Abstract
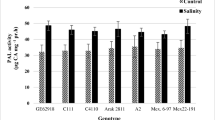
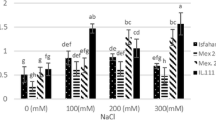
This study investigated the effects of different concentrations of two elicitors, namely chitosan (CHT) (25 and 50 mg L−1) and salicylic acid (SA) (50 and 100 mg L−1) on the production of secondary metabolites (SMs) and antioxidant activity of safflower callus under salinity stress. The content of total phenolics, total flavonoids, total flavonols, anthocyanin and antioxidant activity with 1,1-diphenyl-2 picrylhydrazyl assay (DPPH), significantly increased under salinity stress including 1.5% (w/v) sodium chloride. Under salinity stress, the highest content of total phenolics and total flavonoids were observed under elicitation by 50 (mg L−1) of SA and 25 (mg L−1) of CHT, but the highest callus growth rate (0.048 mm day−1), highest content of total flavonols (4.2 mg RE g−1 FW) and the highest DPPH activity (61.48% of inhibition) were observed under elicitation by 50 (mg L−1) of SA under salinity stress. This indicated partial superiority elicitation of SA over CHT for callus growth and SMs production of safflower under salinity stress. This new elicitation opens new avenues for the selection and exploitation of the best elicitors and their dosages for the enhancement of commercially important SMs in safflower as an important medicinal plant at cellular level.
Key message
Effects of chitosan and salicylic acid on elicitation of SMs of safflower under in vitro salinity stress.


Similar content being viewed by others
Abbreviations
- SA:
-
Salicylic acid
- CHT:
-
Chitosan
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- SMs:
-
Secondary metabolites
- ROS:
-
Reactive oxygen species
- TPC:
-
Total phenolics content
- TFD:
-
Total flavonoids content
- TFL:
-
Total flavonols content
- 2,4-D:
-
2,4-Dichlrophenoxyacetic acid
- Kin:
-
Kinetin
- Ant:
-
Anthocyanin
References
Abdallah SB, Aung B, Amyot L, Lalin I, Lachâal M, Karray-Bouraoui N, Hannoufa A (2016) Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol Plant 38:72
Ahmed IM, Nadira UA, Bibi N, Cao F, He X, Zhang G, Wu F (2015) Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ Exp Bot 111:1–12
Ajungla L, Patil P, Barmukh R, Nikam T (2009) Influence of biotic and abiotic elicitors on accumulation of hyoscyamine and scopolamine in root cultures of Datura metel L. Indian J Biotechnol 8:317–322
Akkol EK, Göger F, Koşar M, Başer KHC (2008) Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem 108:942–949
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Ali MB, Yu KW, Hahn EJ, Paek KY (2006) Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep 25:613–620
Alrawaiq NS, Abdullah A (2014) A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. Int J PharmTech Res 6(3):933–941
Arzani A (2008) Improving salinity tolerance in crop plants: a biotechnological view. In Vitro Cell Dev Biol Plant 44:373–383
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Carretero CL, Cantos M, García JL, Troncoso A (2007) In vitro–ex vitro salt (NaCl) tolerance of cassava (Manihot esculenta Crantz) plants. In Vitro Cell Dev Biol-Plant 43:364–369
Cheng XY, Zhou HY, Cui X, Ni W, Liu CZ (2006) Improvement of phenylethanoid glycosides biosynthesis in Cistanche deserticola cell suspension cultures by chitosan elicitor. J Biotechnol 121:253–260
Danaee M, Farzinebrahimi R, Kadir MA, Sinniah UR, Mohamad R, Taha RM (2015) Effects of MeJA and SA elicitation on secondary metabolic activity, antioxidant content and callogenesis in Phyllanthus pulcher. Braz J Bot 38:265–272
El-Nabarawy M, El-Kafafi S, Hamza M, Omar M (2015) The effect of some factors on stimulating the growth and production of active substances in Zingiber officinale callus cultures. Ann Agric Sci 60:1–9
Falcone Ferreyra ML, Rius S, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222
Gengmao Z, Yu H, Xing S, Shihui L, Quanmei S, Changhai W (2015) Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crops Prod 64:175–181
Golkar P, Taghizadeh M (2018) In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tissue Organ Cult 134:357–368
Golkar P, Amooshahi F, Arzani A (2017) The effects of salt stress on physio-biochemical traits, total phenolic and mucilage content of Plantago ovata Forsk under in vitro conditions. J Appl Bot Food Qual 90:224–231
Govindaraju S, Arulselvi PI (2018) Effect of cytokinin combined elicitors (l-phenylalanine, salicylic acid and chitosan) on in vitro propagation, secondary metabolites and molecular characterization of medicinal herb–Coleus aromaticus Benth (L). J Saudi Soc Agric Sci 17:435–444
Gupta P, Sharma S, Saxena S (2014) Effect of salts (NaCl and Na2CO3) on callus and suspension culture of Stevia rebaudiana for steviol glycoside production. Appl Biochem Biotechnol 172:2894–2906
Hara M, Oki K, Hoshino K, Kuboi T (2003) Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci 164:259–265
Horvath E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300
Karray-Bouraoui N, Harbaoui F, Rabhi M, Jallali I, Ksouri R, Attia H, Msilini N, Lachaâl M (2011) Different antioxidant responses to salt stress in two different provenances of Carthamus tinctorius L. Acta Physiol Plant 33(4):1435–1444
Katiyar D, Hemantaranjan A, Singh B (2015) Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J Plant Physiol 20:1–9
Kim J-S, Lee B-H, Kim S-H, Oh K-H, Cho KY (2006) Responses to environmental and chemical signals for anthocyanin biosynthesis in non-chlorophyllous corn (Zea mays L.) leaf. J Plant Biol 49:16–25
Liu W, Zhang Y, Yuan X, Xuan Y, Gao Y, Yan Y (2016) Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ J Plant Physiol 63:132–142
Ma X, Zheng J, Zhang X, Hu Q, Qian R (2017) Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front Plant Sci 8:600
Malerba M, Cerana R (2016) Chitosan effects on plant systems. Int J Mol Sci 17:996
Martinez V, Mestre TC, Rubio F, Girones-Vilaplana A, Moreno DA, Mittler R, Rivero RM (2016) Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front Plant Sci 7:838
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Namdeo A (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1(1):69–79
Orlita A et al (2008) Application of chitin and chitosan as elicitors of coumarins and furoquinolone alkaloids in Ruta graveolens L.(common rue). Biotechnol Appl Biochem 51:91–96
Philibert T, Lee BH, Fabien N (2017) Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl Biochem Biotechnol 181:1314–1337
Płażek A, Hura K, Żur I (2003) Reaction of winter oilseed rape callus to different concentrations of elicitors: pectinase or chitosan. Acta Physiol Plant 25:83
Putalun W, Luealon W, De-Eknamkul W, Tanaka H, Shoyama Y (2007) Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol Lett 29:1143–1146
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37:91–102
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Salem N, Msaada K, Dhifi W, Limam F, Marzouk B (2014) Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol Plant 36:433–445
Salimgandomi S, Shabrangi A (2016) The effect of Chitosan on antioxidant activity and some secondary metabolites of Mentha piperita L. J Pharm Health Sci 4:135–142
Shaki F, Maboud HE, Niknam V (2018) Growth enhancement and salt tolerance of Safflower (Carthamus tinctorius L.), by salicylic acid. Curr Plant Biol 13:16–22
Sharma V, Ramawat KG (2013) Salinity-induced modulation of growth and antioxidant activity in the callus cultures of miswak (Salvadora persica). 3 Biotech 3(1):11–17
Sharma V, Ramawat KG (2014) Salt stress enhanced antioxidant response in callus of three halophytes (Salsola baryosma, Trianthema triquetra, Zygophyllum simplex) of Thar desert. Biologia 69:178–185
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. In: Stahl U, Donalies UE, Nevoigt E (eds) Food biotechnology. Springer, New York, pp 187–228
Talukder P, Talapatra S, Ghoshal N, Sen Raychaudhuri S (2016) Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. J Sci Food Agric 96:232–244
Tohidi B, Rahimmalek M, Arzani A (2017) Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem 220:153–161
WaSkiewicz A, Muzolf-Panek M, Goliński P (2013) Phenolic content changes in plants under salt stress. In: Ahmad P, Azooz M, Prasad M (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 283–314
Xie Z, Duan L, Tian X, Wang B, Eneji AE, Li Z (2008) Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J Plant Physiol 165:375–384
Zhou X, Tang L, Xu Y, Zhou G, Wang Z (2014) Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol 151:27–43
Acknowledgements
The authors would like to thank Research Institute for Biotechnology and Bioengineering, Isfahan University of Technology, Isfahan, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Nokwanda Pearl Makunga.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Golkar, P., Taghizadeh, M. & Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tiss Organ Cult 137, 575–585 (2019). https://doi.org/10.1007/s11240-019-01592-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01592-9