Abstract
The dried root of Glehnia littoralis is a traditional Chinese herbal medicine mainly used to treat lung diseases and plays an important role in fighting coronavirus disease 2019 pneumonia in China. This study focused on the key enzyme gene GlPS1 for furanocoumarin synthesis in G. littoralis. In the 35S:GlPS1 transgenic Arabidopsis study, the Arabidopsis thaliana-overexpressing GlPS1 gene was more salt-tolerant than Arabidopsis in the blank group. Metabolomics analysis showed 30 differential metabolites in Arabidopsis, which overexpressed the GlPS1 gene. Twelve coumarin compounds were significantly upregulated, and six of these coumarin compounds were not detected in the blank group. Among these differential coumarin metabolites, isopimpinellin and aesculetin have been annotated by the Kyoto Encyclopedia of Genes and Genomes and isopimpinellin was not detected in the blank group. Through structural comparison, imperatorin was formed by dehydration and condensation of zanthotoxol and a molecule of isoprenol, and the difference between them was only one isoprene. Results showed that the GlPS1 gene positively regulated the synthesis of coumarin metabolites in A. thaliana and at the same time improved the salt tolerance of A. thaliana.
Key message
The results demonstrated that GlPS1 positively regulates the biosynthetic pathway of furacoumarin, resulting in the accumulation of coumarin metabolites in transgenic Arabidopsis thaliana and the enhanced tolerance to salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In China, Chinese herbal medicines have been used for thousands of years. As early as ancient times, there was a legend that Shennong tasted all kinds of herbs. His classic book Shennong Bencaojing has been handed down today (Zhao et al. 2020). Until now, Chinese herbal medicine culture has become an inseparable part of Chinese culture.
Nowadays, due to more adverse reactions caused by the use of western medicine, more and more people use Chinese herbal medicines to treat diseases (Liu et al. 2015). The bioactive components of Chinese herbal medicines play an important role in treating diseases. The development of new drugs cannot be separated from the theoretical guidance of traditional and ethnic medicines. Medicinal plants should be selected from the theoretical knowledge of traditional medicine. It is important to develop new drugs by extracting and isolating new bioactive components with in vitro or in vivo activities (Yuan et al. 2016). Bioactive ingredients mainly come from metabolites produced by the secondary metabolic pathway of plants. The secondary metabolites of plants mainly include five categories: alkaloids, flavonoids, phenolic acids, lignans, and terpenes, most of which are phenolic substances. Some compounds have biological activity and are widely used in medicine (Panche et al. 2016; Neag et al. 2018; Wink 2012; Ali et al. 2017; Barker 2019).
Plants produce and accumulate secondary metabolites through different metabolic pathways (Aharoni and Galili 2011). The phenylpropane biological metabolism pathway is important for phenolic substance metabolism, in which aromatic amino acids are finally converted into phenolic acids, flavonoids, stilbenes, coumarins, and other phenolic substances through a series of biochemical reactions (Shahidi and Yeo 2016). Glehnia littoralis is a perennial salt-tolerant plant belonging to the genus Glehnia in Umbelliferae, an endangered plant. The dried root of this plant can be used as medicine, commonly known as G. littoralis root. It is a tonic in traditional Chinese medicine. The whole plant of fresh G. littoralis can be eaten as a vegetable, and its roots can also be dried and processed into medicinal materials. In traditional medicine, G. littoralis root is mostly used to treat respiratory and pulmonary diseases, for example, cough, hemoptysis, dry throat, thirst, and other symptoms. Since the coronavirus disease 2019 (COVID-19) virus has spread worldwide, traditional Chinese medicine has played an important role in treating COVID-19 pneumonia. The therapeutic effect is very remarkable (Zhao et al. 2020). Some traditional Chinese medicine compounds contain G. littoralis root, so some effective ingredients in G. littoralis have played a positive role in fighting COVID-19 pneumonia (Yang et al. 2020). There are many bioactive components in G. littoralis. Phytochemical analysis shows that the main active components of G. littoralis are phenylpropanoids, coumarins, lignans, flavonoids, organic acids and their derivatives, terpenoids, polyacetylenes, steroids, nitrogen-containing compounds, etc. Coumarins are the main bioactive components in G. littoralis (Song et al. 2020).
Sixty-seven coumarins have been isolated from G. littoralis, including 17 simple coumarins and six pyran coumarins. The largest number of furocoumarins is 44 (Yang et al. 2019). Coumarins are widely used in the medical field. They are widely existing natural and synthetic compounds with anti-inflammatory, antioxidant, hepatoprotective, antithrombotic, antiviral, antibacterial, antituberculosis, anticancer, antidepression, antihyperlipidemic, and anticholinesterase activities (Emami and Dadashpour 2015). To sum up, coumarins have very important medical and commercial value in medicinal plants. The amount of high-value secondary metabolites determines the market price of medicinal materials (Chizzola and Lohwasser 2020). Therefore, improving the quality of medicinal plants by technical means is a very meaningful scientific research task. Plants accumulate secondary metabolites under stress conditions. Shu et al. found that salt stress treatment of G. littoralis can increase the content of three furanocoumarins, psoralen, imperatorin, and isoimperatorin, in G. littoralis root. Therefore, appropriate stress is of great economic value in improving the quality of G. littoralis (Shu et al. 2019). With the development of science and technology, molecular breeding technology is widely used in medicinal plant breeding. Transcriptome analysis of medicinal plants is used to screen and obtain key enzyme genes of plant secondary metabolism to construct expression vectors. Agrobacterium-mediated technology is used to transfer the expression vector into recipient cells, and a new medicinal plant is screened and cultivated (Hao and Xiao 2015; Bandurska et al. 2016; Niazian 2019). The ultimate goal is to improve the quality of medicinal plants and increase their metabolite levels (Yang et al. 2021). Some medicinal plants, such as Salvia miltiorrhiza, are mature in terms of molecular breeding (Song et al. 2013).
To improve the quality of G. littoralis, this study preliminarily screened the key enzyme genes of cytochrome P450 epoxygenase furocoumarin in the G. littoralis transcriptome, including PAL, C4H, 4CL, C2H, UDT, PS, MS, and other secondary metabolic key enzyme genes (Song et al. 2020). The key enzyme genes GlUDT (GenBank accession no. MH507300.1) and GlPS1 (GenBank accession no. MF632070.1) of furocoumarin were cloned and analyzed by bioinformatics. The construction of the overexpression vector has been completed (Song et al. 2018). This study further explored the GlPS1 gene of G. littoralis to determine its protein function.
Materials and methods
Plant cultivation
Arabidopsis thaliana (ecotype Columbia, Col-0) seeds were surface-sterilized, stratified by incubation in the dark at 4 °C for 3 days, and transferred to a chamber with 8 h light/16 h darkness at 75% relative humidity (RH) and 22 °C.
Fresh G. littoralis seeds were selected and harvested in July and August, dried in the sun, and packaged. The seeds were soaked in running water, put into a container of tap water overnight, discarding the excess water on the next day, and mixed. The seeds were packed into a black plastic bag, sealed, and treated at 4 °C low-temperature dark treatment for 3 to 4 months. After ~ 3 months, a bulge was observed on the concave surface of G. littoralis seeds. For the swelling point, the shell on the seed was carefully scraped using a scalpel, and the residue was set aside. On a sterile table, the seeds were immersed in a 0.1% HgCl2 solution for 20 min. The HgCl2 solution was recovered, and the seeds were rinsed thrice with sterile water and placed on moist sterile filter paper for later use. A wound was gently cut on the seed convex shaft using an anatomy knife; the wound depth was not too deep. Using two scalpels in the seed pocket, light pressure was applied on both sides of the medial axis of the face to cause the G. littoralis seed to wound from the medial axis. The seed was split and divided into two and a half. At this time, elongated embryo nodules were visible to the naked eye. Structure: The embryo was taken out from the seed using an anatomical needle and placed in a tissue culture bottle or cone on MS medium in a bottle. The bottle was sealed, and the embryo was cultured in an illuminated incubator with 8 h light/16 h darkness at 75% RH and 22 °C. G. littoralis embryos were put into an MS culture medium for ~ 15 days to obtain seedlings for the experiment.
Methyl jasmonate (MeJA) and NaCl-treated G. littoralis seedlings
The MeJA solution was prepared with 50% ethanol solution as the solvent and applied evenly to the upper and lower surfaces of the true leaves of robust sterile seedlings of G. littoralis as the experimental group material (the concentration of MeJA solution was 200 μmol L−1). In the CK group, the real leaves were only coated with 50% ethanol solution, and the treatment method was the same as the experimental group. Dark treatment was carried out at room temperature for 16 h, the true leaves of the aseptic seedlings of the G. littoralis were cut, and the leaves were quickly frozen in liquid nitrogen and stored at − 80 °C.
The filter paper was spread flat in the Petri dish, and the NaCl solution (200 mmol L−1) was prepared with distilled water. After the filter paper in the Petri dish was moistened and the Petri dish was tilted, it was then appropriate to observe a very small amount of effusion on the outer side of the Petri dish. Tweezers were used to gently spread the roots of G. littoralis aseptic seedlings on the filter paper moistened with NaCl solution, and the roots were in full contact with the surface of the filter paper. The samples were incubated at 25 °C for 24 h for the sampling of the whole plant, quickly frozen in liquid nitrogen, and stored at − 80 °C.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the leaves (MeJA-treated samples) and whole plant (NaCl-treated samples) using RNAprep Pure Plant Kit (Tiangen, China). RNA reverse transcription was performed using HiScript® III RT SuperMix for qPCR (Vazyme, China) and qRT-PCR using primer references (Table 1). qRT-PCR was performed using ABI QuantStudio 5 (Thermo Fisher, USA). qRT-PCR analysis was performed in a total volume of 25 µL containing 12.5 μL SYBR Premix ExTaq™ (TaKaRa, China), 0.5 μL of each primer, 1 μL cDNA, and 10.5 μL nuclease-free water. Gene expression was calculated using the Power = 2−ΔΔCt method. qRT-PCR analysis was built on at least three biological replicates of each sample with three technical replicates.
Transformation and regeneration of Arabidopsis
The GlPS1 gene was cloned by PCR from G. littoralis cDNA using KpnI and SalI restriction sites. The amplified fragment was inserted into the pCambia1300-221-3HA vector between the KpnI and SalI sites upstream of the 35S gene to form the vector pCambia1300-221-3HA:GlPS1. The pCambia1300-221-3HA:GlPS1 vector was transformed into Arabidopsis ecotype Columbia using the Agrobacterium tumefaciens-mediated floral dip method (Xie et al. 2012). Transformed Arabidopsis seeds were selected on 1/2 Murashige and Skoog (MS) medium containing 30 µg mL−1 hygromycin. Three independent lines of the T3 generation were randomly chosen for further analysis. Refer to the DNA extraction method of transgenic Arabidopsis by Doyle (1991). The primers used are shown in Table 1.
Semi-qRT-PCR determination 35S:GlPS1 transgenic Arabidopsis
Total RNA was extracted from the whole transgenic Arabidopsis plant using RNAprep Pure Plant Kit. RNA reverse transcription was performed using HiScript® III RT SuperMix for semi-qRT-PCR and qRT-PCR using primer references (Table 1). Semi-qRT-PCR was performed using ABI VeritiPro PCR (Thermo Fisher). Semi-qRT-PCR analysis was performed in a total volume of 25 µL containing 12.5 μL Green™ Premix Ex Taq™ II (TaKaRa), 0.5 μL of each primer, 1 μL cDNA, and 10.5 μL nuclease-free water. The GlPS1 and GlActin gene-specific primers used for semi-qRT-PCR are shown in Table 1. Statistical data were evaluated by Student’s t test.
Phenotypic analysis of transgenic Arabidopsis salt stress
The seeds of three strains randomly selected from CK strains were fully mixed. Similarly, the seeds of three strains of GlPS1 were fully mixed and washed thrice with 75% ethanol for 60 s each time in a sterile environment. The seeds were placed on sterile filter paper for drying. The seeds were sprinkled onto a 1/2 MS solid plate with gradient NaCl concentrations of 0, 100, 150, and 200 mmol L−1. The Petri dish was sealed with a sealing film and placed vertically in an illumination incubator for culture for 2 weeks. The culture conditions of transgenic Arabidopsis were 8 h light/16 h darkness at 75% RH and 22 °C. At the end of the culture, Arabidopsis seedlings were taken out, and the root length (from the hypocotyl to the root tip) was measured and photographed. Statistical data were evaluated by Student’s t test.
Metabolomics analysis of transgenic Arabidopsis
Sample preparation and extraction: The freeze-dried leaf was crushed using a mixer mill (MM 400; Retsch) with a zirconia bead for 1.5 min at 30 Hz. A 100 mg powder was weighted and extracted overnight at 4 °C with 1.0 mL of 70% aqueous methanol. After centrifugation at 10,000×g for 10 min, the extracts were absorbed (CNWBOND Carbon-GCB SPE Cartridge, 250 mg, 3 mL; ANPEL, Shanghai, China; www.anpel.com.cn) and filtrated (SCAA-104, 0.22 μm pore size; ANPEL) before liquid chromatography (LC)-mass spectrometry (MS) analysis.
High-performance LC (HPLC) conditions: The sample extracts were analyzed using an LC-electrospray ionization (ESI)-tandem MS (MS/MS) system (HPLC; Shim-pack UFLC Shimadzu CBM30A System; MS, Applied Biosystems 6500 QTRAP).
Analytical conditions: HPLC: column, Waters ACQUITY UPLC HSS T3 C18 (1.8 μm, 2.1 × 100 mm); solvent system, water (0.04% acetic acid)/acetonitrile (0.04% acetic acid); gradient program, 100:0 (v/v) at 0 min, 5:95 (v/v) at 11.0 min, 5:95 (v/v) at 12 min, 95:5 (v/v) at 12.1 min, 95:5 (v/v) at 15.0 min; flow rate, 0.40 mL min−1; temperature, 40 °C; injection volume: 2 μL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.
ESI-QTRAP-MS/MS: LIT and triple quadrupole (QQQ) scans were acquired on a triple QTRAP-MS, API 6500 QTRAP LC/MS/MS system, equipped with an ESI turbo ion spray interface, operating in a positive-ion mode and controlled by Analyst 1.6.3 software (AB Sciex).
ESI source operation parameters: ion source, turbo spray; source temperature 500 °C; ion spray voltage, 5500 V; ion source gas I, gas II, and curtain gas were set at 55, 60, and 25 psi, respectively; the collision gas was high. Instrument tuning and mass calibration were performed with 10 and 100 μmol L−1 polypropylene glycol solutions in QQQ and LIT modes, respectively. QQQ scans were acquired as MRM experiments with collision gas (nitrogen) set to 5 psi. DP and CE for individual MRM transitions were done with further DP and CE optimization. A specific set of MRM transitions was monitored for each period according to the metabolites eluted within this period.
Results
Effects of MeJA and NaCl stress on GlPS1 gene expression of G. littoralis
Proper salt treatment can induce salt stress and secondary stress in plants. Proper salt stress is helpful in improving the quality of medicinal plants. MeJA plant hormone can induce plants to produce defensive metabolites that can alleviate biotic and abiotic stresses faced by plants. MeJA also positively improves the quality of medicinal materials (Yu et al. 2019; Yang and Guo 2018; Zhou et al. 2021; Gengmao et al. 2014). To verify whether the GlPS1 gene of G. littoralis is involved in secondary metabolism and salt stress, G. littoralis were treated with MeJA and NaCl salt stress. qRT-PCR was used to analyze the GlPS1 gene of G. littoralis (Fig. 1a and b). After 24 h of treatment, GlPS1 gene expression in G. littoralis treated with MeJA and NaCl was significantly higher than in the untreated control. The relative expression was nearly four times that of the control, indicating that the GlPS1 gene successfully participated in the reaction process of secondary metabolism and NaCl stress in G. littoralis.
a GlPS1 gene expression of G. littoralis treated by MeJA. b GlPS1 gene expression of G. littoralis treated by NaCl stress. c Semi-quantitative detection of CK (transgenic Arabidopsis with empty vectors) and GlPS1 (transgenic Arabidopsis with 35S:GlPS1). d Root length measurement and analysis under salt treatment in CK and GlPS1.This experiment was repeated three times. Data represent the mean ± SD, significant difference from the wild type, as indicated by Student’s t test: **0.001 < P < 0.01
GlPS1 overexpression enhanced the NaCl tolerance of transgenic Arabidopsis
It is a good technical means to study gene function through model plants, especially in the study of abiotic stress of plants. Arabidopsis plants that successfully expressed exogenous genes were treated with abiotic stress and compared to the control group in morphology and physiological and biochemical indices. The gene function was deduced (Liu et al. 2020; Li et al. 2012). To speculate whether the GlPS1 gene could improve the salt tolerance of A. thaliana, and the salt tolerance of plants was closely related to the accumulation of plant metabolites (Aharoni and Galili 2011), A. thaliana plants were prepared with 35S overexpression of the GlPS1 gene. DNA detection results showed that the positions of the bands of GlPS1 transgenic A. thaliana strains and the positive control were correct, whereas the negative control had no electrophoresis bands (Fig. S1). The semiquantitative experimental results showed that the semiquantitative electrophoresis band of the GlPS1 gene was not detected in the PCR results of the CK group. The semiquantitative electrophoresis band of the GlPS1 gene was detected in all GlPS1 transgenic Arabidopsis strains, indicating that the GlPS1 gene was successfully expressed in GlPS1 transgenic Arabidopsis strains (Fig. 1c).
To test the salt tolerance of transgenic A. thaliana with 35S:GlPS1, transgenic Arabidopsis seeds were planted with the CK and GlPS1 groups on 1/2 MS plates with different NaCl concentrations, and the root length of the seedlings of the two groups was compared after 2 weeks for comparative analysis. A. thaliana seeds germinated into seedlings successfully in all 1/2 MS media. A. thaliana grew well in the medium supplemented with 0 and 100 mmol L−1 NaCl. The aerial part had two healthy intact cotyledons and true leaves. The root was long. A. thaliana grown on the medium without NaCl was stronger. On the medium supplemented with 150 mmol L−1 NaCl, the hypocotyl length of A. thaliana was obviously shortened. The true leaves and cotyledons showed slight wilting. On the medium supplemented with 200 mmol L−1 NaCl, the length of the A. thaliana plant was < 1 cm, indicating that the plant shrank seriously. The hypocotyl shortening was very serious. The leaves were pale green, and no true leaf structure was found (Fig. 2).
The hypocotyl length of two groups of A. thaliana was measured and analyzed. Results were presented in the form of histograms. When NaCl was not added, the average root length of A. thaliana in the CK and GlPS1 groups was 4.3 and 3.3 cm, respectively. There was a significant difference between the groups. At a NaCl concentration of 100 mmol L−1, the average root length of A. thaliana in the CK and GlPS1 groups was 4.8 and 4.6 cm, respectively. There was no significant difference between the groups. At a NaCl concentration of 150 mmol L−1, the average root length of A. thaliana in the CK and GlPS1 groups was 2.0 and 2.5 cm, respectively, whirly half of the length compared to the previous value. However, the root length of A. thaliana in the GlPS1 group was longer than that in the CK group. Data were reversed with significant differences. At a NaCl concentration of 200 mmol L−1, the root length of A. thaliana in the CK and GlPS1 groups was < 1 cm. There were significant differences between the groups (Fig. 1d).
Results showed that the salt tolerance of A. thaliana root transformed with the GlPS1 gene was better under salt stress conditions. At the same time, it was speculated that the GlPS1 group of A. thaliana was more salt-tolerant because GlPS1 overexpression accumulated metabolites in A. thaliana. Thus, it was necessary to systematically analyze the metabolites in the two groups of A. thaliana plants.
35S:GlPS1 transgenic Arabidopsis metabolomics analysis
To determine the secondary metabolites in the two groups of A. thaliana, plant metabolomics analysis was conducted on the CK and GlPS1 groups, and the 226 detected secondary metabolites were divided into 19 classes. The classification results are shown in Fig. 3a, including 32 coumarins, 29 phenolic acids, 27 flavonols, 27 glucosinolates, 24 alkaloids, 9 flavonoids, 5 plumeranes, 5 phenolamines, 5 lignans, 4 flavonoid carbonosides, 3 dihydroflavones, 3 isoflavones, 2 piperidine alkaloids, 2 sesquiterpenoids, 2 flavanols, 2 anthocyanins, 1 isoquinoline alkaloid, 1 amphetamine alkaloid, and 43 others.
a Classification of the 226 metabolites of CK and GlPS1. b PLS-DA analysis of metabolites of CK and GlPS1. c PCA analysis of metabolites identified from CK and GlPS1. Equal volumes of CK and GlPS1 samples were mixed for use as a quality control (QC). d cluster heat map analysis of metabolites from samples of CK and GlPS1. The colour indicates the level of accumulation of each metabolite, from low (green) to high (red). The Z-score represents the deviation from the mean by standard deviation units. e Volcouldo plot of the 226 metabolites identified. Differential metabolites were defined as metabolites with fold change ≥ − 1 or ≤ 1 in CK compared to GlPS1. A threshold of VIP ≥ 1 was used to separate differential metabolites from unchanged metabolites. (Color figure online)
To compare the accumulation of secondary metabolites in the two groups of A. thaliana, the 226 detected secondary metabolites were analyzed by heat mapping. The secondary metabolites were classified into five clusters marked as Roman numerals I to V, respectively. Compared to the secondary metabolites in the GlPS1 group in clusters I and II, the secondary metabolites in the CK group were upregulated. In cluster III, most secondary metabolites were upregulated, and a few were downregulated. In clusters IV and V, most secondary metabolites were upregulated (Fig. 3d). The PLS-DA analysis showed that the two principal components PC1 and PC2, were 92.1 and 4.8%, respectively, and the cumulative contribution rate was 96.8% (Fig. 3b). Principal component analysis (PCA) showed that the two groups of experimental samples were separated obviously on the first principal component but not on the second principal component, indicating differences in the accumulation of secondary metabolites between the two groups. There was little difference in the types of secondary metabolites (Fig. 3c). PLS-DA, PCA and cluster heat map analysis showed that there were differences in the accumulation of secondary metabolites between the two groups. The content of the total components in the GlPS1 group was higher than in the CK group.
To further analyze the content and types of differential metabolites in the two groups, the logFC (fold change) value was used as the abscissa and the variable importance in projection VIP) value as the ordinate to construct a volcano map. The VIP value of 1 was set as the dividing line to distinguish the differential metabolites. Thirty different metabolites were obtained, of which 26 were upregulated and 4 were downregulated (Fig. 3e). Among the 30 differential metabolites, there were 12 coumarins, 2 glucosinolates, 5 alkaloids, 2 flavonols, 2 phenolic acids, 1 isoflavone, 1 anthocyanin metabolite, 1 phenolamine metabolite, 1 lignan metabolite, 1 flavonoid carbonoside, and 2 others. Eleven compounds, including 8-methoxypsoralen, isopimpinellin, imperatorin, isooxypeucedanine, wedelolactone, esculin, glucosinalbin, caffeoylcholine 5-glucoside, feruloylcholine glucoside, caffeic acid, and isorhamnetin-O-hexoside-O-rhamnoside-O-rhamnoside, had no effective relative peak areas in the CK group, but effective peak areas were detected in the GlPS1 group (Table 2). Therefore, these 11 compounds were unique secondary metabolites of GlPS1 transgenic Arabidopsis, and they were regulated by the GlPS1 exogenous gene.
To analyze the metabolic pathways involved in 30 differential metabolites in the experimental metabolome and the related annotation information in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, differential metabolites were searched in the KEGG pathway, and 11 compounds were annotated. Three were involved in the biosynthesis of phenology, two in metabolic pathways, two in the biosynthesis of secondary metabolites, one in phenology biosynthesis, one in the degradation of aromatic compounds, one in arginine and proline metabolism, and one in anthocyanin biosynthesis (Fig. 4a). Phenylpropanoid biosynthesis was the metabolic pathway that involved the GlPS1 gene. In the literature, this pathway was divided into 11 submetabolic pathways, among which coumarin biosynthesis was the metabolic process that this study focused on.
To analyze the significance and enrichment degree of differential metabolites, an enrichment map of the Rich factor and P values of differential metabolites was drawn (Fig. 4b). The focus was on the three compounds in phenanthroponoid biosynthesis. Compared to the other six metabolic pathways, the enrichment of phenanthroponoid biosynthesis was the most significant. Therefore, the GlPS1 gene was significantly involved in A. thaliana biosynthesis.
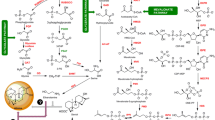
To show the results of this metabolomics experiment more clearly in the published metabolic pathway, the results of the thermogram analysis of different metabolites were put into the published phenylpropanoid biosynthesis (Fig. 5). Phenylpropanoid and coumarin biosyntheses are two submetabolic pathways of phenylpropanoid biosynthesis, also the focus of this experiment. Phenylpropanoid biosynthesis was located upstream of coumarin biosynthesis, and the p-coumarate-CoA provided by it was an important reaction substrate for downstream coumarin biosynthesis. Imperatorin was similar to xanthotoxol in chemical structure, and both had a psoralen structure. It was speculated that accelerin is formed by the dehydration reaction between xanthotoxol and a molecule of isopentenol, but there might be a series of biochemical reactions between them. Therefore, it was speculated that imperatorin is the downstream product of coumarin biosynthesis. Results indicated that the functional protein induced by the GlPS1 gene of G. littoralis played an important role in phenylpropanoid and coumarin biosyntheses.
Phenylpropanoid biosynthesis and Coumarin biosynthesis Pathway in G. littoralis. The red color small rectangle indicates that metabolite content is significantly upregulated, the green small rectangle indicates that metabolite content is significantly down regulated. The blue character and formula represent phenylpropanoid biosynthesis. The green character and formula represent Coumarin biosynthesis. The dotted line part is a speculated coumarin metabolic pathway. (Color figure online)
Discussion
Phenylpropanoid biosynthesis is an important pathway of plant secondary metabolism, which uses several intermediates in the shikimic acid pathway as the core raw materials to synthesize metabolites. The shikimate pathway is located upstream of phenylpropanoid biosynthesis. In this process, hydroxycinnamic acid and some esters undergo a series of cascade reactions, such as reductase, oxygenase, and transferase. Combinatorial decomposition is carried out in different parts of plants, resulting in plant tissue differences (Vogt 2010). Phenylalanine produced by the shikimate pathway provides raw materials for downstream of phenylpropanoid biosynthesis. The catalytic products of l-phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumaric acid CoA ligase (4CL) provide a material basis for all subsequent branching metabolic processes. 4CL introduces the generated p-coumaroyl CoA into different biosynthetic pathways. It is the direct precursor of flavonoid, coumarin, or lignin biosynthesis (D’Auria 2006). When plants are stressed, the change in the concentration of the shikimic acid pathway intermediates may lead to the formation of new phytoalexins, volatiles, flavonoids, and anthocyanins in the whole phenological biosynthesis and protein resynthesis (Schoch et al. 2006). Most metabolites produced by plant secondary metabolism are stored in vacuoles in the form of glycosylation to participate in the subsequent biosynthesis reaction. Phenolic compounds, such as coumarins, are related to the survival strategy of plants. After extracellular information induction, the intermediate in the vacuoles is released into the cytosol for the next metabolism. Vacuoles are important organelles for the accumulation of secondary metabolic intermediates and also important for the biosynthesis and transport of intermediates (Shitan and Yazaki 2013; Xu et al. 2015; Shitan 2016; Park et al. 2017; Martinoia 2018; Brito Francisco and Martinoia 2018; Zhang et al. 2018; Taguchi et al. 2000; Shitan and Yazaki 2013).
MeJA is a plant endogenous hormone molecule usually used as an inducer and elicitor of plants (Zhao et al. 2005; Pauwels et al. 2009; Shi et al. 2017). MeJA has been used for a long time to regulate the secondary metabolism of plants to trigger stress reactions and produce plant secondary metabolites (Rahnamaie-Tajadod et al. 2019). External application of MeJA to plants can upregulate or downregulate genes related to plant secondary metabolism in a short time. At the same time, the amount of secondary metabolites in plant cells is increased. Rahnamaie et al. analyzed the transcriptome of leaves of Polygonum minus plants with MeJA and obtained 2374 differentially expressed genes, of which 1419 were upregulated and 955 were downregulated, including secondary metabolic synthetase gene and transcription factor (Rahnamaie-Tajadod et al. 2017). In agricultural production, the quality of agricultural products can be improved by applying MeJA externally. Using MeJA externally in apples can induce a blush phenomenon in a short time. Many phenolic compounds accumulate in the peel, which improves the apple’s quality (Shafiq et al. 2013). Exogenous MeJA can increase volatile components in tea, especially geraniol, linalool, and their oxides. Furthermore, MeJA can activate the secondary metabolic pathway, especially terpene volatile components (Shi et al. 2017). Spraying healthy Ocimum basilicum L. with 0.5 mM MeJA can accumulate total phenol content in plants and increase significantly (Kim et al. 2006). MeJA-induced treatment can promote the release of volatile organic compounds from leaves of P. minus, and its components are mainly terpenoids. Terpenoids mediated by MeJA are related to plant resistance to biotic and abiotic stresses (Rahnamaie-Tajadod et al. 2019). On the molecular mechanism of MeJA regulation, exogenous MeJA can regulate JA biosynthesis and JA signaling pathway and regulate downstream gene expression, including genes related to the key enzymes of the phenylpropanoid pathway, such as PAL, C4H, 4CL, chalcone synthase, chalcone flavanone isomerase, flavanone 3-hydroxylase, and dihydroflavonol reductase. Many genes encoding transcription factors were induced by MeJA (Rahnamaie-Tajadod et al. 2019).
Salt stress affects the secondary metabolic process of medicinal plants (Yang et al. 2018; Gupta and Huang 2014; Sytar et al. 2018). The increase of salinity in the soil makes plant roots suffer from salt stress. In the initial stage, due to osmotic stress mediated by high salt concentrations (Xu et al. 2016; Munns 2002; Adak et al. 2019), plant roots lose water and the water absorption capacity of roots drops sharply. The roots are dehydrated, vacuoles and cytoplasm lose water, and the cytoplasm becomes thicker (Mahajan and Tuteja 2005). To resist the water loss caused by salt stress, plants accumulate specific metabolites, such as phenolic compounds, to resist biotic and abiotic stresses, resulting in reduced water potential of plants and water absorption in the soil (Yi et al. 2010; Fu et al. 2011; Abideen et al. 2015; Parida et al. 2004; Ksouri et al. 2007; Hanen et al. 2008; Lim et al. 2012; Petridis et al. 2012; Borgognone et al. 2014; Falcinelli et al. 2017; López-Berenguer et al. 2009; Valifard et al. 2017; Imen et al. 2012; Daneshmand et al. 2010; Petrusa and Winicov 1997; Aziz et al. 1998; Pedrazani et al. 2003). The accumulation of secondary metabolites in plants is related to the ability of plants to cope with salt stress. In this experiment, the successful heterologous overexpression of the GlPS1 gene in A. thaliana and the subsequent metabolomics analysis confirmed that the GlPS1 gene might accumulate secondary metabolites in A. thaliana because transgenic Arabidopsis has more obvious salt tolerance. Additionally, the GlPS1 protein may play a direct role.
The psoralen synthase (PS) protein sequence has been cloned and sequenced in many species, but its protein function is rarely published. In 2006, the first PS gene in Ammi majus plant by molecular biology was cloned and verified (Larbat et al. 2007). In 2020, the protein activity of the PS gene CYP71AJ49 of Peucedanum praeruptorum was confirmed by yeast experiments in vivo and in vitro. HPLC-ESI-MS verified that the CYP71AJ49 gene could transform marmesin into psoralen. It was subsequently speculated that CYP71AJ49 might have other activities (Ramos et al. 2018; Larbat et al. 2007; Kim et al. 2017, 2021; Xie et al. 2019; Silva-Junior et al. 2018; Pecrix et al. 2017; Raymond et al. 2018; Bredeson et al. 2022; Hufnagel et al. 2020; Jian et al. 2020). The GlPS1 gene was heterologously expressed in model plant A. thaliana, and metabolomics analysis was carried out. The heterologous expression of the exogenous gene in the model plant A. thaliana through the study of the gene function of plant metabolomics is rarely reported in the literature. This study made a breakthrough in previous metabolomics research methods of A. thaliana and proved the function of the GlPS1 protein of G. littoralis by analyzing relevant metabolomics data (Saito 2013; Meng et al. 2019; Lan et al. 2021; Jaini et al. 2017; Zhu et al. 2020).
Coumarin biosynthesis of G. littoralis is similar to the published metabolic pathway. Based on the analysis of different metabolites between the CK and GlPS1 groups, 11 metabolites were not detected in the CK group. Three metabolites, isopimpinellin, esculin, and caffeic acid, were noted in the metabolic map (Fig. 4). The imperatorin site was a putative pathway. It was speculated that these four coumarin metabolites might not exist in wild A. thaliana. In contrast, the content of the GlPS1 group was significantly higher than the CK group. The unique variable GlPS1 protein played an important biological function. The metabolites of psoralen could not be detected due to the limitation of standard quantity samples; however, the structural formulas of isopimpinellin and imperatorin both contained psoralen units. Thus, it was speculated that the psoralen content in the GlPS1 group was higher. Imperatorin was formed by the dehydration reaction with isopentenol based on psoralen. It was also speculated that this process is completed by isopentenyl transferase (IPT). The IPT gene has been reported in many species. It mainly functions as isoprene tRNA and participates in the de novo synthesis of a cytokine through ADP/ATP-IPT. However, whether IPT plays a role in coumarin biosynthesis is unknown (Chen et al. 2021; Jameson and Song 2015). Therefore, relevant experimental data are needed to confirm it. In addition, the GlPS1 gene is also involved in other metabolic pathways of A. thaliana, upregulating two metabolites (p-coumaroylputrescine and cyanidin 3-O-3″,6″-O-dimalonylglucoside). p-Coumaroylputrescine is a secondary metabolite of phenolic amides induced by biotic and abiotic stresses in plants, especially by trauma. Therefore, these metabolites can be used as antimicrobial, herbivorous compounds to reduce ultraviolet stress (Andama et al. 2020; Alamgir et al. 2016; Shinya et al. 2021).
Conclusions and perspectives
There are few related studies on G. littoralis because it is difficult to study the species. When G. littoralis seeds and plants are stored and grown in the laboratory, the requirements on temperature, air humidity, soil type, water content, light intensity, etc., are strict. The experiment would fail if it was careless. Therefore, it was a good choice to study the genes related to the secondary metabolism of G. littoralis through the model plant A. thaliana and metabolomics; however, genetic engineering and breeding of G. littoralis are also very important scientific research tasks. Based on this study, the experimental method of infecting A. thaliana can be considered to prepare G. littoralis transgenic plants.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MeJA:
-
Methyl jasmonate
- PAL:
-
l-Phenylalanine ammonia-lyase
- C4H:
-
Cinnamic acid 4-hydroxylase
- 4CL:
-
4-Coumaric acid CoA ligase
- C2H:
-
p-Coumaroyl CoA 2′-hydroxylase
- UDT:
-
Umbelliferone dimethylallyl transferase
- PS:
-
Psoralen synthase
- MS:
-
Marmesin synthase
References
Abideen Z, Qasim M, Rasheed A et al (2015) Antioxidant activity and polyphenolic content of Phragmites karka under saline conditions. Pak J Bot 47(3):813–818
Adak S, Roy A, Das P et al (2019) Soil salinity and mechanical obstruction differentially affects embryonic root architecture in different rice genotypes from West Bengal. Plant Physiol Rep. https://doi.org/10.1007/s40502-019-00450-2
Aharoni A, Galili G (2011) Metabolic engineering of the plant primary-secondary metabolism interface. Curr Opin Biotechnol 22(2):239–244. https://doi.org/10.1016/j.copbio.2010.11.004
Alamgir KM, Hojo Y, Christeller JT et al (2016) Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ 39(2):453–466. https://doi.org/10.1111/pce.12640
Ali M, Abbasi BH, Ahmad N et al (2017) Strategies to enhance biologically active-secondary metabolites in cell cultures of Artemisia—current trends. Crit Rev Biotechnol 37:833–851. https://doi.org/10.1080/07388551.2016.1261082
Andama JB, Mujiono K, Hojo Y et al (2020) Nonglandular silicified trichomes are essential for rice defense against chewing herbivores. Plant Cell Environ 43(9):2019–2032. https://doi.org/10.1111/pce.13775
Aziz A, Martin-Tanguy J, Larher F (1998) Stress-induced changes in polyamine and tyramine levels could regulate proline accumulation in tomato leaf discs treated with sodium chloride. Physiol Plant 104:195–202
Bandurska K, Berdowska A, Król M (2016) Transformation of medicinal plants using Agrobacterium tumefaciens. Postepy Hig Med Dosw (Online) 70:1220–1228
Barker D (2019) Lignans. Molecules 24(7):1424. https://doi.org/10.3390/molecules24071424
Borgognone D, Cardarelli M, Rea E et al (2014) Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J Sci Food Agric 94(6):1231–1237. https://doi.org/10.1002/jsfa.6403
Bredeson JV, Lyons JB, Oniyinde IO et al (2022) Chromosome evolution and the genetic basis of agronomically important traits in greater yam. Nat Commun 13(1):2001. https://doi.org/10.1038/s41467-022-29114-w
Brito Francisco R, Martinoia E (2018) The vacuolar transportome of plant specialized metabolites. Plant Cell Physiol 59(7):1326–1336. https://doi.org/10.1093/pcp/pcy039
Chen L, Zhao J, Song J et al (2021) Cytokinin glucosyl transferases, key regulators of cytokinin homeostasis, have potential value for wheat improvement. Plant Biotechnol J 19(5):878–896. https://doi.org/10.1111/pbi.13595
Chizzola R, Lohwasser U (2020) Diversity of secondary metabolites in roots from Conium maculatum L. Plants (Basel) 9(8):939. https://doi.org/10.3390/plants9080939.PMID:32722223;PMCID:PMC7464025
D’Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9(3):331–340. https://doi.org/10.1016/j.pbi.2006.03.016
Daneshmand F, Arvin MJ (2010) Kalantari KM (2010) Physiological responses to NaCl stress in three wild species of potato in vitro. Acta Physiol Plant 32:91–101
Doyle J (1991) DNA protocols for plants. In: Hewitt GM, Johnston AWB, Young JPW (eds) Molecular techniques in taxonomy. NATO ASI series, vol 57. Springer, Berlin. https://doi.org/10.1007/978-3-642-83962-7_18
Emami S, Dadashpour S (2015) Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur J Med Chem 102:611–630. https://doi.org/10.1016/j.ejmech.2015.08.033
Falcinelli B, Sileoni V, Marconi O et al (2017) Germination under moderate salinity increases phenolic content and antioxidant activity in rapeseed (Brassica napus var oleifera Del.) sprouts. Molecules 22(8):1377. https://doi.org/10.3390/molecules22081377
Fu XZ, Ullah Khan E, Hu SS et al (2011) Overexpression of the betaine aldehyde dehydrogenase gene from Atriplex hortensis enhances salt tolerance in the transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Environ Exp Bot 74:106–113. https://doi.org/10.1016/j.envexpbot.2011.05.006
Gengmao Z, Quanmei S, Yu H et al (2014) The physiological and biochemical responses of a medicinal plant (Salvia miltiorrhiza L.) to stress caused by various concentrations of NaCl. PLoS ONE 9(2):e89624. https://doi.org/10.1371/journal.pone.0089624
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. https://doi.org/10.1155/2014/701596
Hanen F, Ksouri R, Megdiche W et al (2008) Effect of salinity on growth, leaf-phenolic content and antioxidant scavenging activity in Cynara cardunculus L. In: Abdelly C, Öztürk M, Ashraf M, Grignon C (eds) Biosaline agriculture and high salinity tolerance. Birkhäuser, Basel. https://doi.org/10.1007/978-3-7643-8554-5_31
Hao DC, Xiao PG (2015) Genomics and evolution in traditional medicinal plants: road to a healthier life. Evol Bioinform (Online) 11:197–212. https://doi.org/10.4137/EBO.S31326
Hufnagel B, Marques A, Soriano A et al (2020) High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nat Commun 11(1):492. https://doi.org/10.1038/s41467-019-14197-9
Imen T, Cristina S, Riccardo I et al (2012) Phenolic acids and total antioxidant activity in Ocimum basilicum L. grown under Na2SO4 medium. J Med Plants Res 6:5868–5875
Jaini R, Wang P, Dudareva N et al (2017) Targeted metabolomics of the phenylpropanoid pathway in Arabidopsis thaliana using reversed phase liquid chromatography coupled with tandem mass spectrometry. Phytochem Anal 28(4):267–276. https://doi.org/10.1002/pca.2672
Jameson PE, Song J (2015) Cytokinin: a key driver of seed yield. J Exp Bot 67(3):593–606. https://doi.org/10.1093/jxb/erv461
Jian X, Zhao Y, Wang Z et al (2020) Two CYP71AJ enzymes function as psoralen synthase and angelicin synthase in the biosynthesis of furanocoumarins in Peucedanum praeruptorum Dunn. Plant Mol Biol 104(3):327–337. https://doi.org/10.1007/s11103-020-01045-4
Kim HJ, Chen F, Wang X et al (2006) Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J Agric Food Chem 54(6):2327–2332. https://doi.org/10.1021/jf051979g
Kim S, Park J, Yeom SI et al (2017) New reference genome sequences of hot pepper reveal the massive evolution of plant disease-resistance genes by retroduplication. Genome Biol 18(1):210. https://doi.org/10.1186/s13059-017-1341-9
Kim MS, Lee T, Baek J et al (2021) Genome assembly of the popular Korean soybean cultivar Hwangkeum. G3 (Bethesda) 11(10):jkab272. https://doi.org/10.1093/g3journal/jkab272
Ksouri R, Megdiche W, Debez A et al (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem 45(3–4):244–249. https://doi.org/10.1016/j.plaphy.2007.02.001
Lan Y, Sun R, Ouyang J et al (2021) AtMAD: Arabidopsis thaliana multi-omics association database. Nucleic Acids Res 49(D1):D1445–D1451. https://doi.org/10.1093/nar/gkaa1042
Larbat R, Kellner S, Specker S et al (2007) Molecular cloning and functional characterization of psoralen synthase, the first committed monooxygenase of furanocoumarin biosynthesis. J Biol Chem 282(1):542–554. https://doi.org/10.1074/jbc.M604762200
Li ZY, Xu ZS, He GY et al (2012) Overexpression of soybean GmCBL1 enhances abiotic stress tolerance and promotes hypocotyl elongation in Arabidopsis. Biochem Biophys Res Commun 427(4):731–736. https://doi.org/10.1016/j.bbrc.2012.09.128
Lim JH, Park KJ, Kim BK et al (2012) Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem 135(3):1065–1070. https://doi.org/10.1016/j.foodchem.2012.05.068
Liu L, Liu C, Wang Y et al (2015) Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol 13(4):481–493. https://doi.org/10.2174/1570159x1304150831122734
Liu Y, Pei L, Xiao S et al (2020) AtPPRT1 negatively regulates salt stress response in Arabidopsis seedlings. Plant Signal Behav 15(3):1732103. https://doi.org/10.1080/15592324.2020.1732103
López-Berenguer C, Martínez-Ballesta M, Moreno DA et al (2009) Growing hardier crops for better health: salinity tolerance and the nutritional value of broccoli. J Agric Food Chem 57(2):572–578. https://doi.org/10.1021/jf802994p
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158. https://doi.org/10.1016/j.abb.2005.10.018
Martinoia E (2018) Vacuolar transporters—companions on a longtime journey. Plant Physiol 176(2):1384–1407. https://doi.org/10.1104/pp.17.01481
Meng L, Zhang T, Geng S et al (2019) Comparative proteomics and metabolomics of JAZ 7-mediated drought tolerance in Arabidopsis. J Proteomics 196:81–91. https://doi.org/10.1016/j.jprot.2019.02.001
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Neag MA, Mocould A, Echeverria J et al (2018) Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front Pharmacol 9:557
Niazian M (2019) Application of genetics and biotechnology for improving medicinal plants. Planta 249(4):953–973. https://doi.org/10.1007/s00425-019-03099-1
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41
Parida AK, Das AB, Sanada Y et al (2004) Effects of salinity on biochemical components of the mangrove Aegiceras corniculatum. Aquat Bot 80:77–87
Park J, Lee Y, Martinoia E et al (2017) Plant hormone transporters: what we know and what we would like to know. BMC Biol 15(1):93. https://doi.org/10.1186/s12915-017-0443-x
Pauwels L, Inze D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 14(2):87–91. https://doi.org/10.1016/j.tplants.2008.11.005
Pecrix Y, Staton SE, Sallet E et al (2017) Whole-genome landscape of Medicago truncatula symbiotic genes. Nat Plants 4(12):1017–1025. https://doi.org/10.1038/s41477-018-0286-7
Pedrazani H, Racagni G, Alemano S et al (2003) Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul 412:149–158
Petridis A, Therios I, Samouris G et al (2012) Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ Exp Bot 79:37–43
Petrusa LM, Winicov I (1997) Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol Biochem 35:303–310
Rahnamaie-Tajadod R, Loke KK, Goh HH et al (2017) Differential gene expression analysis in Polygonum minus leaf upon 24 h of methyl jasmonate elicitation. Front Plant Sci 8:109. https://doi.org/10.3389/fpls.2017.00109
Rahnamaie-Tajadod R, Goh HH, Mohd Noor N (2019) Methyl jasmonate-induced compositional changes of volatile organic compounds in Polygonum minus leaves. J Plant Physiol 240:152994. https://doi.org/10.1016/j.jplph.2019.152994
Ramos AM, Usié A, Barbosa P et al (2018) The draft genome sequence of cork oak. Sci Data 5:180069. https://doi.org/10.1038/sdata.2018.69
Raymond O, Gouzy J, Just J et al (2018) The Rosa genome provides new insights into the domestication of modern roses. Nat Genet 50(6):772–777. https://doi.org/10.1038/s41588-018-0110-3
Saito K (2013) Phytochemical genomics—a new trend. Curr Opin Plant Biol 16(3):373–380. https://doi.org/10.1016/j.pbi.2013.04.001
Schoch G, Morant M, Abdulrazzak N et al (2006) Themeta-hydroxylation step in the phenylpropanoid pathway: a new level of complexity in the pathway and its regulation. Environ Chem Lett 4:127–136
Shafiq M, Singh Z, Kha A (2013) Time of methyl jasmonate application influences the development of ‘Cripps Pink’ apple fruit colour. J Sci Food Agric 93:611–618
Shahidi F, Yeo JD (2016) Insoluble-bound phenolics in food. Molecules 21(9):1216. https://doi.org/10.3390/molecules21091216
Shi J, Ma C, Qi D et al (2017) Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biol 15:233. https://doi.org/10.1186/s12870-015-0609-z. ((Erratum in: BMC Plant Biol 17(1):136))
Shinya T, Miyamoto K, Uchida K et al (2021) Chitooligosaccharide elicitor and oxylipins synergistically elevate phytoalexin production in rice. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01217-w
Shitan N (2016) Secondary metabolites in plants: transport and self-tolerance mechanisms. Biosci Biotechnol Biochem 80(7):1283–1293. https://doi.org/10.1080/09168451.2016.1151344
Shitan N, Yazaki K (2013) New insights into the transport mechanisms in plant vacuoles. Int Rev Cell Mol Biol 305:383–433. https://doi.org/10.1016/B978-0-12-407695-2.00009-3
Shu X, Li N, Tang X et al (2019) Effects of NaCl stress on photosynthetic physiology and active component of different Glehnia littoralis provenance. Jiangsu J Agric 35(4):8
Silva-Junior OB, Grattapaglia D, Novaes E et al (2018) Genome assembly of the Pink Ipê (Handroanthus impetiginosus, Bignoniaceae), a highly valued, ecologically keystone Neotropical timber forest tree. Gigascience 7(1):1–16. https://doi.org/10.1093/gigascience/gix125
Song JY, Luo HM, Li CF et al (2013) Salvia miltiorrhiza as medicinal model plant. Yao Xue Xue Bao 48(7):1099–1106
Song JJ, Luo HM, Jing MX et al (2018) Cloning and expression analysis of GlPS1, GlPS2 gene in Glehnia littoralis. Chin Tradit Herb Drugs 24:1139–1145
Song J, Luo H, Xu Z et al (2020) Mining genes associated with furanocoumarin biosynthesis in an endangered medicinal plant Glehnia Littoralis. J Genet 99:11
Sytar O, Barki S, Zivcak M et al (2018) The involvement of different secondary metabolites in salinity tolerance of crops. In: Kumat V (ed) Salinity responses and tolerance in plants, vol. 2. Springer, Berlin, pp 21–48. https://doi.org/10.1007/978-3-319-90318-72.
Taguchi G, Fujikawa S, Yazawa T et al (2000) Scopoletin uptake from culture medium and accumulation in the vacuoles after conversion to scopolin in 2,4-D-treated tobacco cells. Plant Sci 151(2):153–161. https://doi.org/10.1016/s0168-9452(99)00212-5
Valifard M, Mohsenzadeh S, Kholdebarin B (2017) Salinity effects on phenolic content and antioxidant activity of Salvia macrosiphon. Iran J Sci Technol A 41:295–300
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3(1):2–20. https://doi.org/10.1093/mp/ssp106
Wink M (2012) Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules 17(11):12771–12791
Xie C, Zhang R, Qu Y et al (2012) Overexpression of MtCAS31 enhances drought tolerance in transgenic Arabidopsis by reducing stomatal density. New Phytol 195(1):124–135. https://doi.org/10.1111/j.1469-8137.2012.04136.x
Xie M, Chung CY, Li MW et al (2019) A reference-grade wild soybean genome. Nat Commun 10(1):1216. https://doi.org/10.1038/s41467-019-09142-9
Xu H, Martinoia E, Szabo I (2015) Organellar channels and transporters. Cell Calcium 58(1):1–10. https://doi.org/10.1016/j.ceca.2015.02.006
Xu C, Tang X, Shao H et al (2016) Salinity tolerance mechanism of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr Genom 17(3):207–214. https://doi.org/10.2174/1389202917666160202215548
Yang Y, Guo Y (2018) Unraveling salt stress signaling in plants. J Integr Plant Biol 60(9):796–804. https://doi.org/10.1111/jipb.12689
Yang L, Wen KS, Ruan X et al (2018) Response of plant secondary metabolites to environmental factors. Molecules 23(4):E762. https://doi.org/10.3390/molecules23040762
Yang M, Li X, Zhang L et al (2019) Ethnopharmacology, phytochemistry, and pharmacology of the genus Glehnia: a systematic review. Evid Based Complement Alternat Med. https://doi.org/10.1155/2019/1253493
Yang C, Lv XD, Pang LJ et al (2020) Analysis of novel coronavirus pneumonia treatment with Chinese herbal compound. J Hainan Med Univ 26(13):961–966. https://doi.org/10.13210/j.cnki.jhmu.20200515.002
Yang BS, Huang QL, Xie LX et al (2021) [Pueraria lobate advances in molecular pharmacognosy]. Zhongguo Zhong Yao Za Zhi 46(9):2149–2157. https://doi.org/10.19540/j.cnki.cjcmm.20210225.102
Yi G, Lei Z, Zhong-Ji S et al (2010) Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot Stud 51:325–336
Yu X, Zhang W, Zhang Y et al (2019) The roles of methyl jasmonate to stress in plants. Funct Plant Biol 46(3):197–212. https://doi.org/10.1071/FP18106
Yuan H, Ma Q, Li Y et al (2016) The traditional medicine and modern medicine from natural products. Molecules 21(5):559. https://doi.org/10.3390/molecules21050559
Zhang J, Martinoia E, Lee Y (2018) Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol 59(7):1317–1325. https://doi.org/10.1093/pcp/pcy006
Zhao J, Davis LC, Verpoorate R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333. https://doi.org/10.1016/j.biotechadv.2005.01.003
Zhao Z, Li Y, Zhou L et al (2020) Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine 85:153308. https://doi.org/10.1016/j.phymed.2020.153308
Zhou W, Shi M, Deng C et al (2021) The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic Res 8(1):10. https://doi.org/10.1038/s41438-020-00443-5
Zhu M, Geng S, Chakravorty D et al (2020) Metabolomics of red-light-induced stomatal opening in Arabidopsis thaliana: coupling with abscisic acid and jasmonic acid metabolism. Plant J 101(6):1331–1348. https://doi.org/10.1111/tpj.14594
Acknowledgements
I sincerely thank professor Dong chunhai for providing Arabidopsis seeds and the associate professor Ting Gao experimental team for great help to me. Contributor 1 (Hongwei Ren) performed experiments and analyses, (Hongwei Ren and Ting Gao) wrote this paper; Contributor 2 (Yanchong Yu, Yao Xu, Xinfang Zhang, Xuemei Tian and Ting Gao) designed experiments.
Funding
This work was supported by Youth Fund Project of National Natural Science Foundation of China, Study on the Functions and Regulating Mechanism of Alternative Splicing isoforms of R2R3-MYB Transcription Factor related to Flavonoids Synthesis in Scutellaria baicalensis, Grant Numbers: 81903748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could appeared to influence this work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Any ethical rights have been observed in this article, and the scientific contribution of all individual scientific contributions have been fully clarified and agreed upon by all.
Additional information
Communicated by Danny Geelen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, H., Yu, Y., Xu, Y. et al. GlPS1 overexpression accumulates coumarin secondary metabolites in transgenic Arabidopsis. Plant Cell Tiss Organ Cult 152, 539–553 (2023). https://doi.org/10.1007/s11240-022-02427-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02427-w