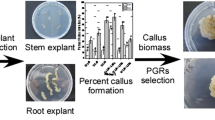
Abstract
The main phenylethanoid glycosides in the Plantago genus are acteoside (verbascoside) and plantamajoside, compounds with broad biological effects. This is a report on Plantago ovata callus induction, proliferation and establishment as well as the content of those phenylethanoids in that cell biomass. In the experimental studies, callus initiated from various seedling explants (roots, hypocotyls and leaves) was cultured on MS (Murashige-Skoog) media augmented with 2,4-D (2,4-dichloroacetic acid) and KIN (kinetin) or NAA (α-naphthaleneacetic acid) and BAP (6-benzylaminopurine). Callus proliferating on MS without NH4NO3 (ammonium nitrate) supplemented with 2,4-D (1.0 mg/l) and KIN (0.5 mg/l or 1.0 mg/l) turned out to be a good growth system for biomass production—mean increase of fresh weigh calculated on three following passages was 9.1 ± 1.8. The phytochemical analyses and antiradical DPPH (1,1-diphenyl-2-picryl-hydrazyl) tests revealed that the antioxidant activity is due to the presence of phenylethanoid glycosides. The quantitative screening of the callus extract by TLC (thin-layer chromatography) video densitometric method showed the highest content of acteoside (9.58 ± 0.75 mg/g dry weight) in root-derived and plantamajoside (8.15 ± 0.81 mg/g d.w.) in hypocotyl-derived callus biomass. In in vitro redifferentiated cultures of P. ovata, compounds with a demonstrated therapeutic effect, can be obtained in a manner that is completely independent of cultivation or harvesting from the wild.
Graphical abstract

Key Message
Three callus lines of Plantago ovata, initiated from roots, hypocotyls and leaves of seedlings, showed good growth on medium without ammonium nitrate and high content of valuable acteoside and plantamajoside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plantago ovata Forssk. (Plantaginaceae), a dicot annual herb popularly known as Ispagula (or Isubgol), is indigenous to the flora of South East Spain, North Africa and South West Asia (Dhar et al. 2005). It is cultivated for seeds and seed husks (Plantaginis ovatae semen, Plantaginis ovatae seminis tegumentum (European Pharmacopoeia 2023a, b)), which are commercially important anticonstipant remedies due to the high content of mucilage (10–30%) (WHO 1999; Fischer et al. 2004; Singh 2007). It has been shown that P. ovata displays hypocholesterolemic, anti-diabetic, anti-diarrhea, antibacterial, and antioxidant activity (Singh 2007; Motamedi et al. 2010; Ahmadi et al. 2012; Gonçalves and Romano 2016; Sarfraz et al. 2017). Moreover, it was applied for the treatment of bowel syndrome symptoms, abdominal pain, wounds, and termination of pregnancy (Sarfraz et al. 2017; Franco et al. 2020). On the other hand, a natural biocoagulant extracted from P. ovata was applied for a water clarification (Ramavandii 2014). Phytochemical analyses showed the presence of iridoids (aucubin, catalpol, 8-epiloganic acid, arborescoside, gardoside, and asperuloside), flavonoids (apigenin 7-glucoside, luteolin 7-glucoside, luteolin 4′-glucoside) and phenylethanoids (acteoside=verbascoside, plantamajoside) in plants from field cultivation (Kawashty et al. 1994; Rønsted et al. 2000; 2003) as well as phenylethanoids (verbascoside, forsythoside B) and flavonoids [rutin, plantaovaside (quercetin 3-O-β-rutinoside 3’-O-β-apioside)] (Nishibe et al. 2001), simple sugars, various organic acids, some amino acids and kaempferol (Frezza et al. 2021) in seeds. The fruits are rich source of primary (fatty acids and amino acids) and secondary metabolites (phenolic compounds, mostly flavonoids) (Patel et al. 2020).
Pharmacological studies have shown that phenylethanoids possess a pletora of pharmacological activities including antibacterial, antifungal, antiviral, antitumor, anti-inflammatory, neuro-protective, antioxidant, hepatoprotective, immunomodulatory, and tyrosinase inhibitory activity (Fu et al. 2008; Fons et al. 2008; Guangmiao et al. 2008; Alipieva et al. 2014; Ravn et al. 2015; Gonçalves and Romano 2016; Galli et al. 2020; Wu et al. 2020; Lou et al. 2021). The most frequently investigated phenylethanoid—acteoside was also included in the International Nomenclature of Cosmetic Ingredients (INCI) database as an antioxidant, bleaching, chelating, and skin protecting agent (CosmileEurope 2023).
In recent times, plant biotechnology offers alternative opportunities for the constant and controlled production of bioactive compounds in medicinal plant-based in vitro systems. Selected lines of homogenous and stabilized undifferentiated cell biomass may represent effective source of the value-added secondary metabolites e.g. pharmaceuticals, food additives, cosmetic compounds. Many biotechnological research has been conducted in order to establish callus cultures with rapidly growing biomass and increased accumulation of desired compounds. Factors, which affect the both parameters, including plant growth regulators exogenously supplementing the culture media, are still being search (Ahmadi-Sakha et al. 2016; Benjamin et al. 2019; Effert 2019).
Recently, we have established callus cultures of P. ovata as an alternative material for obtaining adventitious root culture as a source of phenylethanoid glycosides (Budzianowska et al. 2022). The aim of the present work was the establishment and development of P. ovata callus cultures and their investigation for phenolic compounds pattern. In this paper, the callus established from leaves, roots and hypocotyls of seedlings cultured on MS medium with reduced nitrogen source and supplemented with various plant growth regulators are described. The quantitative content of the main phenylethanoids—acteoside and plantamajoside was determined by HPTLC densitometry. The DPPH radical scavenging activity was also investigated.
Materials and methods
Plant material
The seeds of Plantago ovata were purchased from the local vendor (Herba Studio Mścisz i Wspólnicy Sp., Wysogotowo, Poland).
In vitro culture conditions
The induction, proliferation and establishment of callus cell lines derived from in vitro-originated seedling explants were carried out on artificial media augmented with plant growth regulators (PGRs) under controlled conditions in the growth chamber. The cultures were performed at 22 ± 1 °C, 60–70% relative humidity, 16 h of photoperiod, 60 μm/m2s light intensity provided by Flora fluorescent tubes (16 h light /8 h dark photoperiod). Culture media consisted of Murashige-Skoog media (Murashige and Skoog 1962)—full strength (MS) or half-strength (1/2 MS) or MS with sucrose 30 g/l, solidified with agar 7.2 g/l and variously supplemented with PGRs—cytokinins 6-benzylaminopurine (BAP), kinetin (KIN) and auxins: 2,4-dichloroacetic acid (2,4-D), α-naphthaleneacetic acid (NAA). All media compounds and plant growth regulators were purchased from Sigma–Aldrich.
Seed sterilization and germination
The seeds of P. ovata were surface-sterilized by soaking in 70% ethanol (v/v) for 30 s, then rinsed with autoclaved tap water, soaked in water for 24 h at 24 °C, soaked in 25% commercial bleach with 1 drop of Tween 20 for 20 min. Then, the seeds were rinsed with double-autoclaved water (5×) and placed on 1/2 MS medium in the test tube (5 seeds per test tube) for germination. The axenic seedlings were the source of the explants for further experiments.
Callus initiation and passaging
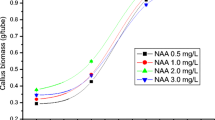
The callus lines were initiated from leaves, roots and hypocotyls excised from 10–20 days-old seedlings. The explants (0.5–1.0 cm length) were placed on culture medium in 100 ml Erlenmeyer flasks (5–10 explants per flask) on 7 variants of MS medium: supplemented with 2,4-D and KIN (1) 1.0 mg/l and 1.0 mg/l, (2) 1.0 mg/l and 0.5 mg/l, (3) 2.0 mg/l and 1.0 mg/l; supplemented with BAP and NAA—(4) 1.0 mg/l and 0.5 mg/l, (5) 2.0 mg/l and 0.1 mg/l; without NH4NO3 and supplemented with 2,4-D and KIN (6) 1.0 mg/l and 1.0 mg/l, (7) 1.0 mg/l and 0.5 mg/l (Table 1). After 4 weeks the calli were passaged onto the same medium. Further passages were performed in 2–3 week intervals onto either the same or different medium variant. Calli growth and texture were recorded.
For each plant material growth index was determined using 10 flasks in triplicate. The quantitative measurement of callus growth was estimated as growth index (GI). For determination of calli index of growth the accurately weighted fresh calli portions of 1 g each (n = 10) were passaged onto MS − NH4NO3 + 2,4-D (1.0 mg/l) + KIN (0.5 mg/l) medium and weighted again after 4 weeks of culturing. The fresh mass of calli (passage X–XII) was weighted immediately after collecting from media and growth indices were calculated from an equation GI = (final mass – initial mass) / initial mass .
Phytochemical analysis
Extraction and sample preparation
The calli from leaves, hypocotyls and roots were dried at 45 °C. The accurately weighted samples of callus from roots (3.903 g), hypocotyls (4.935 g) and leaves (6.753 g) were separately extracted with boiling methanol, 4 × 50 ml, for 1 h each time. The combined extracts were each concentrated to dryness on a vacuum rotary evaporator at the temperature ≤ 40 °C to give extracts of calli derived from leaves (2.550 g), hypocotyls (2.094 g) and roots (1.453 g). The solutions in 70% ethanol were prepared from extracts (0.1 g/ml) and standards (1.0 mg/ml) (section: Qualitative analysis).
The samples of dry methanol extracts (0.1 g) of calli derived from leaves, hypocotyls and roots were each separated over a polyamide MN-SC6 (grain size < 0.07 mm; Macharey-Nagel, Düren, Germany) column (1 × 10 cm) by the sequential elution with water, methanol, methanol with 0.1% ammonia (v/v), and methanol with 0.1% formic acid (v/v) to give the aqueous (IA, IB), methanol (II), ammonium (III) and acidic (IV) fractions. The fractions were evaporated to dryeness.
Qualitative analysis
Solutions of extracts from each callus type in 70% ethanol (0.1 g/1ml), as well as their fractions obtained by column chromatography (section: extraction and sample preparation), were analysed by thin-layer chromatography using band-wise (1D-TLC) or spot (2D-TLC) application of 5 µl samples to the plates on cellulose (Merck, Darmstadt, Germany) (1-butanol-acetic acid-water 4:1:5, upper phase, 15% acetic acid), polyamide 6 (Macherey-Nagel, Düren, Germany) (butan-2-one saturated with water or butan-2-one-ethyl acetate-water 30:10:4) and silica gel (Merck, Darmstadt, Germany) (ethyl acetate-acetic acid-water 4:1:1), using samples (5 µl, 1 mg/ml) of phenylethanoids isolated from P. lanceolata (Budzianowska et al. 2004) for reference (acteoside, plantamajoside, lavandulifolioside, leucosceptoside A, martinoside), and visualization of developed chromatograms under UV (254 and 366 nm) before and after spraying with 0.1% solution of NA (phenylboric acid 2-aminoethanol complex, Roth, Germany) for the general detection of phenolic compounds or 1% aluminium chloride solution in ethanol followed by heating for the detection of flavonoids.
Chromatographic examination of the column chromatography fractions showed the presence of phenolic compounds only in the methanol fractions and their composition was similar for all types of callus. The methanol fraction from the callus derived from the leaves was separated by preparative thin-layer chromatography on a polyamide (Macherey Nagel) home-made plate developed in butan-2-one saturated with water to give four compounds after elution of scrapped bands with methanol. They were identified as phenylethanoid glycosides: acteoside (verbascoside) and plantamajoside as the main constituents, and martinoside and leucosceptoside A as minor constituents by co-chromatography with the reference compounds and UV spectral analysis with diagnostic reagents (Budzianowska et al. 2004).
Quantitative analysis
The solutions of standards in methanol (acteoside and plantamajoside) (1.0 mg/ml) and extracts in 70% ethanol (100 mg/ml) were applied to HPTLC 60 F254 silica gel glass plates 20 × 10 cm (Merck, Darmstadt, Germany) as bands by using Camag Linomat automatic TLC sampler (Camag, Mutenz, Switzerland). The bands of 1.0 cm length were applied 1.0 cm from the lower edge and 1 cm from the left and right edge of the plate. To prepare calibration curves, the standards solutions were each applied in amounts of 2.5, 5, 7.5, 10 and 15 µl, in triplicate. The volumes of 5 µl of each extract were applied in triplicate to the same plate together with 10 µl of the standard also in triplicate. The plates were developed in a vertical TLC chamber for plates 20 × 20 cm (Camag) in a mobile phase consisting of a mixture ethyl acetate-acetic acid-water 4:1:1 (v/v/v) to the distance of 9 cm. The developed plates were dried at room temperature and chromatograms were captured under ultraviolet wavelength of λ = 366 nm with Camag Reprostar 3 device using Videostore computer program (Camag). For quantitative evaluation the images of chromatograms were scanned with Videoscan program (Camag) using previously described integration parameters (Budzianowska et al. 2019). The five-point calibration curves were constructed by plotting the TLC band average area against the amount of the standard. The acteoside and plantamajoside content was determined by comparing the band area of the compound in the extract with that in the calibration curve using polynomial regression y = − 916x2 + 31963x + 54,102, correlation coefficient R2 = 0.9936, for acteoside, and polynomial regression y = − 1385x2 + 44668x + 6044, correlation coefficient R2 = 0.9986, for plantamajoside.
Analysis of DPPH scavenging activity
The free radical scavenging activity of the fractions was estimated using the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) standard method as described by (Molyneux 2004). The water fractions (IA, IB) were dissolved in 50% ethanol, while the methanol (II), ammonia (III), and acidic (IV) fractions and reference compounds—acteoside (isolated from Plantago lanceolata (Budzianowska et al. 2004)) or BHA - tert-butylhydroxyanisole (Aldrich, USA) in methanol (each 200 µg/ml). One millilitre of the fraction or the reference solution dilution (final concentration of 1, 2.5, 5, 10, 20, 40, 80, 100 µg/ml) was mixed with 1.0 ml of 200 µM DPPH (Aldrich, USA) in methanol (final concentration 100 µM). The absorbance was read at 517 nm against a blank. The negative control was a mixture of the DPPH reagent and the solvent used.
For each sample, the measurement was performed three times. The values were expressed as the mean of three replications ± SD. The percentage of DPPH free radical reduction inhibition was calculated using the following equation: % DPPH reduction = (A – Ax) / A × 100, where A is the absorbance of the DPPH solution with solvent, Ax is the absorbance of the DPPH solution with a sample solution.
The DPPH scavenging activity was expressed as the concentration of the fraction required to decrease the DPPH absorbance by 50% (IC50). The IC50 value was determined from the graph of the %DPPH reduction plotted against the sample final concentration (Budzianowski and Budzianowska 2006).
The antioxidant activity index (AAI) was calculated from the equation: AAI = DPPH final concentration (µg/ml)/IC50 (µg/ml) (Scherer and Godoy 2009). This parameter was claimed to allow for the reliable comparisons of IC50 values determined by using different concentrations of DPPH mixed in various proportions with the solutions of tested extracts or compounds (Scherer and Godoy 2009).
Statistical analysis
Differences between means were tested for statistical significance with the one-way ANOVA test and multivariate analysis of variance, followed by the Tukey’s test. Data were calculated using Statistica 13.3 (TIBCO, Palo Alto, CA, USA) and MS-Excel 2016 (Microsoft Sp. z o. o., Warsaw, Poland).
Results and discussion
In vitro cultures
The species propagates from seeds and does not have ability for vegetative propagation. Nevertheless, in vitro regeneration through induction of axillary shoots (Barna and Wakhlu 1988; Budzianowska et al. 2022), indirect organogenesis (Wakhlu and Barna 1989) and somatic embryogenesis (Chowdhury et al. 1996) was described for P. ovata (Fons et al. 2008). Moreover, the reliable protocol for adventitious root culture characterized by good biomass growth parameters and effective acteoside production was developed recently (Budzianowska et al. 2022).
The callus cultures of P.ovata were initiated from hypocotyls (Wakhlu and Barna 1989), also from seeds, shoots and roots (Mahmood et al. 2012), leaves of seedlings (Gupta et al. 2015) and shootlets (Talukder et al. 2016). More recently, callus biomass was obtained, which was found to produce gallic acid and flavonoids, like rutin and possess anti-oxidative properties (Talukder et al. 2016).
In the present study, in vitro cultures of P. ovata were initiated from seeds, which successfully (90%) germinated on MS medium with reduced concentrations of mineral components and vitamins. The same result (90% seeds germinated) was observed by Mahmood et al. 2012), when the aseptic seeds were placed on 1/2 MS medium, however, supplemented with 2 mg/l gibberellic acid. The callus occurred in each case between 7th to 14th day of culturing leaves, roots and hypocotyls excised from 20-days-old seedlings. This observation is consistent with previous studies on this species, which described callus induction on hypocotyls excised from 5-day-old seedlings within 7 days of culturing (Wakhlu and Barna 1989). The experiment was evaluated for optimum biomass production in response to the medium variant (full medium or reduction of NH4NO3 content), types and concentration of plant growth regulators as well as seedling explant.
Readily achieved callus on all used explants was maintained on media supplemented with 2,4-D/KIN (three variants) or NAA/BAP (two variants) in various concentrations. The morphological aspects—colour and texture of the resulting callus on different media and from various explants are shown in Table 1; Fig. 1. The calli morphology was very similar in the case of each explant employed. However, it had a different consistency depending on the phytohormonal supplementation of the medium (Table 1). Callus grown in the presence of NAA/BAP was more green and compact, while the one on the media with the addition of 2,4-D/KIN—yellow–green, watery and gelatinous. In turn, the reduction of NH4NO3 content in the medium made callus more soft and friable (Table 1). This approach has appeared to be useful in the case of callus cultures of related species—P. lanceolata (Budzianowska et al. 2004; Gonda et al. 2014).
Plantago ovata in vitro cultures: a seedlings on 1/2 MS medium, b callus initiation on root explants on MS medium with 2,4-D 1.0 mg/l + KIN 1.0 mg/l, c callus initiation on leaf explants on MS medium with BAP 2.0 mg/l + NAA 0.1 mg/l; d callus from root (after 6th passage), e callus from hypocotyl (after 12th passage), f callus from leaf (after 6th passage)—(d–f) on MS medium without NH4NO3 with 2,4-D 2.0 mg/l + KIN 1.0 mg/l
As a result of the conducted experiments, it was found that although all hormonal variants of the MS medium were good for P. ovata callus induction, the supplementation with 2,4-D 1.0 mg/l and KIN 1.0 mg/l and the reduced concentration of NH4NO3 content was the best for callus morphology and growth. In the present study, the best increase in callus proliferation was observed when the concentration ratio of 2,4-D to KIN was 2:1 (auxin predominated)—similar to the research of Talukder et al. 2016), while in the case of Gupta studies the opposite observation was made—the ratio of 2,4-D to KIN was 1:2 (cytokinin predominated) (Gupta et al. 2015). In the study of Wakhlu and Barna (1989), the highest P. ovata callus index and growth was achieved on MS medium with 2,4-D 1.0 mg/l and KIN 1.0 mg/l, which was in accordance with our results. In the study of Mahmood et al. 2012) an increase in the concentration of 2,4-D beyond 4.0 mg/l has a negative impact on P. ovata callus induction and proliferation. It was shown that the lowest concentrations of 2,4-D (0.5–2.0 mg/l) influenced the highest percentage of callus proliferation, which was characterized by creamy or yellow colour and fragile or compact texture depending on the explant used (seed or shoot) (Mahmood et al. 2012).
In the present study, a tendency towards organogenesis was shown by callus formed from seedling explants on medium MS with BAP (2.0 mg/l) and NAA (0.1 mg/l) as well as with BAP (1.0 mg/l) and NAA (0.1 mg/l) due to the significant predominance of cytokinin in the culture medium. The combination of 2,4-D and KIN greatly stimulated the proliferation of callus cells, which was also confirmed in other studies on this taxon. However, these authors carried out the process of inducing callus to in vitro regenerate shoots by indirect organogenesis (Wakhlu and Barna 1989; Pramanik et al. 1996).
In passages X, XI and XII the growth indices were measured (Table 2). The calli of best growth and morphology occurred on MS medium without NH4NO3 and supplemented with 2,4-D (1.0 mg/l) + KIN (0.5 mg/l or 1.0 mg/l), induced from the root explants. The growth indices for the root-derived calli in passages X, XI and XII were 5.5 ± 0.5, 13.0 ± 2.0 and 8.9 ± 1.7, respectively (Table 2).
Other scientists dealing with the induction and proliferation of callus of this species did not estimate the rate of biomass gain, partly because this callus was intended for other purposes e.g. regeneration of organs. In this study, for the first time, the growth index determining the rate of proliferation of callus cells depending on the explant used was determined.
Phytochemical analyses
The analyses of the crude extracts of calli biomass (Fig. 2), and their fractions from column chromatography, showed the presence of four phenylethanoids—acteoside and plantamajoside, as major compounds, and leucosceptoside A and martynoside, as minor constituents. Both compounds—acteoside and plantamajoside are in an approximately equal ratio. Moreover, lavandulifolioside—a constituent of P. lanceolata (intact plants, shoot culture) (Murai et al. 1995; Budzianowska et al. 2004) was completely absent in callus cultures of P. ovata. Intact, fresh plants of P. ovata were found to contain phenylethanoid glycosides, like acteoside and plantamajoside (Rønsted et al. 2003), which are considered to be active principles of other medicinally useful Plantago species, like e.g. P. lanceolata, P. major or P. asiatica (Gonçalves and Romano 2016). Moreover, seeds from intact plants were found to contain two phenylethanoid glycosides, like acteoside and forsythoside B (Nishibe et al. 2001) also being typical of the genus Plantago (Rønsted et al. 2003).
TLC video densitometric analysis. a Chromatogram with extracts of calli derived from roots (CR), hypocotyls (CH) and leaves (CL) (A—acteoside, P—plantamajoside); b–d densitograms (1—plantamajoside, 2—acteoside) applied for video densitometric determination in extracts from calli b CR, c CH, and d CL; e, f polynomial calibration graphs
The quantification of selected phenylethanoid glycosides was performed by TLC video densitometric method (Mustoe and McCrossen 2001; Popovic and Sherma 2014; Fichou and Morlock 2018), which is particularly useful for the simultaneous determination of metabolites, including phenylethanoid glycosides, in different samples of crude plant extracts (Agatanovic-Kustrin et al. 2013; Budzianowska et al. 2019; 2022). The quantitative screening showed the content of acteoside (from 0.43 ± 0.04% to 0.96 ± 0.08% of d.w.) and plantamajoside (from 0.75 ± 0.03 to 0.81 ± 0.08% of d.w.) in in vitro cultured calli. The highest content of acteoside and plantamajoside was detected in in vitro root-derived and hypocotyl-derived calli, respectively, i.e. 9.60 ± 0.75 mg/g and 8.15 ± 0.81 mg/g, respectively (Table 3; Fig. 3). In P. ovata adventitious roots culture the respective content of these compounds was 33.02 ± 1.14 mg/g d.w. and 6.39 ± 0.20 mg/g d.w. (Budzianowska et al. 2022).
Graphical comparison of the acteoside and plantamajoside content in the calli of Plantago ovata (Table 3)
Callus cultures of P. ovata were not investigated for the presence of phenylethanoid compounds, which are considered to be active principles of other medicinally useful Plantago species, like e.g. P. lanceolata or P. major. In the study of callus initiation and stabilization of P. ovata performed by Wakhlu and Barna (1989), Pramanik et al. 1996), Mahmood et al. 2012), Gupta et al. 2015) the phytochemical analysis of phenylethanoids in in vitro-derived biomass was not performed.
No flavonoids could be detected in all three kinds of callus—neither in crude extracts nor in their fractions obtained by column chromatography. This class of phenol metabolites has been reported from intact plants of P. ovata. Two structurally different groups of flavonoids have been found in this species—flavones (glycosides of apigenin and luteolin) in aerial parts (Kawashty et al. 1994) and flavonols (glycosides like rutin (quercetin 3-O-β-rutinoside) and plantaovaside (quercetin 3-O-β-rutinoside 3′-O-β-apioside)) in seeds (Nishibe et al. 2001). The obvious ability of P. ovata for the synthesis of flavonoids was lost in the case of callus cultures and the similar phenomenon has been observed for the callus cultures of P. lanceolata (Budzianowska et al. 2004).
Talukder et al. 2016) have reported P. ovata shootlets-derived callus culture producing flavonoids, like rutin and quercetin, besides gallic acid, cinnamic acid, p-coumaric acid, caffeic acid and chlorogenic acid (5-O-caffeoylquinic acid), but not phenylethanoids. The content of those metabolites, as well as the phenylalanine ammonia lyase (PAL) activity—a key enzyme in the phenylpropanoid and flavonoid biosynthesis pathway (Fig. 4), could be increased by additives in the medium such as casein hydrolysate or coconut water.
It is remarkable, that callus cultures of P. ovata may have biosynthesis capability restricted to either phenylethanoid glycosides (current work) or flavonoids (Talukder et al. 2016) among phenylpropanoid secondary metabolites. Both classes of these compounds have common precursor, i.e. phenylalanine, while phenylethanoid biosynthesis utilizes additionally tyrosine (Ellis 1983; Saimaru and Orihara 2010; Hu et al. 2014; Zhou et al. 2020) and flavonoids formation requires malonyl-Coenzyme A (Davies and Schwinn 2006; Ververidis et al. 2007) (Fig. 5). It is difficult to indicate the factor determining such a switch in the biosythethic pathway directed to either phenylethanoids or flavonoids. It has been shown that culture conditions of P. lanceolata callus, such as nitrogen level and NH4+/NO3− ions ratio, significantly influenced (Gonda et al. 2014).
Simplified scheme of relationships of biosynthesis pathways leading to phenolic compounds found in callus cultures of Plantago ovata (shown in boxes) in the present and previous studies (Talukder et al. 2016). Compiled from (Muir et al. 2011) (benzoic acids), (Ellis 1983; Saimaru and Orihara 2010; Hu et al. 2014) (phenylethanoid glycosides), (Davies and Schwinn 2006; Ververidis et al. 2007) (flavonoids). Dashed line arrows indicate multistep processes. PAL phenylalanine ammonia lyase
The in vitro cultures of P. ovata appear to be an interesting source of phenylethanoids, which have been shown to have various, very promising, medicinal properties—e.g., antioxidant, antibacterial, anti-inflammatory, anticancer, antimicrobial and antiviral (Guangmiao et al. 2008).
DPPH anti-radical analysis
Taking into consideration the anti-oxidative activity as an important prerequisite feature of the biological activity of constituents of the produced calli, the anti-DPPH tests were performed for the fractioned extracts obtained by column chromatography on polyamide. The results of these DPPH tests for all studied fractions are presented in the Table 4. The anti-DPPH activity was found only in the methanol fractions and, significantly lower (less than 50%), in ammoniacal fractions. It was possible to determine the IC50 value only for the methanol fractions, which contained phenylethanoid glycosides. On the basis of this value, it was found that the activity of the methanol fraction of callus extract from roots (15.94 ± 0.72 µg/ml), hypocotyls (16.71 ± 0.54 µg/ml) and leaves (19.24 ± 4.13 µg/ml) is similar. Determination of the DPPH scavenging activity for the reference compounds showed that the IC50 value for acteoside was 5.94 ± 0.36 µg/ml (9.51 ± 0.58 µM) and for BHA was 5.66 ± 0.43 µg/ml (31.40 ± 2.38 µM). The reported anti-DPPH IC50 values for acteoside and plantamajoside were 13.0 µM and 11.8 µM, respectively (Skari et al. 1999). Thus, all three methanol fractions were less potent than the acteoside and BHA standards (Table 4). In turn, based on the Pearson correlation coefficient analysis, the IC50 values of the methanol fractions showed statistically significant strong correlation with the content of acteoside (r = − 0.8101) and the sum of acteoside and plantamajoside (r = − 0.9373) (Fig. 4) determined in calli. It should be noted, that the negative sign for the correlation coefficient values points to the inverse correlationthe higher the content of phenylethanoids, the lower IC50 value, i.e. the stronger anti-DPPH activity.
Studies of the anti-DPPH activity of fractionated extracts from P. ovata callus have not been carried out so far, however, research on the anti-radical activity for unfractionated extracts of in vitro-derived callus can be found in the literature (Talukder et al. 2016). The supplementation of the medium had a significant influence on the antioxidant capacity of callus extracts. The DPPH inhibition of callus from the basal medium ranged from 26.7 to 36.7%, and from the enriched media it ranged from 57.2 to 70.1%. These calli contained flavonoids such as rutoside and quercetin, and phenolic acids, such as benzoic acid derivative—gallic acid, and phenylpropanoid derivtives—cinnamic, p-coumaric, caffeic acids and chlorogenic acid, but not phenylethanoids (Talukder et al. 2016). According to the literature data, the DPPH radical scavenging activity was determined for a number of extracts from various organs of ground plants of several related Plantago species - P. major, P. bellardi, P. asiatica, P. afra, P. coronopus, P. lagopus, P. lanceolata, P. serraria or P. reniformis (Galvez et al. 2005a, b; Choi et al. 2008; Beara et al. 2012; Kartini et al. 2014). Most of these extracts (methanol or water) from ground plant organs (aerial parts or leaves or roots or seeds) showed weaker anti-DPPH activity than the methanol fractions of P. ovata callus extracts. The exception is a methanol extract from aerial parts of P. serraria, whose IC50 = 7.60 ± 0.46 µg/ml (Galvez et al. 2005b) and the calculated AAI = 5.17, and from P. reniformis, whose IC50 = 11.07 ± 0.43 µg/ml (Beara et al. 2012) but the calculated AAI = 1.07 rather points to weaker activity.
Conclusions
The present study showed the successful attempt for the excellent callus biomass proliferation and phenolic compounds, with several health benefits, enhanced production. The callus lines retained the ability to produce four phenylethanoids—acteoside and plantamajoside as major compounds and leucosceptoside A and martynoside as minor constituents. The homogenous and stabilized callus with good growth characteristic offers a promising source for suspension cell culture initiation.
Data availability
All data generated or analysed during this study are included in this article.
References
Agatonovic-Kustrin S, Loescher CM, Singh R (2013) Quantification of phenylpropanoids in commercial Echinacea products using TLC with video densitometry as detection technique and ANN for data modelling. Phytochem Anal 4:303–308. https://doi.org/10.1002/pca.2411
Ahmadi R, Kalbasi-Ashtari A, Gharibzahedi SMT (2012) Physical properties of psyllium seed. Int Sgrophys 26:91–93. https://doi.org/10.2478/v10247-012-0013-y
Ahmadi-Sakha S, Sharifi M, Niknam V (2016) Bioproduction of phenylethanoid glycosides by plant cell culture of Scrophularia Striata Boiss.: from shake-flasks to bioreactor. Plant Cell Tiss Organ Cult 124:275–281. https://doi.org/10.1007/s11240-015-0891-3
Alipieva K, Korkina L, Orhan IE, Georgiev MI (2014) Verbascoside—a review of its occurrence, (bio)synthesis and pharmacological significance. Biotech Adv 32:1065–1076. https://doi.org/10.1016/j.biotechadv.2014.07.001
Barna KS, Wakhlu AK (1988) Axillary shoot induction and plant regeneration in Plantago ovata Forssk. Plant Cell Tiss Organ Cult 15:169–173. https://doi.org/10.1007/BF00035758
Beara IN, Lesjak MM, Cetojevic-Simin DD, Orcic DZ, Jankovic T, Anackov GT, Mimica-Dukic ND (2012) Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of endemic P. Reniformis G. Beck. Food Res Int 49(1):501–507. https://doi.org/10.1016/j.foodres.2012.08.006
Benjamin ED, Ishaku GA, Peingurta FA, Afolabi AS (2019) Callus culture for the production of therapeutic compounds. Am J Plant Biol 4(4):76–84. https://doi.org/10.11648/j.ajpb.20190404.14
Budzianowska A, Skrzypczak L, Budzianowski J (2004) Phenylethanoid glucosides from in vitro propagated plants and callus cultures of Plantago lanceolata L. Planta Med 70:834–840. https://doi.org/10.1055/s-2004-827232
Budzianowska A, Kikowska M, Małkiewicz M, Karolak I, Budzianowski J (2019) Phenylethanoid glycosides in Plantago media L. organs obtained in in vitro cultures. Acta Biol Cracov Series Bot 61(1):75–86. https://doi.org/10.24425/118060
Budzianowska A, Kikowska M, Budzianowski J (2022) Adventitious root culture of Plantago ovata Forssk. As a source of phenylethanoid glycosides. Ind Crops Prod 180:114773. https://doi.org/10.1016/j.indcrop.2022.114773
Budzianowski J, Budzianowska A (2006) Chromatographic and spectrophotometric analyses of the DPPH free radical scavenging activity of the fractionated extracts from Lamium album L., Lamium purpureum L. and Viscum album L. Herba Pol, 52(1/2):51–57
Choi SY, Jung SH, Lee HS, Park KW, Yun BS, Lee KW (2008) Glycation inhibitory activity and the identification of an active compound in Plantago asiatica extract. Phytother Res 22(3):323–329. https://doi.org/10.1002/ptr.2316
Chowdhury AR, Kundu S, Raychawdhuri SS (1996) Regeneration of Plantago ovata Forssk through somatic embryogenesis. Cytobios 85:255–261
CosmileEurope V (2023) https://cosmileeurope.eu/inci/detail/16830/verbascoside/. Accessed 1 May, 2023
Davies KM, Schwinn KE (2006) Molecular biology and biotechnology of flavonoid biosynthesis. In: Andersen OM, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC press, Boca Raton. https://doi.org/10.1201/9781420039443
Dhar MK, Kaul S, Sarreen S, Koul AK (2005) Plantago ovata: genetic diversity, cultivation, utilization and chemistry. Plant Gen Res 3(2):252–263. https://doi.org/10.1079/PGR200582
Effert T (2019) Biotechnology application of plant callus culture. Engineering 5:50–59. https://doi.org/10.1016/j.eng.2018.11.006
Ellis BE (1983) Production of hydroxyphenylethanol glycosides in suspension cultures of Syringa vulgaris. Phytochemistry 22:1941–1943. https://doi.org/10.1016/0031-9422(83)80018-1
European Pharmacopoeia (2023a) Ispaghula Seed. Plantaginis ovatae semen. Monograph 11.0-1570. 11th edn. European Directorate for the Quality of Medicines and Health, Strasbourg
European Pharmacopoeia (2023b) Ispaghula Husk. Plantaginis ovatae seminis tegumentum. Monograph 11.3–5135. 11th edn. European Directorate for the Quality of Medicines and Health, Strasbourg
Fichou D, Morlock GE (2018) QuanTLC, an online open-source solution for videodensitometric quantification. J Chromatogr A 1560:78–81. https://doi.org/10.1016/j.chroma.2018.05.027
Fischer MH, Yu N, Gray GR, Ralph J, Anderson L, Marlett JA (2004) The gelforming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohyd Res 339:2009–2017. https://doi.org/10.1016/j.carres.2004.05.023
Fons F, Gargadennec A, Rapior S (2008) Culture of Plantago species as bioactive components resources: a 20-year review and recent applications. Acta Biol Gallica 155:277–300. https://doi.org/10.1080/12538078.2008.10516109
Franco E, Nazario A, Sanches-Silva FA, Ribeiro-Santos R, Ramosde MN (2020) Psyllium (Plantago ovata Forsk): from evidence of health benefits to its food application. Trends Food Technol 96:166–175. https://doi.org/10.1016/j.tifs.2019.12.006
Frezza C, Sciubba F, Tomai P, Gentili A, Bianco A, Serafini M, Golkar P (2021) Phytochemical analysis on the seeds of a new Iranian Plantago ovata Forssk. population specimen. Nat Prod Res. https://doi.org/10.1080/14786419.2021.1881960
Fu G, Pang H, Wong YH (2008) Naturally Occurring Phenylethanoid glycosides: potential leads for new therapeutics. Curr Med Chem 15:2592–2613. https://doi.org/10.2174/092986708785908996
Galli A, Marciani P, Marku A, Ghislanzoni S, Bertuzzi F, Rossi R, Di Giancamillo A, Castagna M, Perego C (2020) Verbascoside protects pancreatic β-Cells against ER-Stress. Biomedicines 8(12):582. https://doi.org/10.3390/biomedicines8120582
Galvez M, Martin-Condero C, Houghton PJ, Ayuso MJ (2005a) Antioxidant activity of Plantago bellardi all. Phytother Res 19(12):1074–1076. https://doi.org/10.1002/ptr.1797
Galvez M, Martin-Condero C, Houghton PJ, Ayuso MJ (2005b) Antioxidant activity of methanol extracts obtained from Plantago species. J Agricult Food Chem 53(6):1927–1933. https://doi.org/10.1021/jf048076s
Gonçalves S, Romano A (2016) The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind Crops Prod 83:213–226. https://doi.org/10.1016/j.indcrop.2015.12.038
Gonda S, Kiss-Szikszai A, Szucs Z, Máthé C, Vasas G (2014) Effects of N source concentration and NH4+/NO3– ratio on phenylethanoid glycoside pattern in tissue cultures of Plantago lanceolata L.: a metabolomics driven full-factorial experiment with LC–ESI–MS3. Phytochemistry 106:44–54. https://doi.org/10.1016/j.phytochem.2014.07.002
Guangmiao F, Haihong P, Yung HW (2008) Naturally occurring phenylethanoid glycosides: potential leads for new therapeutics. Curr Med Chem 15:2592–2613. https://doi.org/10.2174/092986708785908996
Gupta M, Kour B, Kaul S, Dhar MK (2015) Mucilage synthesis in callus cultures of Plantago ovata Forsk. Nat Acad Sci Lett 38(2):103–106. https://doi.org/10.1007/s40009-014-0303-y
Hu GS, Jia JM, Kim DH (2014) Effects of feeding tyrosine and phenylalanine on the accumulation of phenylethanoid glycosides to Cistanche deserticola cell suspension culture. Chin J Nat Med 12(5):367–372. https://doi.org/10.1016/S1875-5364(14)60045-5
Kartini Piyaviriyakul S, Siripong P, Vallisuta O (2014) HPTLC simultaneous quantification of triterpene acids for quality control of Plantago major and evaluation of their cytotoxic and antioxidant activities. Ind Crops Prod 60:239264. https://doi.org/10.1016/j.indcrop.2014.06.020
Kawashty SA, Gamal-el-Din E, Abdalla MF, Saleh NAM (1994) Flavonoids of Plantago species in Egypt. Biochem Syst Ecol 22(7):729–733. https://doi.org/10.1016/0305-1978(94)90058-2
Luo S, Jiang X, Yin G, Liu Y, Liu Z, Meng L, Wu J, Wu H (2021) The herbal agent plantamajoside, exerts a potential inhibitory effect on the development of hepatocellular carcinoma. Exp Th Med 21(6):573. https://doi.org/10.3892/etm.2021.10005
Mahmood T, Jameel A, Abbasi BH, Munir F, Naqvi Syed MS (2012) In vitro callogenesis and detection of somaclonal variations in Plantago ovata L. J Crop Sci Biotech 15:289–295. https://doi.org/10.1007/s12892-012-0014-1
Molyneux P (2004) The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Tech 26:211–219
Motamedi H, Darabpour E, Gholipour M, Seyyed Nejad SM (2010) Antibacterial effect of ethanolic and methanolic extracts of Plantago ovata and Oliveria decumbens endemic in Iran against some pathogenic bacteria. Int J Pharm 6:117–122. https://doi.org/10.3923/ijp.2010.117.122
Muir RM, Ibáñez AM, Uratsu SL, Ingham ES, Leslie CA, McGranahan GH, Batra N, Goyal S, Joseph J, Jemmis ED, Dandekar AM (2011) Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia). Plant Mol Biol 75(6):555–565. https://doi.org/10.1007/s11103-011-9739-3
Murai M, Tamayama Y, Nishibe S (1995) Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema. Planta Med 61:479–480. https://doi.org/10.1055/s-2006-958143
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiol Planta 15:473–479. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Mustoe SP, McCrossen SD (2001) A comparison between slit densitometry and video densitometry for quantitation in thin layer chromatography. Chromatographia 53:S474–S477. https://doi.org/10.1007/BF02490381
Nishibe S, Kodama A, Noguchi Y, Han Y (2001) Phenolic compounds of Plantago ovata and P.psyllium. Nat Med 55:258–261
Patel MK, Mishra A, Jaiswar S, Jha B (2020) Metabolic profiling and scavenging activities of developing circumscissile fruit of psyllium (Plantago ovata Forssk.) Reveal variation in primary and secondary metabolites. BMC Plant Biol 20:116. https://doi.org/10.1186/s12870-020-2318-5
Popovic N, Sherma J (2014) Comparative study of the quantification of thin-layer chromatograms of a model dye using three types of commercial densitometers and image analysis with ImageJ. Trends Chromatograph 9:21–28
Pramanik S, Raychaudhuri SS, Chakraborty S (1996) Changes in esterase and superoxide dismutase isozymes during in vitro morphogenaesis in Plantago ovata Forssk. Plant Cell Tiss Organ Cult 44:123–127. https://doi.org/10.1007/BF00048189
Ramavandii B (2014) Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovata. Water Res Ind 6:36–50. https://doi.org/10.1016/j.wri.2014.07.001
Ravn HW, Mondolot L, Kelly MT, Lykke AM (2015) Plantamajoside—a current review. Phytochem Lett 12:42–53. https://doi.org/10.1016/j.phytol.2015.02.002
Rønsted N, Göbel E, Franzyk H, Jensen SR, Olsen CE (2000) Chemotaxonomy of Plantago. Iridoid glucosides and caffeoyl phenylethanoid glycosides. Phytochemistry 55:337–348. https://doi.org/10.1016/S0031-9422(00)00306-X
Rønsted N, Franzyk H, Mølgaard P, Jaroszewski JW, Jensen SR (2003) Chemotaxonomy and evolution of Plantago L. Plant Syst Evol 242:63–82. https://doi.org/10.1007/s00606-003-0057-3
Saimaru H, Orihara Y (2010) Biosynthesis of acteoside in cultured cells of Olea europaea. J Nat Med 64:139–145. https://doi.org/10.1007/s11418-009-0383-z
Sarfraz RM, Khan H, Maheen S, Afzal S, Akram MR, Mahmood A, Afzal K, Abrar MA, Akram MA, Andaleeb M, Haider I, Abbas K, Yasmeeni T (2017) Plantago ovata: a comprehensive review on cultivation, biochemical, pharmaceutical and pharmacological aspects. Acta Pol Pharm 74:739–746
Scherer R, Godoy HT (2009) Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem 112:654–658. https://doi.org/10.1016/j.foodchem.2008.06.026
Singh B (2007) Psyllium as therapeutic and drug delivery agent. Int J Pharmaceut 334:1–14. https://doi.org/10.1016/j.ijpharm.2007.01.028
Skari KP, Malterud KE, Haugli T (1999) Radical scavengers and inhibitors of enzymatic lipid peroxidation from Plantago major, a medicinal plant. In: Kumpulainen JT, Salonene JT (Eds.) Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease. Proceedings of the Second International Conference on Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease, Royal Society of Chemistry, Helsinki, Cambridge, 200–202 June 1998
Talukder P, Talapatra S, Ghoshal N, Sen Raychaudhuri S (2016) Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. J Sci Food Agricult 96:232–244. https://doi.org/10.1002/jsfa.7086
Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (2007) Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: chemical diversity, impacts on plant biology and human health. Biotechnol J 2:1214–1234. https://doi.org/10.1002/biot.200700084
Wakhlu AK, Barna KS (1989) Callus initiation, growth and plant regeneration in Plantago ovata Forsk. Cv. GI-2. Plant Cell Tiss Organ Cult 17:235–241. https://doi.org/10.1007/BF00046870
WHO monographs on selected medicinal plants (1999) Semen Plantaginis, vol 1. World Health Organisation, Geneva, pp 202–212
Wu L, Georgiev MI, Cao H, Nahar L, El-Seedi HR, Sarker SD, Xiao J, Lu B (2020) Therapeutic potential of phenylethanoid glycosides: a systematic review. Med Res Rev 40(6):2605–2649. https://doi.org/10.1003/med.21717
Zhou Y, Zhu J, Shao L, Guo M (2020) Current advances in acteoside biosynthesis pathway elucidation and biosynthesis. Fitoterapia 142:104495. https://doi.org/10.1016/j.fitote.2020.104495
Funding
This research was financially supported by Poznan University of Medical Sciences (502-01-03303407-03567).
Author information
Authors and Affiliations
Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization: AB; methodology: AB, JB; software: MK, JB; formal analysis: AB, JB, MK; investigation: AB, JB; resources: AB, JB data curation: MK, AB, JB writing—original draft preparation: MK, AB, JB; writing—review and editing: JB, MK; visualization: MK, AB, JB; supervision: AB, JB; project administration: JB. All authors read and approved the final manuscript”.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Agnieszka Szopa.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Budzianowska, A., Kikowska, M. & Budzianowski, J. Phenylethanoid glycosides accumulation and antiradical activity of fractionated extracts of Plantago ovata Forssk. callus cultures lines. Plant Cell Tiss Organ Cult 156, 54 (2024). https://doi.org/10.1007/s11240-023-02635-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02635-y