Abstract
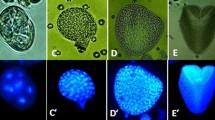
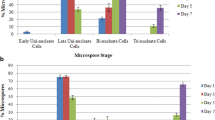
The improvement of androgenic induction efficiency of anther cultures is an important goal for plant biotechnology. Although n-butanol has been proven to enhance the androgenic induction, the structural background of this effect has not been investigated in detail. In the present study, the cytological and ultrastructural alterations triggered by two treatments that improve androgenic induction, n-butanol (0.2 % n-butanol for 6 h) and cold pretreatment (7 °C for 10 days) were studied in maize anther cultures. Both treatments increased the frequency of responding microspores, and the highest embryo yield (20.9 embryos per 100 plated anthers compared to 0.5/100 anthers in control) was achieved when a combination of both treatments was applied. To study the effect of the treatments on the cytoskeleton, we labeled microtubules using indirect immunofluorescence and actin filaments by rhodamine phalloidin. Cold pretreatment increased the quantity of actin filaments, whereas the microtubule network remained unaffected. In contrast, n-butanol treatment triggered the reversible depolymerization of microtubules, without having any effect on the actin network. Transmission electron microscopy revealed that n-butanol induced the formation of irregular cell walls. Autophagy-related structures were present during the early development of embryogenic microspores following both treatments, but autophagy was only sustained after fourteen days in microspore-derived structures treated with n-butanol. The results support the concept that the androgenic developmental switch is assisted by cytoskeletal rearrangements, which may facilitate androgenic induction through the promotion of symmetric divisions. The longer duration of autophagic processes may also play a role in the elevated embryo induction after n-butanol treatment.






Similar content being viewed by others
References
Abdrakhamanova A, Wang QY, Khokhlova L, Nick P (2003) Is microtubule disassembly a trigger for cold acclimation? Plant Cell Physiol 44:676–686
Alché JD, Castro AJ, Solymoss M et al (2000) Cellular approach to the study of androgenesis in maize anthers: immunocytochemical evidence of the involvement of the ubiquitin degradative pathway in androgenesis induction. J Plant Physiol 156:146–155
Barnabás B (2003) Anther culture of maize (Zea mays L.). In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. Springer, Netherlands, pp 103–108
Barnabás B, Obert B, Kovács G (1999) Colchicine, an efficient genome-doubling agent for maize (Zea mays L.) microspores cultured in anthero. Plant Cell Rep 18:858–862
Barnabás B, Szakács É, Karsai I, Bedő Z (2001) In vitro androgenesis of wheat: from fundamentals to practical application. Euphytica 119:211–216
Barton DA, Cantrill LC, Law AMK et al (2014) Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant, Cell Environ 37:2781–2794
Binarova P, Hause G, Cenklová V et al (1997) A short severe heat shock is required to induce embryogenesis in late bicellular pollen of Brassica napus L. Sex Plant Reprod 10:200–208
Broughton S (2011) The application of n-butanol improves embryo and green plant production in anther culture of Australian wheat (Triticum aestivum L.) genotypes. Crop Pasture Sci 62:813–822
Castillo AM, Nielsen NH, Jensen A, Vallés MP (2014) Effects of n-butanol on barley microspore embryogenesis. Plant Cell, Tissue Organ Cult 117:411–418
Collings DA (2008) Crossed-wires: interactions and cross-talk between the microtubule and microfilament networks in plants. In: Nick P (ed) Plant microtubules. Springer, Berlin, pp 47–79
Corral-Martínez P, Parra-Vega V, Seguí-Simarro JM (2013) Novel features of Brassica napus embryogenic microspores revealed by high pressure freezing and freeze substitution: evidence for massive autophagy and excretion-based cytoplasmic cleaning. J Exp Bot 64:3061–3075
De Storme N, Copenhaver GP, Geelen D (2012) Production of diploid male gametes in Arabidopsis by cold-induced destabilization of postmeiotic radial microtubule arrays. Plant Physiol 160:1808–1826
Dhonukshe P, Laxalt AM, Goedhart J et al (2003) Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell Online 15:2666–2679
Dunwell JM (2010) Haploids in flowering plants: origins and exploitation. Plant Biotechnol J 8:377–424
Eady C, Lindsey K, Twell D (1995) The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell Online 7:65–74
Eleftheriou EP, Palevitz BA (1992) The effect of cytochalasin D on preprophase band organization in root tip cells of Allium. J Cell Sci 103:989–998
Földesiné Füredi PK, Ambrus H, Barnabás B (2011) The effect of n-butanol and 2-amino-ethanol on the in vitro androgenesis of maize. Acta Biol Szeged 55:77–78
Földesiné Füredi PK, Ambrus H, Barnabás B (2012) Development of cultured microspores of maize in the presence of n-butanol and 2-aminoethanol. Acta Agron Hung 60:183–189
Gaillard A, Vergne P, Beckert M (1991) Optimization of maize microspore isolation and culture conditions for reliable plant regeneration. Plant Cell Rep 10:55–58
Gardiner J, Collings DA, Harper JDI, Marc J (2003) The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol 44:687–696
Genovesi AD, Collins GB (1982) In vitro production of haploid plants of corn via anther culture. Crop Sci 22:1137
Gervais C, Newcomb W, Simmonds DH (2000) Rearrangement of the actin filament and microtubule cytoskeleton during induction of microspore embryogenesis in Brassica napus L. cv. Topas. Protoplasma 213:194–202
Higaki T, Kutsuna N, Sano T et al (2010) Quantification and cluster analysis of actin cytoskeletal structures in plant cells: role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J 61:156–165
Hirase A, Hamada T, Itoh TJ et al (2006) n-Butanol induces depolymerization of microtubules in vivo and in vitro. Plant Cell Physiol 47:1004–1009
Höfer M (2004) In vitro androgenesis in apple—improvement of the induction phase. Plant Cell Rep 22:365–370
Holmsen JD, Hess FD (1985) Comparison of the disruption of mitosis and cell plate formation in oat roots by DCPA, colchicine and propham. J Exp Bot 36:1504–1513
Hong Y, Zhang W, Wang X (2010) Phospholipase D and phosphatidic acid signalling in plant response to drought and salinity. Plant, Cell Environ 33:627–635
Huang S, Gao L, Blanchoin L, Staiger CJ (2006) Heterodimeric capping protein from Arabidopsis is regulated by phosphatidic acid. Mol Biol Cell 17:1946–1958
Inoué S (1952) The effect of colchicine on the microscopic and submicroscopic structure of the mitotic spindle. Exp Cell Res Suppl 2:305–318
Islam SMS, Tuteja N (2012) Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci 182:134–144
Kost B, Chua N-H (2002) The plant cytoskeleton: vacuoles and cell walls make the difference. Cell 108:9–12
Kuo C, Lu W, Kui Y (1986) Corn (Zea mays L.): production of pure lines through anther culture. In: Bajaj Y (ed) Biotechnology in agriculture and forestry, vol 2. Crops I. Springer, Berlin, pp 152–164
Lalonde S, Beebe DU, Saini HS (1997) Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sex Plant Reprod 10:40–48
Lanza M, Garcia-Ponce B, Castrillo G et al (2012) Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Dev Cell 22:1275–1285
Li F, Vierstra RD (2012) Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci 17:526–537
Li J, Henty-Ridilla JL, Huang S et al (2012) Capping protein modulates the dynamic behavior of actin filaments in response to phosphatidic acid in Arabidopsis. Plant Cell Online 24:3742–3754
Liscovitch M, Czarny M, Fiucci G, Tang X (2000) Phospholipase D: molecular and cell biology of a novel gene family. Biochem J 345:401–415
Liu B, Palevitz BA (1992) Organization of cortical microfilaments in dividing root cells. Cell Motil Cytoskeleton 23:252–264
Liu B, Hotta T, Ho C-MK, Lee Y-RJ (2011) Microtubule organization in the phragmoplast. In: Liu B (ed) The plant cytoskeleton. Springer, New York, pp 207–225
Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK (2005) Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221:95–104
Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) (2003) Doubled haploid production in crop plants: a manual. Kluwer Academic Publishers, Dordrecht
Maraschin SF, Gaussand G, Pulido A et al (2005a) Programmed cell death during the transition from multicellular structures to globular embryos in barley androgenesis. Planta 221:459–470
Maraschin SF, de Priester W, Spaink HP, Wang M (2005b) Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J Exp Bot 56:1711–1726
Maraschin SdeF, Caspers M, Potokina E et al (2006) cDNA array analysis of stress-induced gene expression in barley androgenesis. Physiol Plant 127:535–550
Mascarenhas JP (1990) Gene activity during pollen development. Annu Rev Plant Biol 41:317–338
Massonneau A, Coronado M-J, Audran A et al (2005) Multicellular structures developing during maize microspore culture express endosperm and embryo-specific genes and show different embryogenic potentialities. Eur J Cell Biol 84:663–675
Mitykó J, Andrásfalvy A, Csilléry G, Fári M (1995) Anther-culture response in different genotypes and F1 hybrids of pepper (Capsicum annuum L.). Plant Breed 114:78–80
Motes CM, Pechter P, Yoo CM et al (2005) Differential effects of two phospholipase D inhibitors, 1-butanol and N-acylethanolamine, on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma 226:109–123
Munnik T, Arisz SA, Vrije TD, Musgrave A (1995) G protein activation stimulates phospholipase D signaling in plants. Plant Cell Online 7:2197–2210
Nishikawa S, Zinkl GM, Swanson RJ et al (2005) Callose (β-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22
Obert B, Barnabás B (2004) Colchicine induced embryogenesis in maize. Plant Cell, Tissue Organ Cult 77:283–285
Petrášek J, Schwarzerová K (2009) Actin and microtubule cytoskeleton interactions. Curr Opin Plant Biol 12:728–734
Pleskot R, Potocký M, Pejchar P et al (2010) Mutual regulation of plant phospholipase D and the actin cytoskeleton. Plant J 62:494–507
Pleskot R, Pejchar P, Žárský V et al (2012) Structural insights into the inhibition of actin-capping protein by interactions with phosphatidic acid and phosphatidylinositol (4,5)-bisphosphate. PLoS Comput Biol 8:e1002765
Pleskot R, Li J, Žárský V et al (2013) Regulation of cytoskeletal dynamics by phospholipase D and phosphatidic acid. Trends Plant Sci 18:496–504
Rasmussen CG, Wright AJ, Müller S (2013) The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J 75:258–269
Reed SM, Trigiano RN, Gray DJ (2004) Haploid cultures. In: Trigiano RN, Gray DJ (eds) Plant development and biotechnology. CRC Press, Boca Raton, pp 225–234
Ruelland E, Cantrel C, Gawer M et al (2002) Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol 130:999–1007
Schmiedel G, Reiss H-D, Schnepf E (1981) Associations between membranes and microtubules during mitosis and cytokinesis in caulonema tip cells of the moss Funaria hygrometrica. Protoplasma 108:173–190
Seguí-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol Plant 134:1–12
Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127:519–534
Sheahan MB, Rose RJ, McCurdy DW (2004) Organelle inheritance in plant cell division: the actin cytoskeleton is required for unbiased inheritance of chloroplasts, mitochondria and endoplasmic reticulum in dividing protoplasts. Plant J 37:379–390
Siegrist SE, Doe CQ (2007) Microtubule-induced cortical cell polarity. Genes Dev 21:483–496
Simmonds DH, Keller WA (1999) Significance of preprophase bands of microtubules in the induction of microspore embryogenesis of Brassica napus. Planta 208:383–391
Smertenko A, Franklin-Tong VE (2011) Organisation and regulation of the cytoskeleton in plant programmed cell death. Cell Death Differ 18:1263–1270
Solís M-T, Rodríguez-Serrano M, Meijón M et al (2012) DNA methylation dynamics and MET1a-like gene expression changes during stress-induced pollen reprogramming to embryogenesis. J Exp Bot 63:6431–6444
Soriano M, Cistué L, Castillo AM (2008) Enhanced induction of microspore embryogenesis after n-butanol treatment in wheat (Triticum aestivum L.) anther culture. Plant Cell Rep 27:805–811
Soriano M, Li H, Boutilier K (2013) Microspore embryogenesis: establishment of embryo identity and pattern in culture. Plant Reprod 26:181–196
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Szakács É, Barnabás B (1995) The effect of colchicine treatment on microspore division and microspore-derived embryo differentiation in wheat (Triticum aestivum L.) anther culture. Euphytica 83:209–213
Tischner T, Kőszegi B, Veisz O (1997) Climatic programs used in the Martonvasar phytotron most frequently in recent years. Acta Agron Hung 45:85–104
Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:297–302
Uváčková L’, Takáč T, Boehm N et al (2012) Proteomic and biochemical analysis of maize anthers after cold pretreatment and induction of androgenesis reveals an important role of anti-oxidative enzymes. J Proteomics 75:1886–1894
Varnier AL, Jacquard C, Clément C (2009) Programmed cell death and microspore embryogenesis. In: Touraev DA, Forster DBP, Jain DSM (eds) Advances in Haploid Production in Higher Plants. Springer, Netherlands, pp 147–154
Wang X, Guo L, Wang G, Li M (2014a) PLD: Phospholipase Ds in plant signaling. In: Wang X (ed) Phospholipases in plant signaling. Springer, Berlin, pp 3–26
Wang X, Su Y, Liu Y et al (2014b) Phosphatidic acid as lipid messenger and growth regulators in plants. In: Wang X (ed) Phospholipases in plant signaling. Springer, Berlin, pp 69–92
Zagorska NA, Shtereva A, Dimitrov BD, Kruleva MM (1998) Induced androgenesis in tomato (Lycopersicon esculentum Mill.) I. Influence of genotype on androgenetic ability. Plant Cell Rep 17:968–973
Zhang Q, Lin F, Mao T et al (2012) Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell Online 24:4555–4576
Zheng MY, Weng Y, Sahibzada R, Konzak CF (2003) Isolated microspore culture in maize (Zea mays L.), production of doubled-haploids via induced androgenesis. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. Springer, Netherlands, pp 95–102
Acknowledgments
This work was supported by Hungarian Scientific Research Fund grant No. OTKA 80260 and the GENPROF IF-18/2012 Research Infrastructure Grant from the Hungarian Academy of Sciences. The authors wish to thank Victor Žárský, Lukas Synek and Roman Pleskot for kindly sharing methods for the fluorescent visualisation of the plant cytoskeleton, and Erika Gondos, Emese Bék and Szilvia Fodor for their excellent technical assistance.
Author contributions
AF planned and performed the cytological and ultrastructural analysis, carried out the statistical analysis, interpreted the results and drafted the manuscript. BB conceived the study aims, and critically reviewed the manuscript; KJ contributed to the drafting and critically reviewed the manuscript. PKFF and HA performed and interpreted the results of the anther culture experiments, LSZ contributed to the ultrastructural analysis and critically reviewed the manuscript. All authors approved the final version of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fábián, A., Földesiné Füredi, P.K., Ambrus, H. et al. Effect of n-butanol and cold pretreatment on the cytoskeleton and the ultrastructure of maize microspores when cultured in vitro. Plant Cell Tiss Organ Cult 123, 257–271 (2015). https://doi.org/10.1007/s11240-015-0829-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0829-9