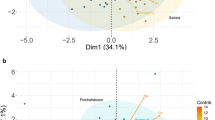
Abstract
Climate change and the increasing world population will lead to an increased water shortage. This gives rise to the need for plant cultivars which are drought tolerant. Solanum tuberosum L. is important not only as a nutritive rich food, but starch potatoes are of great value for the industry. Two starch genotypes of S. tuberosum L. divergently responding to osmotic stress were subjected to medium containing 0.2 M sorbitol in vitro. A targeted metabolomics approach was performed in which 42 metabolites were analysed 11 days after the transfer of the plants to the experimental medium. The sensitive genotype displayed stress responses comprising higher abundant metabolites such as phenylalanine, proline and sucrose and a decrease e.g. in GABA and fumaric acid. These can be used for protein build up, nitrogen storage and the protection through osmotic active compounds. In contrast, the tolerant genotype showed a higher abundance in compounds used as osmolytes (citric acid and proline), which might give rise to acclimatisation to the stress. Interestingly, in chromatograms of both genotypes a high sorbitol peak was detected, whereas control plants or plants treated with 4.8 % PEG 8000 did not accumulate this substance. Conclusively, sorbitol is taken up during in vitro growth, which raises the question for the fate and effect of the incorporated sorbitol.





Similar content being viewed by others
References
Bardzik JM, HV Marsh Jr, Havis J (1971) Effects of water stress on the activities of three enzymes in maize seedlings. Plant Physiol 47(6):828–831
Bouché N, Fait A, Bouchez D, Møller SG, Fromm H (2003) Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. PNAS 100(10):6843–6848
Bündig C, Jozefowicz AM, Mock H-P, Winkelmann T (2016a) Proteomic analysis of two divergently responding potato genotypes (Solanum tuberosum L.) following osmotic stress treatment in vitro. J Proteom. doi:10.1016/j.jprot.2016.04.048
Bündig C, Vu TH, Schum A, Meise P, Seddig S, Winkelmann T (2016b) Variability in osmotic stress tolerance of starch potato genotypes (S. tuberosum L.) as revealed by an in vitro screening: role of proline, osmotic adjustment and drought response in pot trials. J Agron Crop Sci (under revision)
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought: from genes to the whole plant. Funct Plant Biol 30(3):239–264
Chia DW, Yoder TJ, Reiter W-D, Gibson SI (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211:743–751
Criel B, Hausman J-F, Oufir M, Swennen R, Panis B, Renaut J (2006) Proteome and sugar analysis of abiotic stress underlying cryopreservation in potato. Comm Appl Biol Sci, Ghent University 71(1):3–6
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Davies WJ, Zhang J (1991) Root signal and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Delauney AJ, Verma DPS (2008) Proline biosynthesis and osmoregulation in plants. Plant J 4(2):215–223
El-Bashiti T, Hamamci H, Oktem HA, Yucel M (2005) Biochemical analysis of trehalose and its metabolizing enzymes in wheat under abiotic stress conditions. Plant Sci 169:47–54
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M (1997) Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201:502–518
Gibon Y, Usadel B, Blaesing OE, Kamlage B, Hoehne M, Trethewey R, Stitt M (2006) Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol 7(8):Article R76
Gopal J, Iwama K (2007) In vitro screening of potato against water-stress mediated through sorbitol or PEG. Plant Cell Rep 26:693–700
Haverkort AJ, Verhagen A (2008) Climate change and its repercussions for the potato supply chain. Potato Res 51:223–237
Hsiao TC, Acevedo E (1974) Plant response to water deficits, water-use efficiency, and drought resistance. Agric Meteorol 14:59–84
Iwama K, Yamaguchi J (2006) Abiotic stresses. In: Gopal J, Khurana SM (eds) Handbook of potato production: improvement and postharvest management. Food Product Press, New York, pp 231–278
Karimi S, Yadollahi A, Arzani K (2013) Responses of almond genotypes to osmotic stress induced in vitro. J Nuts 4(4):1–7
Khelil A, Menu T, Ricard B (2007) Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivar. Plant Physiol Biochem 45:551–559
Kim Y-S, Jones LS, Dong A, Kendrick BS, Chang BS, Manning MC, Randolph TW, Carpenter JF (2003) Effects of sucrose on conformational equilibria and fluctuations within the native-state ensemble of proteins. Protein Sci 12:1252–1261
Lipavska H, Vreugdenhil D (1996) Uptake of mannitol from the media by in vitro grown plants. Plant Cell Tissue Organ Cult 45:103–107
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1:387–396
Luedemann A, Strassburg K, Erban A, Kopka J (2008) TagFinder for the quantitative analysis of gas chromatography and mass spectrometry (GC-MS)-based metabolite profiling experiments. Bioinformatics 24:732–737
Mousavi A, Hotta Y (2005) Glycine-rich proteins: a class of novel proteins. Appl Biochem Biotechnol 120:169–174
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niessen N, Krause K, Horst I, Staebler N, Klaus S, Gaertner S, Kebeish R, Araujo WL, Fernie AR, Peterhansel C (2012) Two alanine aminotranferases link mitochondrial glycolate oxidation to the major photorespiratory pathway in Arabidopsis and rice. J Exp Bot 63(7):2705–2716
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Novitskaya L, Trevanion SJ, Driscoll S, Foyer CH, Noctor G (2002) How does photorespiration modulate leaf amino acid contents? a dual approach through modelling and metabolite analysis. Plant, Cell Environ 25:821–835
Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 69:3225–3243
Ohkama-Ohtsu N, Oikawa A, Zhao P, Xiang C, Saito K, Oliver DJ (2008) A γ-Glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant Physiol 148:1603–1613
Price KR, Howard B, Coxon DT (1976) Stress metabolite production in potato tubers infected by Phytophthora infestans, Fusarium avenaceum and Phoma exigua. Physiol Plant Pathol 9(2):189–197
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 22(4):375–383
Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 23(1):131–142
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signalling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Sauter A, Davies WJ, Hartung W (2001) The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot 52(363):1991–1997
Schafleitner R, Gaudin A, Gutierrez R, Alvarado C, Bonierbale M (2007) Proline accumulation and real time PCR expression analysis of genes encoding enzymes of proline metabolism in relation to drought tolerance in andean potato. Acta Physiol Plant 29:19–26
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4(11):446–452
Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21:197–203
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279
Smillie RM, Hetherington SE (1999) Photoabatement by anthocyanin shilds photosynthetic system from light stress. Photosynthetica 36(3):451–463
Sperdouli I, Moustakas M (2012) Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J Plant Physiol 169:577–585
Taji T, Chieko Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29(4):417–426
Veselov DS, Mustafina AR, Sabirjanova IB, Akhiyarova GR, Dedov AV, Veselov SU, Kudoyarova GR (2002) Effect of PEG-treatment on the leaf growth response and auxin content in shoots of wheat seedlings. Plant Growth Regul 38:191–194
Wang H-L, Lee P-D, Liu L-F, Su J-C (1999) Effect of sorbitol induced osmotic stress on the changes of carbohydrate and free amino acid pools in sweet potato cell suspension cultures. Bot Bull Acad Sin 40:219–225
Wingler A (2002) Molecules of interest. The function of trehalose biosynthesis in plants. Phytochemistry 60:437–440
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Zhang X, Gou M, Liu C-J (2013) Arabidopsis kelch repeat F-Box proteins regulate phenylpropanoid biosynthesis via controlling the turnover of phenylalanine ammonia-lyase. Plant Cell 25:4994–5010
Acknowledgments
The study was conducted with funding provided by the (BMEL) through the Agency of Renewable Resources (FNR e.V.), funding no Federal Ministry of Food and Agriculture: 22023511. The authors would like to thank Birgit Lippmann for GC/MS support and Dr. A. Schum for in vitro cultures of the studied potato genotypes.
Authors contribution
C. Bündig: Planned and performed experiments, analysed data, prepared all figures and wrote the manuscript. C. Blume: Standard curve preparation, manuscript correction and final approval. C. Peterhänsel: Helped in experimental design, manuscript correction and final approval. T. Winkelmann: Helped in planning experiment, contribution to writing and correction of the manuscript and final approval.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bündig, C., Blume, C., Peterhänsel, C. et al. Changed composition of metabolites in Solanum tuberosum subjected to osmotic stress in vitro: Is sorbitol taken up?. Plant Cell Tiss Organ Cult 127, 195–206 (2016). https://doi.org/10.1007/s11240-016-1042-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1042-1