances both in the presence of mono and divalent solutions. However, from the batch studies, it could be observed that adsorption of humic acid onto the sand media occurred at the experimental pH value and for this reason, the sites available for bacteria gets reduced resulting in a decreased deposition. The change in surface properties of porous media has been stated as a major mechanism in few studies [20]. In studies involving metal-coated collector media, charge reversal has been reported to be the cardinal reason for increased transport. Also, some of the earlier work testify the absence of change in surface charge of collector media [13]. In the present study, there was no variation in surface charge of sand media and hence there was no involvement of this phenomenon to the enhanced transport of biocolloid in the presence of humic substances.
The effect of mono and divalent cations on the transport of bacteria in the presence of humic substances which exists in environmentally relevant conditions is important. It is so because most colloids get charged owing to ionization of their surface functional groups. And, these charges are substantial as they encourage electrostatic repulsion between particles thereby preventing aggregation. It could be observed that the bacterial breakthrough (Fig. 3) reduced in the presence of humic acid and divalent cation compared to the transport in the presence of monovalent ion and humic acid. Also, it was noted the increase in bacterial transport in the presence of humic acid is also true in the presence of divalent Ca2+ ions in solution. Humic acid substances being complex, anionic polyelectrolytes form complexes with divalent calcium ion thereby reducing the negative charge on the humic molecule. It has been reported earlier that calcium could act as a destabilizer and could promote adhesion causing what is called as “Ca bridging” [27]. Also, calcium has also been noted to increase the straining and interception effect leading to reduced transport. Ca2+, a divalent cation, steers significant aggregation of humic substances. And the resulting rise in valency would enhance charge screening, decreasing the diffuse layer and the repulsive force, thereby reducing the transport. However, we do not find a deep decrease because humic acid works to counterbalance the destabilizing effect of the divalent calcium ion caused decline in repulsion. It has also been reported that humic acid could bring upon stabilization because of the increased electrostatic repulsion as seen above and in addition, a steric hindrance that occurs due to the attached humic substances on the surface of the porous media [20]. Thereby, it could be understood that humic acid substances reduce cell deposition irrespective of the prevailing chemistry (valency).
To understand further, experimental studies were performed by pre-treating the column with the humic acid solution and then passing the bacterial suspension through the column. Enhanced transport of the bacteria was observed with pre-treating the column than compared to the studies with no pre-treatment of humic acid (Fig. 3). It could be inferred that some of the sites might be occupied by humic acid that decreases bacterial attachment and hence more transport could be observed. Humic acid pre-treatment affects bacterial attachment as it could be found that there was approximately 3% of bacteria that was not able to attach and eluted from the column. A similar trend was reported by [20] wherein 20% was reported unattached. The higher percentage in that study was due to the goethite coating on sand media where ligand exchange occurs when pre-treated with humic acid.
The transport of humic acid was performed to investigate the deposition behavior of the substance (Fig. 4a, b). The breakthrough was rapid and smooth without significant retardation. It was found that the breakthrough curve appeared similar to that of a tracer with a C/C 0 value of 0.95. Unlike the previous experiment, this particular experiment was performed at pH values 4.5, 6.7, 7.5, and 8. It was observed that with the increase in pH, the attachment/sorption of humic acid to the collector media reduced. Similar observations have been described in earlier studies [25]. The transport of humic acid was found to be affected by a change in pH, ionic strength, and grain size of the matrix.
Neither the cell surface property like hydrophobicity nor the electrophoretic mobility (zeta potential) had a marked change with or without the humic acid substances. Additionally, there were no variations in the surface property of the collector media on the addition of humic acid. Similar observations have been described earlier [13]. However, in a study by [20], the silica sand was coated with ferric oxyhydride, and it was observed that the charge of the collector media reversed from negative to positive. This happened because the humic acid readily absorbed to the charged Fe-coated sand. Increase in concentration of the humic acid resulted in the decrease of the retention of negatively charged biocolloids. Here, in this study, from the column experiment with the prior passage of humic acid and from the batch sorption experiments, it could be understood that the site competition and better attachment of humic acid than bacteria could collectively lead to the enhanced transport of bacteria. Experiments conducted at different pH values throw more light on this, as it is observed that maximum humic acid attachment occurred at pH 4.5. In addition to all this, steric stabilization by humic acid has also been suggested to play an important role in the observed decrease in colloid attachment efficiency [28].
Effect of Clay Colloids on the Transport of Bacteria
Transport experiments were performed by addition of clay colloids and bacterial suspension at the inlet to the column packed with river sand under saturated flow-through conditions. Breakthrough curves of bacteria were obtained by monitoring the effluent samples that were analyzed for bacterial concentration and the removal was quantified from these curves. Batch studies were performed to examine the adhesion of bacteria to sand media in the presence of clay colloids.
Batch Studies for Sorption of Bacteria in the Presence of Clay Colloids onto Porous Media
Batch sorption studies were conducted in the presence of clay colloids. The isotherms are presented in Fig. 5. In general, the extent of bacterial adsorption onto plain river sand in the batch experiments were less compared to the presence of clay colloids and similar results have been observed in earlier studies [29]. The study reported the adsorption of bacteria to porous media to be below the limit of detection. In the presence of clay colloids, it was observed that in all three cases of bentonite clay, silicate, and kaolinite, the sorption process was found to influence the attachment of bacteria. Increased surface area and charge heterogeneity of clay colloids led to more attachment of bacteria onto the media. Further, the adsorption of bacteria onto bentonite clay was found to be lesser than in the case of silicate and kaolinite. A similar trend has been observed earlier [30]. It was identified that the lower adsorption on bentonite clay was due to more negative charge that caused a strong repulsion of the bacteria. This indicated the importance of electrostatic properties of clay minerals in the sorption of bacteria. The values of the adsorption coefficients for these batch runs are given in Table 2. The Freundlich constant K f for silicate and kaolinite were smaller than that for bentonite clay, indicating that more energy was released for the adsorption of E. coli on silicate and kaolinite than on bentonite clay. Similar trends were reported earlier (D. [30]). From Table 2, it could also be inferred that the sorption capacity of silicate and kaolinite were greater than that of bentonite clay colloids.
Influence of Bentonite Clay Colloids on the Transport of Bacteria
The transport of E. coli was observed in the presence of bentonite clay at two valence conditions (mono and di). Flat breakthrough plateaus were witnessed at both solution conditions. The bacterial breakthrough curves (Fig. 6) in the presence of bentonite clay colloids are of lower peak values owing to enhanced cell deposition in the presence of clay particles. It is evident from the batch studies that adsorption of bacteria onto the porous media is enhanced in the presence of bentonite clay owing to their high surface area and high cation exchange capacity.
Increased valency leads to lower breakthrough with less negative zeta potential is in agreement with DLVO theory. Similar findings were reported earlier [31]. The breakthrough curve with bacteria, clay, and NaCl had decreased by 12%, i.e., from 0.68 to 0.6, whereas the breakthrough curve in the case of bacteria, clay, and CaCl2 decreased by 58% from C/C 0 value of 0.68 to 0.28. This indicated that the presence of bentonite clay colloids on the transport of bacteria was more noteworthy in the case of divalent ion solution.
Earlier research has shown that the surface charge heterogeneity that was present on the clay colloid surface caused interaction between bacteria and clay colloids [32, 33]. In addition to charge heterogeneity, the change in zeta potential of the collector media was observed. The zeta potential of the collector media was lower in the presence of bentonite clay that caused a reduction in electrostatic repulsive force which leads to more deposition of biocolloid in the presence of bacteria. Co-deposition and adsorption in the presence of bentonite clay and NaCl and formation of bentonite clay bacteria clusters in presence of bentonite and CaCl2 might be the suggested mechanisms taking place. Also, the charge heterogeneity present on the surface of clay particles could cause to adhere to sand which in turn leads to more interaction between bacteria and the clay colloids [34]. And this effect is further enhanced in the presence of divalent cations as it has been found that CaCl2 could lead to aggregation of colloids increasing the straining and interaction effect thereby reducing the transport. The above observation shows that the presence of clay colloids impede the movement of biocolloid in porous media. In places where they appear in surplus, the microbes will be at once held in porous media to the discharge sites and thus their travel distance will be shorter.
Influence of Bentonite Clay and Humic Acid on the Transport of Bacteria
In natural environments, one witnesses the presence of both organic and inorganic colloids. Therein, the current work focuses on the combined effect of clay colloids and organic matter on the transport of bacteria which has not been looked into before. The breakthrough of bacteria in the presence of humic acid, bentonite clay, and divalent cation (Fig. 7) is reduced compared to the transport in the presence of bentonite clay and CaCl2 due to the complex interaction between these substances. Humic and fulvic acid substances adsorb to mineral surfaces by a variety of mechanisms. Electrostatic attraction and specific adsorption via ligand exchange are the dominant mechanisms for humic matter binding to oxide surfaces and edge of clay minerals. In the presence of clay colloids, the humic substances are found to form “humic clay complexes” [34]. Humic substances interact with the surface of these clay colloids and form these complexes owing to the charge heterogeneity present on the surface of the clay complexes. This leads to affecting the transport of bacteria. Also, these reactions are further enhanced in the presence of a divalent cation. Earlier researchers have reported that there could be adsorption and aggregation of humic substances to porous media depending on the concentration in which humic substances are present [20]. This could, in turn, affect the contaminant mobilizing or stabilizing them.
Influence of Silicate on the Transport of Bacteria
The influence of silicate on the transport of bacteria in river sand, the most common, has not been studied and requires investigation. The breakthrough of bacteria in the presence of silicate (Fig. 8) shows reduced transport. A reduction of around 36% was observed when silicate and NaCl were present. The zeta potential of bacteria was measured in the presence and absence of silicate clay colloids. And it was observed that the zeta potential of bacteria negatively reduced in the presence of silicate colloids. This could have led to less repulsive interaction between the sand and the bacteria causing more deposition and lesser transport. The chemical conditions play a vital role in the transport process as they govern the electrostatic properties as the processes happening at the pore-scale. It could be seen from the figure (Fig. 8) that the breakthrough curves are sensitive to the solution chemistry as indicated by a decrease in a breakthrough with increasing ion valence. Low zeta potentials were observed at high ion valence which is in agreement with DVLO theory. In addition to increased attachment, the addition of clay colloids may also affect the pore size distribution and hence cause an effect on the transport behavior of bacteria.
Influence of Kaolinite on the Transport of Bacteria
Transport experiments of bacteria were performed in saturated columns with and without kaolinite clay colloids. Kaolinite, a principal clay colloid, is produced by the weathering process of feldspar and muscovite and is widely distributed in most weathered soils and natural waters Kaolinite (Al2Si2O5(OH)4) is a 1:1 alumina silicate compromising a tetrahedral silica sheet bonded to an octahedral alumina sheet by shared oxygen atoms [35]. It has a cation exchange capacity (CEC) of 17 meq/100 g and density of 2.3 g/cm3 [36]. The results are presented in the figure (Fig. 9).
In the absence of kaolinite colloids, the transport of bacteria appeared faster. Mass recovery of bacteria was reduced in the presence of kaolinite suggesting colloid supported bacterial reduction. The presence of kaolinite clay particles inhibited the transport of bacteria in these column experiments. A study by [37] compared the adsorption of bacteria Pseudomonas putida on kaolinite and montmorillonite. It was observed that the bacteria possessed high affinity for kaolinite compared to montmorillonite. The sorption onto kaolinite was found to be more enthalpically favorable and more bacteria appeared to form aggregates with kaolinite. Here too, we witness a higher attachment of bacteria onto kaolinite than to bentonite clay. Also, earlier work has exhibited from the batch experiments conducted with kaolinite and bacteria that they showed attachment which was adequately described by Langmuir isotherm [10]. In that study, the observed reduction of the bacteria Pseudomonas putida recovery in the column outflow was attributed to bacteria attachment onto kaolinite particles, which were retained in the solid matrix (glass beads) of the column. In the study, it was also suggested that the decrease in transport in the presence of kaolinite might be because the bacteria could be attached to kaolinite clay colloids which in turn could be retained by solid matrix. Here also, we witness a decrease in transport of bacteria in the presence of kaolinite and NaCl and an increased decrease in the presence of kaolinite and CaCl2.
Deposition Rates for the Transport Experiments
Deposition rate gives an idea of the quantitative comparison of the bacterial deposition kinetics in different experimental conditions. The single collector efficiency equation is based on a rigorous numerical solution of convective-diffusion equation with all particle-removal mechanisms and interaction forces considered [38]. The single collector efficiency equation finds application in determining attachment efficiency for given physiochemical conditions (like porous medium and solution chemistry) [38]. The above application has been used in this study to understand the attachment of bacteria to collector media in the presence of different colloids.
The deposition rate of these bacteria in porous media can be quantified by single collector efficiency, η, which is defined as a ratio of the particle deposition rate to the rate at which the particles approach the collector [39]. Values for η were calculated from the single collector efficiency expression:
where a c is the radius of the sand grains, L is the length of the bed, f is the porosity of the bed, c/co is the near-steady state value for the concentration ratio. The values of the collector efficiency are given in Table 3. The values indicate increased attachment and decreased transport as the complexity (valency, clay colloids) increases.
Conclusion
The results from this study have many implications for better understanding the transport and attachment behavior of bacteria in saturated porous media. Natural soils are made up of humus substances and clay components. The study throws light on the transport of bacteria in the presence of these components. The study exhibits the importance of the effect of other organic and inorganic colloids on the transport of bacteria in the environment. It also emphasizes the importance of chemical conditions (divalent ions) on the transport process. The humic substances enhanced the transport of bacteria by site competition making the attachment of bacteria unfavorable in their presence. These naturally occurring organic colloids could increase the bioactivity of beneficial soil organisms and could work to attenuate these contaminating microbes when present, in a sustainable way. Looking at the mobility of bacteria in the presence of clay colloids, it was found to be reduced. And when the valence increased, the interactions became complex and led to more reduced transport. Thereby, it can be understood that in the natural soil system, the presence of calcium ion can help the mobilized colloids and its associated contaminants to travel to uncontaminated zones of porous media. The outcome of this work establishes that the presence of other organic and inorganic colloids and the solution properties play a crucial role in governing the bacterial transport in porous media and aquifer systems. The results of the study can be further used in designing systems which can enhance removal at the contamination source itself or at the downgradient of the well. More attention needs to be paid to the risk of microbial contamination due to their mobility issues in the presence of other organic and inorganic colloids and further work is required to investigate their transport behavior in unsaturated conditions.
References
Krauss, Stephen, and Christian Griebler. 2011. Georessource Wasser-Herausforderung Globaler Wandel Pathogenic Microorganisms and Viruses in Groundwater
Griebler C, Avramov M (2015) Groundwater ecosystem services: a review. Freshw Sci 34(1):355–367. https://doi.org/10.1086/679903
Cheng T, Saiers JE (2009) Mobilization and transport of in situ colloids during drainage and imbibition of partially saturated sediments. Water Resour Res 45(8):1–14
Sen TK (2010) Processes in pathogenic biocolloidal contaminants transport in saturated and unsaturated porous media: a review. Water Air Soil Pollut 216(1–4):239–256 http://link.springer.com/10.1007/s11270-010-0531-9 (June 25, 2014)
Tufenkji N (2007) Modeling microbial transport in porous media: traditional approaches and recent developments. Adv Water Resour 30(6–7):1455–1469 http://linkinghub.elsevier.com/retrieve/pii/S030917080600128X (June 25, 2014)
Pang L (2008) Microbial removal rates in subsurface media estimated from published studies of field experiments and large intact soil cores. J Environ Qual 38(4):1531–1559
Jansen S, Vereecken H, Klumpp E (2010) On the role of metabolic activity on the transport und deposition of Pseudomonas fluorescens in saturated porous media. Water Res 44(4):1288–1296 http://www.ncbi.nlm.nih.gov/pubmed/20153499 (June 25, 2014)
Zhao W, Walker SL, Huang Q, Cai P (2014) Adhesion of bacterial pathogens to soil colloidal particles: influences of cell type, natural organic matter, and solution chemistry. Water Res 53:35–46. https://doi.org/10.1016/j.watres.2014.01.009
Sen TK, Khilar KC (2006) Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv Colloid Interface Sci 119(2–3):71–96 http://www.ncbi.nlm.nih.gov/pubmed/16324681 (June 8, 2014)
Vasiliadou IA, Chrysikopoulos CV (2011) Cotransport of Pseudomonas putida and kaolinite particles through water-saturated columns packed with glass beads. Water Resour Res 47(2):1–14
Ryan JN, Elimelech M (1996) Colloid mobilization and transport in groundwater. Colloids Surf A Physicochem Eng Asp 107(95):1–56. https://doi.org/10.1016/0927-7757(95)03384-X
Jiang, Shu. 2012. Clay minerals from the perspective of oil and gas exploration. Clay minerals in nature—their characterization, modification and application: 21–38. http://www.intechopen.com/books/clay-minerals-in-nature-their-characterization-modification-and-application/clay-minerals-from-the-perspective-of-oil-and-gas-exploration
Yang H, Kim H, Tong M (2012a) Influence of humic acid on the transport behavior of bacteria in quartz sand. Colloids Surf B Biointerfaces 91:122–129 http://www.sciencedirect.com/science/article/pii/S0927776511006424 (June 11, 2015)
Dong Z, Yang H, Wu D, Ni J, Kim H, Tong M (2014) Influence of silicate on the transport of bacteria in quartz sand and iron mineral-coated sand. Colloids Surf B: Biointerfaces 123:995–1002. https://doi.org/10.1016/j.colsurfb.2014.10.052
Yang, Haiyan, Meiping Tong, and Hyunjung Kim. 2012b. Influence of bentonite particles on representative gram negative and gram positive bacterial deposition in porous media
Park S-J, Kim S-B (2011) Bacterial adhesion to metal oxide-coated surfaces in the presence of silicic acid. Water Environ Res 83(5):470–476
Iler, R K. 1979. The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. Lavoisierfr: 892 pp. http://books.google.com.hk/books?id=eKwOKQEACAAJ
Yamamoto H, Liljestrand HM, Shimizu Y, Morita M (2003) Effects of physical-chemical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environ Sci Technol 37(12):2646–2657. https://doi.org/10.1021/es026405w
Hu J-D, Zevi Y, Kou XM, Xiao J, Wang XJ, Jin Y (2010) Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci Total Environ 408(16):3477–3489. https://www.scopus.com/inward/record.uri?eid=2-s2.0-77953703572&partnerID=40&md5=020aac6b78f74e159a17a96cfc1b6d99. https://doi.org/10.1016/j.scitotenv.2010.03.033
Foppen JW, Liem Y, Schijven J (2008) Effect of humic acid on the attachment of Escherichia coli in columns of goethite-coated sand. Water Res 42(1–2):211–219. https://doi.org/10.1016/j.watres.2007.06.064
Dai X, Hozalski RM (2002) Effect of NOM and biofilm on the removal of cryptosporidium Parvum oocysts in rapid filters. Water Res 36(14):3523–3532. https://doi.org/10.1016/S0043-1354(02)00045-3
Parent ME, Velegol D (2004) E. coli adhesion to silica in the presence of humic acid. Colloids Surf B: Biointerfaces 39(1–2):45–51. https://doi.org/10.1016/j.colsurfb.2004.08.020
Lenhart JJ, Saiers JE (2002) Transport of silica colloids through unsaturated porous media: experimental results and model comparisons. Environ Sci Technol 36(4):769–777. https://doi.org/10.1021/es0109949
Shashidhar T, Philip L, Murty Bhallamudi S (2006) Bench-scale column experiments to study the containment of Cr(VI) in confined aquifers by bio-transformation. J Hazard Mater 131(1–3):200–209. https://doi.org/10.1016/j.jhazmat.2005.09.034
Wei X, Shao M, Horton R, Han X (2009) Humic acid transport in water-saturated porous media. Environ Model Assess 15(1):53–63 http://www.springerlink.com/index/10.1007/s10666-008-9186-y
Technology, O F. 1999. Sorption of humic substances and microbial activity in the course of artificial recharge of groundwater
Davis CJ, Eschenazi E, Papadopoulos KD (2002) Combined effects of Ca2+ and humic acid on colloid transport through porous media. Colloid Polym Sci 280(1):52–58. https://doi.org/10.1007/s003960200007
Darmawan, Hariyanto. 2011. Transport of a pathogenic bacterium and its non-pathogenic variant strain through a granular porous medium: from a simple system to a real system. (October)
Ams DA, Fein JB, Dong H, Maurice PA (2004) Experimental measurements of the adsorption of Bacillus subtilis and Pseudomonas mendocina onto Fe-oxyhydroxide-coated and uncoated quartz grains. Geomicrobiol J 21(8):511–519 http://www.informaworld.com/openurl?genre=article&doi=10.1080/01490450490888172&magic=crossref%7C%7CD404A21C5BB053405B1A640AFFD44AE3
Jiang D, Huang Q, Cai P, Rong X, Chen W (2007) Adsorption of Pseudomonas putida on clay minerals and iron oxide. Colloids Surf B: Biointerfaces 54(2):217–221. https://doi.org/10.1016/j.colsurfb.2006.10.030
Yang H, Tong M, Kim H (2012c) Influence of bentonite particles on representative gram negative and gram positive bacterial deposition in porous media. Environ Sci Technol 46(21):11627–11634. https://doi.org/10.1021/es301406q
Tombacz E, Szekeres M (2004) Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl Clay Sci 27(1−2):75−94
Tombacz E, Szekeres M (2006) Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl Clay Sci 34(1−4):105−124
Kloster N, Avena M (2015) Interaction of humic acids with soil minerals: adsorption and surface aggregation induced by Ca2+. Environ Chem 12(6):731–738. https://doi.org/10.1071/EN14157
Mitchell, J K, and K Soga. 2005. Fundamentals of soil behavior Fundamentals of Soil Behavior
Demirbaş Ö, Alkan M, Demirbaş A (2013) Surface properties of catalase and casein on kaolinite and design of experiments. Microporous Mesoporous Mater 172:151–160. https://doi.org/10.1016/j.micromeso.2012.12.050
Rong X, Huang Q, He X, Chen H, Cai P, Liang W (2008) Interaction of pseudomonas putida with kaolinite and montmorillonite: a combination study by equilibrium adsorption, ITC, SEM and FTIR. Colloids Surf B: Biointerfaces 64(1):49–55. https://doi.org/10.1016/j.colsurfb.2008.01.008
Tufenkji N, Elimelech M (2004) Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ Sci Technol 38(2):529–536. https://doi.org/10.1021/es034049r
Deshiikan SR, Eschenazi E, Papadopoulos KD (1998) Transport of colloids through porous beds in the presence of natural organic matter. Colloids Surf A Physicochem Eng Asp 145(1):93–100
Acknowledgements
The author would like to express her gratitude to guides Dr. Ligy Philip and Dr. S Murty Bhallamudi, IIT Madras for their continued support. The author thanks the reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madumathi, G. Transport of E. coli in Presence of Naturally Occuring Colloids in Saturated Porous Media. Water Conserv Sci Eng 2, 153–164 (2017). https://doi.org/10.1007/s41101-017-0037-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-017-0037-z