Abstract
Auxins induce adventitious roots (ARs) in numerous culture-systems, and indole-3-butyric acid (IBA) is frequently the best AR-inducer. Vitamin requirements vary according to species, explant, and culture-conditions. Arabidopsis thaliana thin cell layers (AtTCLs) are uncapable of AR-formation on hormone-free medium containing thiamine and myo-inositol, whereas ARs are induced when IBA (10 μM), with/without kinetin (Kin, 0.1 μM), is added. The research first aim was to determine whether a synergism between IBA and myo-inositol and thiamine was necessary for AR-formation. Results showed that IBA induced AR-formation without myo-inositol and thiamine, but better when both vitamins were also present. Deciphering hormonal action on AR formation under optimal vitamin content would be essential for improving the AR process. Ethylene (ET)/jasmonic acid (JA) signaling cross-talk has been demonstrated as being involved in AR-formation in IBA + Kin-cultured AtTCLs, by using ein3eil1 and coi1-16 mutants. ETHYLENE INSENSITIVE3 (EIN3)/EIN3-LIKE1 (EIL1) are positive regulators of ethylene (ET)-signaling, whereas CORONATINE INSENSITIVE1 (COI1) is involved in JA-signaling. The ETHYLENE INSENSITIVE2 (EIN2) protein activates EIN3/EIL1 in ET-presence. To understand whether EIN2 was also involved, the AR-response of ein2-1 and coi1-16 TCLs was evaluated adding the ET-precursor 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) and/or the JA-donor methyl jasmonate (JAMe, 0.01 μM) to IBA + vitamins-containing medium. AR-formation was enhanced by JAMe, reduced by ACC, but unchanged by JAMe + ACC in the wild type TCLs, whereas remained similarly low in ein2-1 and coi1-16 under all treatments. Collectively, these results demonstrate that the antagonism between JA and ET in AR-formation from AtTCLs involves a cross-talk by EIN2 and COI1.
Key message
The reciprocal regulation of ethylene and jasmonate perception through EIN2 and COI1 cross-talk is involved in root formation and branching in Arabidopsis stem explants, cultured with IBA, myo-inositol and thiamine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant root system is formed by a primary root of embryonic origin, and by lateral roots (LRs) formed post-embryonically by the pericycle of the primary root (Fahn 1990). However, there are several plants with a fibrous root system formed in large part by adventitious roots (ARs), and by LRs developed from the ARs. Various tissues of the aerial organs are able to form ARs (Fahn 1990). In plants with a fibrous root system, AR-formation is essential for survival, contributing to water and inorganic salts uptake from the soil and to anchorage to it. Cereals are examples of plants of economic value with a root system largely formed by ARs. The formation of ARs is also essential for successful micropropagation programs (Ricci et al. 2016, and references therein), and for various biotechnological applications, as, for example, the production of antifungals from ARs-derived extracts (Simonetti et al. 2016).
Indole-3-acetic acid (IAA) is universally known as the main inducer of ARs and LRs in planta and in some in vitro systems, however its natural precursor indole-3-butyric acid (IBA), combined or not with a low level of cytokinin, is frequently a better inducer in the in vitro systems (Fattorini et al. 2017b, and references therein). In Arabidopsis thaliana, a very limited number of ARs is formed in planta without exogenous hormones (Falasca and Altamura 2003). However, Arabidopsis stem thin cell layers (AtTCLs) form a large amount of ARs when cultured in darkness on MS (Murashige and Skoog 1962) salt medium supplemented with 10 μM IBA and 0.1 μM kinetin (Kin) (Falasca et al. 2004). It has been recently shown that in AtTCLs IBA affects AR-formation by its conversion into IAA (Fattorini et al. 2017b). However, other hormones may be also important in the control of the AR-response, e.g., jasmonates (Fattorini et al. 2018). In addition, other components of the culture medium, such as specific vitamins, might be necessary to optimize the process.
In tissue culture, requirements for vitamin concentration/type change according to the species and type of explant and culture (Abrahamian and Kantharajah 2011). On the basis of the research of Falasca et al. (2004), AtTCLs are usually cultured on MS medium in the presence of myo-inositol and thiamine, at the same concentrations used for tobacco AR-forming TCLs (Tran Thanh Van et al. 1974). However, investigations on the effects of IBA alone or in combination with myo-inositol, on in vitro AR-formation of cherry rootstocks, grown on MS medium, showed that myo-inositol suppressed the positive effects of IBA on root length (Sarropoulou et al. 2013), suggesting a negative interaction with the hormone. By contrast, a positive interaction was shown by the hypocotyl cuttings of Phaseolus aureus, which, pre-treated with IBA, developed ARs in response to myo-inositol (Jarvis and Booth 1981).
Plant cells are able to metabolize myo-inositol, absorbed from the culture medium, by different pathways leading to phytate production, conjugation with phytohormones, oxidation to glucuronate, and lipids formation, as described by Biffen and Hanke (1990, and references therein). The same authors reported that soybean callus cells, although grow and divide more rapidly in the presence of exogenous myo-inositol, still grow and divide in its absence, suggesting that this vitamin might be excluded from the medium (Biffen and Hanke 1990). Possible interactions between myo-inositol and cytokinin have been described. In particular, an additive action of applied myo-inositol and Kin has been suggested following the promotion of Kin translocation to the roots of freshly rooted peach cuttings (Gur et al. 1987).
Thiamine is another vitamin with an important role in rooting. In fact, AR-formation in Taxus brevifolia cuttings was doubled after treatment with thiamine (Chee 1995). Synergistic interactions between thiamine and auxins have been reported for different plant rooting systems, as Jatropha curcas and Tectona grandis cuttings (Dhillon et al. 2011, and references therein), and GF677 (peach x almond hybrid) shoot microcuttings. In the latter, significant rooting increments were detected at different IBA levels, but always when thiamine was present even at low levels (Sepahvand et al. 2012). Thiamine may also interact positively with cytokinins. In fact, a Kin low level in the medium sustains the growth of tobacco callus cultures only if thiamine is added (Digby and Skoog 1966). Moreover, a positive interaction between thiamine and myo-inositol has been observed on in vitro root development, i.e. from Matteuccia struthiopteris shoot explants cultured on MS/2 medium containing both vitamins (Dikeman and Cumming 1985).
Synergistic and antagonistic interactions between jasmonate (JA) and ethylene (ET) occur in many morphogenic events (Fattorini et al. 2018, and references therein). In AtTCLs cultured with IBA and Kin, JA, applied as the JA-donor methyl jasmonate (i.e., the jasmonic acid methyl ester, JAMe) at 0.01 μM concentration, enhances AR-formation, whereas ET, applied as its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) at 0.1 μM, reduces it. Moreover, AR production does not change under the combined presence of ACC and JAMe in comparison with their absence, suggesting a compensatory effect by ET and JA (Fattorini et al. 2018). The JASMONATE ZIM-DOMAIN (JAZ) proteins are the target of CORONATINE INSENSITIVE1 (COI1) protein, and COI1–JAZ is a co-receptor of jasmonoyl-isoleucine (JA-Ile, the active form of JA). Mutations affecting the F-box motiv of COI1 compromise the formation of the JA-Ile receptor complex as occurs in coronatine insensitive1-1 (coi1-1) and impairs male fertility in Arabidopsis (Xie et al. 1998). coi1-16 does not respond to JA similarly to coi1-1, however, the male sterility of coi1-16 can be overcome with an adequate temperature regulation during plant growth and it can be maintained as a homozygous line (Ellis and Turner 2002). For this reason coi1-16 is often preferred to coi1-1 in studies on jasmonate perception (Adams and Turner 2010; Fattorini et al. 2018). coi1-16 mutant is JA/JAMe-insensitive (Ellis and Turner 2002), but IBA + Kin-cultured TCLs excised from this mutant are also insensitive to ACC (Fattorini et al. 2018). The transcription factors (TFs) ETHYLENE INSENSITIVE 3 (EIN3) and its closest homolog EIN3-LIKE 1 (EIL1), known to be positively involved in ET-signaling, physically interact with JAZ1, JAZ3 and JAZ9 repressors of JA-signaling, resulting in the suppression of their activity (Zhu et al. 2011). However, in the presence of JA-Ile, JAZs are degraded, and, consequently, EIN3/EIL1 become free and require ET for their stabilization (Wasternack and Hause 2013). In accordance, ein3eil1 mutant is insensitive to both JA and ET during AR-formation from IBA + Kin-cultured AtTCLs, suggesting that EIN3/EIL1 may be a critical link between JA and ET in AR-formation from this type of explants (Fattorini et al. 2018). However, these results do not exclude that other factors upstream to EIN3/EIL1 may be involved. ETHYLENE INSENSITIVE 2 (EIN2) may be a possible candidate. EIN2, an integral membrane protein of the endoplasmic reticulum, participates in ET signalling by acting downstream of the ET-receptor family, but upstream of the EIN3/EIL TF family.
In the absence of ET, EIN2 activity is repressed by phosphorylation at its C-terminus, mediated by the ET receptor-interacting CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1) protein (Ju et al. 2012). In ET presence, an EIN2 C-terminal fragment, after dephosphorylation, is cleaved and translocated into the nucleus, where it transduces signals to EIN3 and EIL1 (Qiao et al. 2012). It is known that JAMe treatments affect the cleavage and nuclear translocation of EIN2-C-terminal end (Zhang et al. 2016a). The latter is sufficient to constitutively activate ET-responses and restores responsiveness to JA in seedlings of numerous Arabidopsis ein2 mutants (Alonso et al. 1999). EIN2 is also directly involved in the regulation of histone acetylation in ET-responses, interacting with the histone-binding protein EIN2 NUCLEAR ASSOCIATED PROTEIN 1 (ENAP1), and promoting more EIN3 binding to the common targets with ENAP1, so accelerating the transcriptional regulation of ET-responsive genes (Zhang et al. 2016b, 2017). Even if petunia transgenic plants with reduced EIN2 levels show an AR-response reduced compared to the wild type (Shibuya et al. 2004), the function of EIN2 in AR-formation remains to date widely unknown.
EIN2 is encoded by a single gene copy in Arabidopsis and numerous proofs of its importance in plant growth have been obtained by using ET-completely insensitive ein2 mutants (Alonso et al. 1999). In particular, ein2-1 has been well characterized and the mutation compromises completely the EIN2 function, causing the lacking of a large portion of the intra-cellular domain containing the Nuclear Localization Signals (Alonso et al. 1999; Li et al. 2015), from which an EIN2 C-terminal fragment is cleaved in the WT and translocated into the nucleus (Qiao et al. 2012) to allow the ET signal transduction. It has been found that the inhibitory effects of cytokinin on root elongation in Arabidopsis seedlings are partially blocked in the ein2-1 mutant. Moreover, the cytokinin-resistant mutant ckr1 is ET-resistant and allelic to ein2 (Cary et al. 1995). However, the ein2 mutants, even leading to cytokinin-insensitive cell elongation and root growth, do not interfere with the cytokinin effect on the root meristem (Růžička et al. 2009), increasing the difficulty to exactly define the relationship between cytokinin and ET by using these mutants.
Arabidopsis seedlings exhibit a very limited AR-formation when cultured on MS medium with thiamine and myo-inositol but without hormones (Della Rovere et al. 2013), and AtTCLs produce no AR under the same culture condition (Fattorini et al. 2017b). However, AR-formation occurs not only with IBA plus Kin, but also with IBA alone both in planta and in TCLs (Veloccia et al. 2016; Fattorini et al. 2017b), whereas Kin exhibits an inhibitory role when applied alone (Della Rovere et al. 2013; Fattorini et al. 2017b). Up to now the AR-promoting effect of JA has been demonstrated only in the IBA + Kin condition, not only in Arabidopsis, but also in tobacco TCLs, and always in the presence of thiamine and myo-inositol in the medium (Fattorini et al. 2009, 2018).
Following these premises, the first aim of the research was to determine whether a synergistic role of both vitamins with IBA (with/without Kin) was necessary for inducing AR-formation in AtTCLs. Results showed that IBA was able to induce the AR-process and to support root elongation and AR-branching in the absence of thiamine, myo-inositol and Kin in the medium. However, the two vitamins were needed for optimizing the IBA-promoting action on AR-formation and branching.
Based on these results, and on the possible negative effect of Kin on the AR process, the second aim was to prove that EIN2 is the molecular link in the mutual antagonism of JA and ET perception in AR-formation of AtTCLs induced by IBA alone in the presence of the two vitamins. To reach the aim, the AR-response of TCLs from ein2-1 and coi1-16 mutants was compared with that of the corresponding wild types (WTs) under treatments with IBA (10 μM) and thiamine (0.1 μM) and myo-inositol (0.55 mM), with/without the addition of either ACC (0.1 μM) or JAMe (0.01 μM) or both the compounds.
The results showed an antagonism between ET and JA in the IBA-caused AR-formation. EIN2 was the functional link in the perception of the two hormones, acting through a cross-talk with COI1.
Materials and methods
Plant material and growth conditions
Seeds of Arabidopsis thaliana (L.) Heynh Col-0 and Col-gl1 ecotypes, and of their mutants ein2-1 (Guzmán and Ecker 1990) [provided by Joseph R. Ecker (Salk Institute for Biological Studies, La Jolla, USA)], and coi1-16 (Ellis and Turner 2002) [provided by John G. Turner (University of East Anglia, UK)] were used for the experiments. In ein2-1 the mutation affects the gene At5G03280 (encoding a membrane protein involved in ethylene signal transduction), while in coi1-16 the mutation affects the gene AT2G39940 (encoding a F-box protein involved in jasmonate reception). Both mutants were obtained by EMS mutagenesis and show complete (ein2-1), or almost complete (coi1-16), loss of sensitivity to either ET (Alonso et al. 1999) or JA (Ellis and Turner 2002; Devoto et al. 2005). The seeds were stratified for 3 days at 4 °C under continuous darkness and sown on a commercial soil. Plant growth took place in a growth chamber, under long days photoperiod, at 22 ± 2 °C, 70% humidity and white light (22 W m−2 intensity). Plants were used for the excision of the TCL explants about 40 days after seed germination.
Thin cell layer culture
Superficial TCLs (about 0.5 × 8 mm) composed by epidermis, three layers of cortical parenchyma, endodermis and 1–2 layers of fibers (Della Rovere et al. 2013), were excised aseptically from stem internodes and placed, epidermal side up, in Phytatray II sterile vessels (Sigma-Aldrich, Milan, Italy) (10 TCLs per vessel), each containing 70 ml of agarized medium. In order to study morphogenesis and root productivity in Col-0 TCLs, a full-strength MS (Murashige and Skoog 1962) medium (Duchefa, Haarlem, The Netherlands) was firstly prepared, containing 10 µM IBA (Duchefa, Haarlem, The Netherlands) and 1% (w/V) sucrose (Sigma-Aldrich, Milan, Italy), supplemented or not with 0.1 µM Kin (Sigma-Aldrich, Milan, Italy) and/or 0.55 mM myo-inositol (Fluka, Buchs, Switzerland) plus 0.1 µM thiamine-HCl (Sigma-Aldrich, Milan, Italy) (the last two compounds subsequently referred to as “vitamins”). The medium was sterilized by autoclaving at 120 °C for 20 min. Before autoclaving, pH was adjusted to 5.7 with 1 M NaOH, and 0.8% agar (w/V) (Sigma-Aldrich, Milan, Italy) was added. In each replicate, the explants were cultured under continuous darkness, at 22 ± 2 °C, for 15 days for evaluating AR productivity, and other 7 days for evaluating the presence of LRs on the ARs.
For ein2-1 and coi1-16 (in addition to their WTs) TCL culture, a medium without Kin but containing all the other compounds described above, including the two vitamins, was prepared and supplemented with 0.1 µM ACC (Sigma-Aldrich, Milan, Italy) and/or 0.01 µM JAMe (Duchefa, Haarlem, The Netherlands). Stock solutions at concentration 1 mM were prepared by dissolving ACC in bidistilled water and JAMe in ethanol (≥ 99.8%, Sigma Aldrich, Milan Italy). The same volumes of water or ethanol used to reach the final ACC/JAMe concentrations in the media were also added to the control medium (containing IBA, sucrose, and vitamins, at the concentrations indicated above) in order to verify the absence of effects of the solvent on TCL response. ACC and JAMe were added after autoclaving, by filtering (with a 0.22 µm pore filter) stock-solutions, as described in Fattorini et al. (2018). In each replicate, one hundred TCLs per genotype and treatment were cultured in vitro, under continuous darkness, at 22 ± 2 °C, for 15 days.
Root scoring
At the end of the culture, 60 TCLs per genotype and treatment were randomly chosen and examined under a LEICA MZ8 stereomicroscope. The images were acquired in digital form using a ZEISS AxioCam camera, applied to the stereomicroscope, and AxioVision Release 4.7.2 software. Different stages of morphogenesis were detected and quantified as percentage of initial-stage TCLs, swollen TCLs, TCLs with callus only, and TCLs with callus and ARs. AR-productivity was expressed as mean number (± SE) of ARs per rooting explant. The mean percentage of LR-forming ARs was also determined.
Histological analysis
Five Col-0 TCLs per replicate, cultured for 15 days with 10 µM IBA or 10 µM IBA + 0.1 µM ACC, both in the presence of vitamins, were dehydrated and embedded in Technovit 7100 resin (Heraeus Kulzer, Hanau, Germany). The embedded samples were longitudinally sectioned, coloured, observed under light microscopy and photographed as indicated in Fattorini et al. (2018).
Statistical analysis
All the data were expressed by mean values (± SE). One-way or two-way analysis of variance (ANOVA, p < 0.05) was used to compare the effects of different treatments, or genotypes and treatments, respectively. If ANOVA showed significant effects, Tukey’s post-test was applied (GraphPad Prism 6.0). Three replicates of each experiment were performed during two following years and very similar results were obtained.
Results
Exogenous IBA induces AR-formation in the absence of myo-inositol and thiamine, but the presence of the two vitamins with IBA highly enhances AR-response
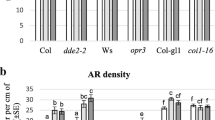
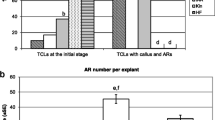
The comparison between TCLs of Col-0 genotype cultured for 15 days on MS medium including the two vitamins myo-inositol and thiamine, either in the presence of IBA alone or IBA and Kin, showed that there was no significant difference caused by the presence of Kin for each type of the morphogenic responses obtained. In fact, the percentage of explants alive but with no growth (initial-stage explants) was similar in the two treatments, and in both cases no swollen explant (that is poorly proliferated explant) was observed (Fig. 1). The percentage of explants with ARs was also similar (Fig. 1), but the AR production on such explants differed, the same as the development stage reached by the ARs (Figs. 2, 3). In fact, the IBA-alone cultured TCLs produced almost twice as much ARs per explant than the IBA + Kin TCLs (p < 0.01; Fig. 3), and ARs were more elongated under IBA than under IBA + Kin (Fig. 2a, b in comparison). Under the latter treatment the most frequent developmental stage shown by the ARs was the primordium one (Fig. 2b inset). Moreover, the IBA + Kin explants were more callused than IBA alone explants (Fig. 2b, a in comparison). When IBA was applied in the absence of both Kin and the two vitamins, a strong increase in the number of explants remaining at the initial stage occurred (Figs. 1, 2c inset), and swollen explants appeared (Fig. 1). However, even if significantly (p < 0.0001) reduced in comparison with the treatments with vitamins, about one quarter of the explants showed ARs (Figs. 1, 2c). Very interestingly, the mean AR number was as high as under IBA + vitamins treatment (Fig. 3), and ARs showed a similar elongation (Fig. 2a, c, in comparison).
Morphogenic responses of Col-0 TCLs. Mean percentage (± SE) of Col-0 TCLs at different stages of morphogenesis after 15 days of culture, under darkness, on MS medium containing IBA (10 µM), vitamins (0.1 µM thiamine and 0.55 mM myo-inositol), in the presence of Kin (0.1 µM) (IBA + Kin + vit) or without Kin (IBA + vit), or IBA alone without vitamins (IBA). Statistical comparisons within the same stage of morphogenesis. Different letters indicate significantly different values (p < 0.0001). n = 3 replicates (60 TCLs per treatment)
Rooting from Col-0 TCLs after 15 (a–c) or 22 days (d) of culture under darkness and with different IBA-treatments. a–d Images under the stereomicroscope. a A TCL, cultured in the presence of IBA and vitamins (thiamine and myo-inositol), which shows mature ARs and a low amount of callus. b A TCL, cultured with IBA, Kin and vitamins, which shows ARs at primordium stage and a conspicuous amount of callus, as also magnified in the inset. c Adventitious rooting from an explant cultured with IBA but in the absence of Kin and vitamins. The inset shows an explant that did not proliferate and remained at the initial-stage. d A TCL with elongated ARs after further 7 days of culture on a medium containing IBA and vitamins. The presence of LRs, at primordium stage, on ARs is highlighted by arrows in the inset. Bars: 1 mm (a–c, c inset, d), 500 μm (b inset), 200 μm (d inset)
AR formation in Col-0 TCLs. Mean number (± SE) of ARs per explant after 15 days of culture, under darkness, on MS medium containing IBA (10 µM), thiamine (0.1 µM) and myo-inositol (0.55 mM), in the presence of Kin (0.1 µM) (IBA + Kin + vit) or without Kin (IBA + vit), or IBA alone without vitamins (IBA). Different letters indicate significant differences between treatments at least at p < 0.05 level. Further statistical details are described in the text. N = 60
To further investigate the effects of treatments on AR development, the capacity of the ARs to produce LRs was also investigated under the different IBA conditions. To this aim, the culture period was prolonged up to day 22. Results showed that the two treatments with IBA without Kin showed significantly (p < 0.0001 and p < 0.01 for IBA with or without vitamins, respectively) higher percentages of ARs with LRs in comparison with the IBA + Kin + vitamins treatment (Fig. 4). However, the presence of the two vitamins together with IBA caused an about three-fold increase in LR production (Fig. 4), showing that it was the best condition for optimal AR-formation and branching (Fig. 2d and inset). Based on these results, the subsequent experiments were performed in the presence of IBA and the two vitamins in the medium, but without Kin.
LR production from ARs in Col-0 TCLs. Mean percentage (± SE) of ARs with LRs in TCLs after 22 days of culture, under darkness on MS medium containing IBA (10 µM), thiamine (0.1 µM) and myo-inositol (0.55 mM), with Kin (0.1 µM) (IBA + Kin + vit) or without Kin (IBA + vit), or IBA alone without vitamins (IBA). Different letters indicate significant differences between treatments at least at p < 0.05 level. Further statistical details are described in the text. n = 3 replicates (200 ARs per treatment)
ET reduces AR-formation, with ET signaling by EIN2, and JA signaling by COI1, involved
After 15 days of culture, TCLs from the two WT genotypes Col-0 and Col-gl1 showed a significant (p < 0.001 and p < 0.05, respectively) reduction in AR formation when the ET precursor ACC was applied together with IBA (Fig. 5a). The histological analysis showed conspicuous xylary cell differentiation in WT explants treated with IBA + ACC in comparison with the explants treated with IBA alone (Fig. 5b, c). AR production under IBA alone was significantly lower in ein2-1 and coi1-16 TCLs (p < 0.001 and p < 0.05, respectively) in comparison with the corresponding WT (Fig. 5a), suggesting a reduction in IBA perception in both mutants. The AR-response was not significantly changed by the addition of ACC in the ein2-1 TCLs (Fig. 5a), confirming the ET insensitivity of this mutant (Alonso et al. 1999). Interestingly also coi1-16 TCLs did not show a significantly different response with and without ACC, suggesting ET insensitivity also in this JA-perception mutant (Fig. 5a).
AR formation and xylogenesis in TCLs under IBA + ACC treatment. a Mean number (± SE) of ARs per explant in TCLs of the mutant lines ein2-1 and coi1-16, and the corresponding WTs Col-0 and Col-gl1, after 15 days of culture under darkness on MS medium containing IBA (10 µM), thiamine (0.1 µM) and myo-inositol (0.55 mM), with or without 0.1 µM ACC. Different letters indicate significant differences between treatments within the same genotype, or between each mutant and its WT within the same treatment, at least at p < 0.05 level. Further statistical details are described in the text. N = 60. b Detail of a histological section of a Col-0 TCL cultured with IBA, showing AR formation in the explant. c Histological image of a Col-0 TCL cultured with IBA + ACC showing a conspicuous differentiation of isolated or grouped xylary cells (arrows). b, c Toluidine blue staining. Bars = 10 µm (b), 30 µm (c)
JA enhances AR-formation, with the JA signaling by COI1 and the ET signaling by EIN2 involved
In both the WT genotypes, the mean percentage of explants producing ARs in the presence of JAMe, together with IBA for 15 days of culture, was higher [88.9 (± 1.5) for Col-0 and 87.2 (± 1.5) for Col-gl1 TCLs] than in JAMe absence [84.4 (± 2) for Col-0 and 83.3(± 2) for Col-gl1 TCLs], but the increase was not statistically significant. However, the application of JAMe resulted into a significant (p < 0.05) increase in AR mean number in comparison with IBA alone (Fig. 6). The AR production of ein2-1 and coi1-16 TCLs under IBA + JAMe treatment was not significantly different in comparison with jasmonate absence (Fig. 6), suggesting JA insensitivity in both mutants. Moreover, the AR response in TCLs of both mutants, under IBA alone, was significantly lower (p < 0.05) in comparison with the corresponding WT (Fig. 6), confirming the reduction in IBA perception in both mutants observed in Fig. 5a.
AR formation in ein2-1 and coi1-16 TCLs treated with JAMe. Mean number (± SE) of ARs per explant in TCLs of the mutant lines ein2-1 and coi1-16, and the corresponding WTs Col-0 and Col-gl1, after 15 days of culture under darkness on MS medium containing IBA (10 µM), thiamine (0.1 µM) and myo-inositol (0.55 mM), with or without 0.01 µM JAMe. Different letters indicate significant differences between treatments within the same genotype, or between each mutant and its WT within the same treatment, at least at p < 0.05 level. Further statistical details are described in the text. N = 60
ET and JA are antagonist in AR-formation from TCLs, with the perception by EIN2 and by COI1 involved
The combined application of ACC and JAMe did not change significantly the AR response in comparison with the IBA alone treatment (ACC 0 µM + JAMe 0 µM) in TCLs from both WT genotypes (Fig. 7). The AR-response of ein2-1 and coi1-16 TCLs was significantly lower with respect to their WTs, not only in the presence of IBA without ACC and JAMe (p < 0.05) but also under the combined treatment ACC + JAMe (p < 0.01 and p < 0.05 for ein2-1 and coi1-16, respectively). Moreover, in the explants of these mutants AR formation remained unaffected by ACC + JAMe treatment with respect to the absence of both compounds (Fig. 7).
AR formation in ein2-1 and coi1-16 TCLs treated with ACC and JAMe. Mean number (± SE) of ARs per explant in TCLs of the mutant lines ein2-1 and coi1-16, and the corresponding WTs Col-0 and Col-gl1, after 15 days of culture, under darkness, on MS medium containing IBA (10 µM), thiamine (0.1 µM) and myo-inositol (0.55 mM), with or without 0.1 µM ACC plus 0.01 µM JAMe. Different letters indicate significant differences between treatments within the same genotype, or between each mutant and its WT within the same treatment, at least at p < 0.05 level. Further statistical details are described in the text. N = 60
Collectively, the results showed that the exogenous application of the two compounds in combination did not induce any significant change in AR-formation, showing a balance between their antagonist effects. Results also confirmed the insensitivity of the mutants to both ET and JA.
Discussion
Results showed that IBA alone was able to induce the AR-process in AtTCLs in the absence of any support by thiamine and myo-inositol in the medium. However, the two vitamins were needed for optimizing the IBA-promoting action on AR-formation and branching. For this reason, a medium containing IBA as the only exogenously applied auxin, and the two vitamins, was chosen for the further experiments. The IBA-induced AR-response in TCLs from ein2-1 and coi1-16 mutants, evaluated in the presence of the ET precursor ACC or the JA-releasing compound JAMe, or of both compounds, showed an antagonism between ET and JA in AR-formation, with EIN2 acting as a functional link, through a cross-talk with COI1, in the perception of the two hormones.
Myo-inositol and thiamine cooperate with exogenous IBA in enhancing AR elongation and branching, whereas exogenous Kin counteracts these events
In the pioneer paper of Tran Thanh Van et al. (1974), the importance of a combination of exogenous IBA and Kin, at the same concentrations here used, was considered the optimal exogenous hormonal input for AR-formation in dark-grown tobacco TCLs. The same concentrations of the two hormones were successfully used in combination also in Arabidopsis TCLs (Falasca et al. 2004). However, histological analyses in the hypocotyls of dark-grown Arabidopsis seedlings showed that exogenous Kin counteracted auxin action on AR-formation in planta, affecting both auxin transport and auxin maximum definition in the AR-apex (Della Rovere et al. 2013). Based on these results, in following experiments IBA was applied alone to intact seedlings, but also to TCLs of Arabidopsis, and AR-formation was successfully obtained in both cases (Veloccia et al. 2016; Fattorini et al. 2017b). IBA alone favoured AR formation in Arabidopsis TCLs in comparison with IBA + Kin, whereas, in the presence of Kin alone, any morphogenic/organogenic response was obtained, i.e. the explants remained at the initial stage (Fattorini et al. 2017b). When added with IBA, Kin favours cell division per se, leading to an increased callus formation and limiting the AR induction due to IBA (Fattorini et al. 2017b; Fig. 2b). The present research confirms the negative impact of Kin on AR production in AtTCLs (Fig. 3) and that exogenous IBA is able per se to induce AR formation. In fact, even if the percentage of AR-forming explants was similar with/without Kin (Fig. 1), the mean number of ARs per explant was higher with IBA alone than with IBA plus Kin (Fig. 3). Moreover, the role of myo-inositol and thiamine was of interest. It is known that AtTCLs are unable to grow and produce ARs on a medium containing these vitamins, but without hormones (Fattorini et al. 2017b). However, here it was shown that these vitamins have a positive role on AR-formation when combined with IBA. In fact, in the absence of the two vitamins, exogenous IBA induced less ARs, and mainly, less LRs from the ARs (i.e., AR-branching) (Fig. 4). The induction of AR-branching is interesting, because, independently of the species, a successful micropropagation depends not only by AR-induction, but also by the AR capability to form LRs, because both are necessary for the building up of an efficient root system in in vitro culture.
As for LR-formation from the primary root (Fahn 1990), successful AR-branching depends on the activity of the pericycle, which, in turn, needs to be differentiated. The differentiation of the AR primary vascular structure, including the pericycle, occurs after the elongation of the AR primordium (Della Rovere et al. 2015). Based on our results, it is possible that IBA positively interacts with the two vitamins promoting AR-elongation as the necessary premise for AR-branching. In accordance, a positive thiamine and IBA interaction in favouring AR-elongation has been demonstrated in the micropropagation of GF677 (peach × almond hybrid) (Sepahvand et al. 2012). Moreover, AR-elongation was also favoured by specific combinations of IBA and myo-inositol in cherry rootstocks CAB-6P and Gisela 6 (Sarropoulou et al. 2013).
The present results show that Kin might counteract the cooperative action of IBA and vitamins. In fact, the ARs remain at primordium stage under IBA + Kin + vitamins treatment, with elongation sporadic, and AR-branching rare (Figs. 2, 4). In accordance, Kin is widely known to inhibit root elongation in Arabidopsis (Cary et al. 1995). Therefore, in order to optimize the conditions for investigating the effects of ET and JA on AR-formation, all subsequent experiments were carried out in the presence of IBA and the two vitamins, and in the absence of Kin.
The negative role of ET on AR-formation in TCLs requires EIN2 action
We observed that the addition of the ET-precursor ACC to the medium with IBA resulted into a significant reduction of mean AR-number in the two WT genotypes (Fig. 5a), in accordance with our recent data obtained with IBA + Kin treatments in the same culture system (Fattorini et al. 2018). Alltogether, present and past data support that Kin has not a key role in the ET-caused reduction in AR response, even if a possible interaction of Kin with ET pathway may not be excluded.
AtTCLs are capable of xylogenesis instead of AR-formation under the same IBA + Kin input (Falasca et al. 2004; Fattorini et al. 2018). The same as AR-formation, xylogenesis is an auxin-induced program, and may be a concomitant/alternative program to AR-formation (Ricci et al. 2016, and references therein). In Arabidopsis, the competitive realization of xylogenesis is known to reduce AR-formation in seedlings and TCLs (Fattorini et al. 2017a, 2018). In WT TCLs treated with IBA + ACC a conspicuous production of xylary cells was observed (Fig. 5c). Thus, in accordance with our histological analyses in IBA + Kin + ACC-cultured TCLs (Fattorini et al. 2018), xylogenesis may be responsible for the observed reduction in AR-formation in the IBA + ACC-treated explants (Fig. 5).
It has been shown that ein2-1 mutant is hyposensitive to IBA (Zolman et al. 2000). Moreover, petunia transgenic plants with low levels of the homolog of Arabidopsis EIN2 show reduced IBA-induced AR-formation (Shibuya et al. 2004). In accordance, data of Fig. 5 show that the loss of ET sensitivity of this mutant (Alonso et al. 1999) causes also resistance to auxin in AtTCLs, with this resulting into a reduced, but similar, AR-response in the presence of IBA ± ACC. Taken together, the response of the ein2-1 TCLs supports the existence of an antagonistic interaction between IBA and ET in AR-formation from this culture system.
EIN2 operates downstream of the ET-receptor family, but upstream of the EIN3/EIL TF family. By the use of the ein3eil1 AtTCLs, it has been recently shown that ET action involves the activity of the EIN3/EIL1 network in the IBA + Kin-induced AR-formation (Fattorini et al. 2018). The same network is positively involved in the IBA-induced xylogenesis in planta (Fattorini et al. 2017a). Moreover, EIN3/EIL1 protein levels are known to be very low in ein2 (Zhu 2014). Because the absence of EIN2 function causes very low levels in the downstream compounds of ET signaling, present data indirectly confirm the essential role of the EIN3/EIL1 network in the AR-process, and also show for the first time a role for the upstream protein EIN2. The importance of the increases in histone acetylation has been demonstrated in the regulatory mechanism leading to LR-formation (Wakeel et al. 2018), but not yet for AR-formation. EIN2 appears to be required for ET-induced elevation of histone acetylation (Zhang et al. 2017) and its role might be important in the epigenetic changes necessary also for AR-formation, even if this aspect requires further investigation.
JA-ET antagonistic interaction in AR-formation requires signaling cross-talk by EIN2 and COI1
JA and ET are known to mutually antagonize some of their common functions. For example, ET promotes apical hook formation in dark-grown seedlings, whereas JA greatly reduces it even in the presence of ET (Zhu 2014, and references therein). Our results show that JAMe, applied at 0.01 μM, enhances AR-formation in IBA-treated WT AtTCLs (Fig. 6), increasing the number of AR formation sites in the rooting explants without affecting significantly the number of explants induced to produce ARs, which depends by IBA. The ethylene precursor ACC, applied at 0.1 μM, reduces AR production (Fig. 5a), and the JAMe + ACC treatment does not change the response in comparison with IBA alone (Fig. 7). In accordance, the same concentration of exogenous JAMe enhanced AR-formation in the IBA + Kin-treated AtTCLs, and the addition of ACC with JAMe nullified this enhancement (Fattorini et al. 2018). Taken together, present and past results indicate that both the positive effect of JAMe and the negative effect of ACC on AR-formation in TCLs are independent of the presence of Kin in the culture medium.
MYC2 is a primary TF in JA signaling, and JA activates it by a de-repression mechanism. In the plant cells, in the absence of JA of endogenous origin and/or derived from the demethylation of applied JAMe (Fattorini et al. 2009), MYC2 is repressed by interacting with a family of JAZ proteins. JA-Ile, the bioactive JA form, promotes the interaction between JAZs and the F-box protein COI1, which causes the proteasomal JAZ degradation to nullify their repression of MYC2 (Zhu 2014, and references therein). Thus, JAZ-COI1 acts as a JA co-receptor (Katsir et al. 2008; Yan et al. 2009; Sheard et al. 2010). In coi1-16 AtTCLs, the JA reception mechanism is compromised, thus JA is not perceived (Fattorini et al. 2018). In accordance, there is no JA-promotion of the IBA-induced AR-formation (Fig. 6), confirming the importance of the positive interaction between IBA and JA in the control of the process. In addition, present and past results together show a reduction in AR-response by coi1-16 TCLs specifically related to an IBA hyposensitivity, because both with IBA alone (Figs. 5, 6, 7), and with IBA + Kin (Fattorini et al. 2018), the AR-response remains reduced in comparison with the WT. The low and unchanged AR-response of ein2-1 AtTCLs, under either ACC or JAMe (Figs. 5, 6), together with the compensation between the promoting AR-action by JA and the reducing action by ET, observed in the combined treatment in the WT TCLs (Fig. 7), suggests that EIN2 is the link between the action of the two hormones. In accordance, JAMe treatments are known to affect the cleavage and nuclear translocation of EIN2-C-terminal end, event sufficient to activate ET-responses (Zhang et al. 2016a).
It has been shown that JA-activated MYC2 TF interacts negatively with EIN3/EIL1 TFs, which function downstream to EIN2, repressing their DNA binding ability and, consequently, reducing the expression of their target genes in many morphogenic processes (Song et al. 2014; Zhu 2014). In JA presence, it is widely known that COI1 activity leads to MYC2 de-repression, but how COI1 or MYC2 may interact with EIN2 is presently unknown. Future research should focus on identifying the protein domains essential for this interaction and on clarifying how the reciprocal repression occurs. This would help advance the understanding in the reciprocal regulation of JA and ET perception in AR-formation which would be useful for successful micropropagation programs and biotechnological applications.
References
Abrahamian P, Kantharajah A (2011) Effect of vitamins on in vitro organogenesis of plant. Am J Plant Sci 2:669–674. https://doi.org/10.4236/ajps.2011.25080
Adams E, Turner J (2010) COI1, a jasmonate receptor, is involved in ethylene-induced inhibition of Arabidopsis root growth in the light. J Exp Bot 61:4373–4386. https://doi.org/10.1093/jxb/erq240
Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284:2148–2152. https://doi.org/10.1126/science.284.5423.2148
Biffen M, Hanke DE (1990) Reduction in the level of intracellular myo-inositol in cultured soybean (Glycine max) cells inhibits cell division. Biochem J 265:809–814
Cary AJ, Liu W, Howell SH (1995) Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol 107:1075–1082
Chee PP (1995) Stimulation of adventitious rooting of Taxus species by thiamine. Plant Cell Rep 14:753–757. https://doi.org/10.1007/BF00232916
Della Rovere F, Fattorini L, D’Angeli S, Veloccia A, Falasca G, Altamura MM (2013) Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann Bot 112:1395–1407. https://doi.org/10.1093/aob/mct215
Della Rovere F, Fattorini L, D’Angeli S, Veloccia A, Del Duca S, Cai G, Falasca G, Altamura MM (2015) Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers. Ann Bot 115:617–628. https://doi.org/10.1093/aob/mcu258
Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T, Turner JC (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58:497–513. https://doi.org/10.1007/s11103-005-7306-5
Dhillon RS, Hooda MS, Pundeer JS, Ahlawat KS, Chopra I (2011) Effects of auxins and thiamine on the efficacy of techniques of clonal propagation in Jatropha curcas L. Biomass Bioenergy 35:1502–1510. https://doi.org/10.1016/j.biombioe.2010.12.017
Digby J, Skoog F (1966) Cytokinin activation of thiamine biosynthesis in tobacco callus cultures. Plant Physiol 41:647–652. https://doi.org/10.1104/pp.41.4.647
Dikeman BW, Cumming BG (1985) In vitro propagation of the ostrich fern (Matteuccia struthiopteris) Can. J Plant Sci 65:1025–1032
Ellis C, Turner JG (2002) A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta 215:549–556. https://doi.org/10.1007/s00425-002-0787-4
Fahn A (1990) Plant anatomy. Pergamon Press, Oxford
Falasca G, Altamura MM (2003) Histological analysis of adventitious rooting in Arabidopsis thaliana (L.) Heynh seedlings. Plant Biosyst 137:265–274. https://doi.org/10.1080/11263500312331351511
Falasca G, Zaghi D, Possenti M, Altamura MM (2004) Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep 23:17–25. https://doi.org/10.1007/s00299-004-0801-3
Fattorini L, Falasca G, Kevers C, Mainero Rocca L, Zadra C, Altamura MM (2009) Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 231:155–168. https://doi.org/10.1007/s00425-009-1035-y
Fattorini L, Della Rovere F, Andreini E, Ronzan M, Falasca G, Altamura MM (2017a) Indole-3-butyric acid induces ectopic formation of metaxylem in the hypocotyl of Arabidopsis thaliana without conversion into indole-3-acetic acid and with a positive interaction with ethylene. Int J Mol Sci 18:2474. https://doi.org/10.3390/ijms18112474
Fattorini L, Veloccia A, Della Rovere F, D’Angeli S, Falasca G, Altamura MM (2017b) Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol 17:121. https://doi.org/10.1186/s12870-017-1071-x
Fattorini L, Hause B, Gutierrez L, Veloccia A, Della Rovere F, Piacentini D, Falasca G, Altamura MM (2018) Jasmonate promotes auxin-induced adventitious rooting in dark-grown Arabidopsis thaliana seedlings and stem thin cell layers by a cross-talk with ethylene signalling and a modulation of xylogenesis. BMC Plant Biol 18:182. https://doi.org/10.1186/s12870-018-1392-4
Gur A, Altman A, Stern R, Sigler T, Wolowitz B (1987) Interactions between myo-inositol and cytokinins: their basipetal transport and effect on peach roots. Physiol Plant 69:633–638. https://doi.org/10.1111/j.1399-3054.1987.tb01977.x
Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2:513–523. https://doi.org/10.1105/tpc.2.6.513
Jarvis BC, Booth A (1981) Influence of indole-butyric acid, boron, myo-inositol, vitamin D2 and seedling age on adventitious root development in cuttings of Phaseolus aureus. Physiol Plant 53:213–218. https://doi.org/10.1111/j.1399-3054.1981.tb04489.x
Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Groth G, Tucker ML, Cooper B, Kieber JJ, Chang C (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA 109:19486–19491. https://doi.org/10.1073/pnas.1214848109
Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105:7100–7105. https://doi.org/10.1073/pnas.0802332105
Li W, Ma M, Feng Y, Li H, Wang Y, Ma Y, Li M, An F, Guo H (2015) EIN2-directed translational regulation of ethylene signaling in Arabidopsis. Cell 163:670–683. https://doi.org/10.1016/j.cell.2015.09.037
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Qiao H, Shen Z, Huang SC, Schmitz RJ, Urich MA, Briggs SP, Ecker JR (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338:390–393. https://doi.org/10.1126/science.1225974
Ricci A, Rolli E, Brunoni F, Dramis L, Sacco E, Fattorini L, Ruffoni B, Díaz-Sala C, Altamura MM (2016) 1,3-di(benzo[d]oxazol-5-yl) urea acts as either adventitious rooting adjuvant or xylogenesis enhancer in carob and pine microcuttings depending on the presence/absence of exogenous indole-3-butyric acid. Plant Cell Tiss Organ Cult 126:411–427. https://doi.org/10.1007/s11240-016-1010-9
Růžička K, Šimášková M, Duclercq J, Petrášek J, Zažímalová E, Simon S, Friml J, Van Montagu MCE, Benková E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA 106:4284–4289. https://doi.org/10.1073/pnas.0900060106
Sarropoulou V, Dimassi-Theriou K, Therios I (2013) Indole-3-butyric acid and myo-inositol impacts on in vitro rooting of the cherry rootstocks CAB-6P and Gisela 6. Biol Plant 57:613–619. https://doi.org/10.1007/s10535-013-0352-y
Sepahvand S, Ebadi A, Kamali K, Ghaemmaghami SA (2012) Effects of myo-inositol and thiamine on micropropagation of GF677 (peach × almond hybrid). J Agric Sci 4:275–280. https://doi.org/10.5539/jas.v4n2p275
Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, He SY, Rizo J, Howe GA, Zheng N (2010) Jasmonate perception by inositol phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405. https://doi.org/10.1038/nature09430
Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, Ecker JR, Klee HJ, Clark DG (2004) The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiol 136:2900–2912. https://doi.org/10.1104/pp.104.046979
Simonetti G, Tocci N, Valletta A, Brasili E, D’Auria FD, Idoux A, Pasqua G (2016) In vitro antifungal activity of extracts obtained from Hypericum perforatum adventitious roots cultured in a mist bioreactor against planktonic cells and biofilm of Malassezia furfur. Nat Prod Res 30:544–550. https://doi.org/10.1080/14786419.2015.1028059
Song S, Huang H, Gao H, Wang J, Wu D, Liu X, Yang S, Zhai Q, Li C, Qi T, Xie D (2014) Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26:263–279. https://doi.org/10.1105/tpc.113.120394
Tran Thanh Van M, Dien NT, Chlyah A (1974) Regulation of organogenesis in small explants of superficial tissue of Nicotiana tabacum L. Planta 119:149–159. https://doi.org/10.1007/BF00390888
Veloccia A, Fattorini L, Della Rovere F, Sofo A, D’Angeli S, Betti C, Falasca G, Altamura MM (2016) Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J Exp Bot 67:6445–6458. https://doi.org/10.1093/jxb/erw415
Wakeel A, Ali I, Khan AR, Wu M, Upreti S, Liu D, Liu B, Gan Y (2018) Involvement of histone acetylation and deacetylation in regulating auxin responses and associated phenotypic changes in plants. Plant Cell Rep 37:51–59. https://doi.org/10.1007/s00299-017-2205-1
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058. https://doi.org/10.1093/aob/mct067
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094. https://doi.org/10.1126/science.280.5366.1091
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236. https://doi.org/10.1105/tpc.109.065730
Zhang Y, Liu J, Chai J, Xing D (2016a) Mitogen-activated protein kinase 6 mediates nuclear translocation of ORE3 to promote ORE9 gene expression in methyl jasmonate-induced leaf senescence. J Exp Bot 67:83–94. https://doi.org/10.1093/jxb/erv438
Zhang F, Qi B, Wang L, Zhao B, Rode S, Riggan ND, Ecker JR, Qiao H (2016b) EIN2-dependent regulation of acetylation of histone H3K14 and non-canonical histone H3K23 in ethylene signaling. Nat Commun 7:13018. https://doi.org/10.1038/ncomms13018
Zhang F, Wang L, Qi B, Zhao B, Ko EE, Riggan ND, Chin K, Qiao H (2017) EIN2 mediates direct regulation of histone acetylation in the ethylene response. Proc Natl Acad Sci USA 114:10274–10279. https://doi.org/10.1073/pnas.1707937114
Zhu Z (2014) Molecular basis for jasmonate and ethylene signal interactions in Arabidopsis. J Exp Bot 65:5743–5748. https://doi.org/10.1093/jxb/eru349
Zhu Z, An F, Feng Y, Li P, Xue L, Mu A, Jiang Z, Kim JM, To TK, Li W, Zhang X, Yu Q, Dong Z, Chen WQ, Seki M, Zhou JM, Guo H (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108:12539–12544. https://doi.org/10.1073/pnas.1103959108
Zolman BK, Yoder A, Bartel B (2000) Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156:1323–1337
Acknowledgements
We thank Dr. Joseph R. Ecker and Dr. John G. Turner for the generous gift of the seeds of ein2-1 and coi1-16 mutants, respectively. We thank Dr. Angela Veloccia for kindly providing preliminary materials for the research. Funds from Sapienza University of Rome (Grant Number RM118164286B32D4 to LF).
Author information
Authors and Affiliations
Contributions
CB planned the experiments, carried out the statistical evaluation of the data, interpreted the results, and contributed to write the manuscript. FDR and MR equally contributed to carry out the experiments. LF collaborated to analyze and discuss the results, and was a major contributor in writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors read, approved the manuscript and declare that there is no conflict of interest.
Additional information
Communicated by Nokwanda Pearl Makunga.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Betti, C., Della Rovere, F., Ronzan, M. et al. EIN2 and COI1 control the antagonism between ethylene and jasmonate in adventitious rooting of Arabidopsis thaliana thin cell layers. Plant Cell Tiss Organ Cult 138, 41–51 (2019). https://doi.org/10.1007/s11240-019-01601-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01601-x