Abstract
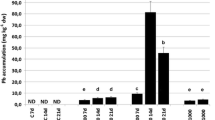
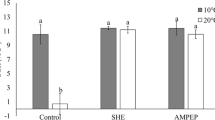
Cell suspension cultures of Sonchus oleraceus L. provide a continuous, efficient system for the production of low molecular weight antioxidants (LMWAs) unhindered by climatic and phenological effects. Because climatic and edaphic factors are known to increase LMWA levels in intact plants, our aim was to study possible effects of abiotic stressors on extractable LMWA activities in vitro. Cell cultures were initiated from in vitro shoots obtained from seedlings of two ecotypes originating from Acacia Bay (ACB) and Oamaru (OAM) in New Zealand. Cells were exposed to chilling, salinity or the combination of both, and concentrations of principal phenolic compounds measured in cell extracts. The cell suspensions yielded higher concentrations of phenolic LMWAs as compared with leaf extracts from in vivo plants. All stressor treatments increased LMWA activities and concentrations of total phenolics, ascorbate and chlorogenic acid relative to the controls. For OAM, all stressors were equally effective; for ACB, the chilling × salinity combination had the greatest effect. Extracts of ACB cells were consistently richer sources of LMWAs than were those from OAM, irrespective of the stressor. The data indicate that the judicious use of abiotic stressors can effectively augment key antioxidant yields from S. oleraceus cell suspensions.




Similar content being viewed by others
References
Blount JW, Masoud S, Sumner LW, Huhman D, Dixon RA (2002) Over-expression of cinnamate 4-hydroxylase leads to increased accumulation of acetosyringone in elicited tobacco cell-suspension cultures. Planta 214:902–910
Cai Z, Kastell A, Mewis I, Knorr D, Smetanska I (2012a) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult 108:401–409
Cai Z, Knorr D, Smetanska I (2012b) Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culture by indanoyl-isoleucine, N-linolenoyl-l-glutamine and insect saliva. Enzym Microb Technol 50:29–34
Cambie RC, Ferguson LR (2003) Potential functional foods in the traditional Māori diet. Mutat Res/Fundam Mol Mech Mutagen 523:109–117
Dávalos A, Gómez-Cordovés C, Bartolomé B (2004) Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem 52:48–54
Doncheck JA, Huss RJ, Running JA, Skatrud TJ (1996). l-ascorbic acid containing biomass of chlorella pyrenoidosa. US Patent 5,521,090, 28 May 1996
Ehlting J, Hamberger B, Million-Rousseau R, Werck-Reichhart D (2006) Cytochromes P450 in phenolic metabolism. Phytochem Rev 5:239–270
Ferguson LR, Yee RL, Scragg R, Metcalf PA, Harris PJ (1995) Differences in intake of specific food plants by Polynesians may explain their lower incidence of colorectal cancer compared with Europeans in New Zealand. Nutr Cancer 23:33–42
Gatto MA, Ippolito A, Linsalata V, Cascarano NA, Nigro F, Vanadia S, Di Venere D (2011) Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol Technol 61:72–82
Gould KS, Thodey K, Philpott M, Ferguson LR (2006) Antioxidant activities of extracts from traditional Māori food plants. NZ J Bot 44:1–4
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine. Oxford University, Oxford
Hussein EA, Aqlan EM (2011) Effect of mannitol and sodium chloride on some total secondary metabolites of fenugreek calli cultured in vitro. Plant Tissue Culture Biotechnol 21:35–43
Jacobo-Velázquez DA, Cisneros-Zevallos L (2012) An alternative use of horticultural crops: stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2:259–271
Kokotkiewicz A, Bucinski A, Luczkiewicz M (2014) Light and temperature conditions affect bioflavonoid accumulation in callus cultures of Cyclopia subternata Vogel (honeybush). Plant Cell Tissue Organ Cult 118:589–593
Kolewe ME, Gaurav V, Roberts SC (2008) Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm 5:243–256
Kweon M-H, Hwang H-J, Sung H-C (2001) Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J Agric Food Chem 49:4646–4655
Mawalagedera SMMR (2014) Antioxidant activities of Sonchus oleraceus L. Doctoral dissertation, School of Biological Sciences, Victoria University of Wellington, New Zealand
Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A, Shinozaki K (2004) Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol 55:327–342
Niknam V, Meratan AA, Ghaffari SM (2011) The effect of salt stress on lipid peroxidation and antioxidative enzymes in callus of two Acanthophyllum species. In Vitro Cell Dev Biol Plant 47:297–308
NIWA (2012) CliFlo: NIWA’s National Climate Database on the Web. http://cliflo.niwa.co.nz
Ou Z, Schmierer DM, Rades T, Larsen L, McDowell A (2013) Application of an online post-column derivatization HPLC-DPPH assay to detect compounds responsible for antioxidant activity in Sonchus oleraceus L. leaf extracts. J Pharm Pharmacol 65:271–279
Pérez-López FR, Chedraui P, Haya J, Cuadros JL (2009) Effects of the mediterranean diet on longevity and age-related morbid conditions. Maturitas 64:67–79
Philpott M, Gould KS, Markham KR, Lewthwaite SL, Ferguson LR (2003) Enhanced coloration reveals high antioxidant potential in new sweetpotato cultivars. J Sci Food Agric 83:1076–1082
Radhakrishnan R, Leelapriya T, Kumari BDR (2012) Effects of pulsed magnetic field treatment of soybean seeds on calli growth, cell damage, and biochemical changes under salt stress. Bioelectromagnetics 33:670–681
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Rashid KI, Ibrahim KM, Hamza SJ (2011) Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from rosemary (Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (HPLC). J Al-Nahrain Univ 14:104–109
Riley M, Enting B (1994) Māori healing and herbal: New Zealand ethnobotanical sourcebook. Viking Sevenseas NZ
Rivelli DP, da Silva VV, Ropke CD, Miranda DV, Almeida RL, Sawada TCH, de Barros SBM (2007) Simultaneous determination of chlorogenic acid, caffeic acid and caffeine in hydroalcoholic and aqueous extracts of Ilex paraguariensis by HPLC and correlation with antioxidant capacity of the extracts by DPPH· reduction. Revista Brasileira de Ciências Farmacêuticas 43:215–222
Rossetto M, Vanzani P, de Marco V, Zennaro L, Scarpa M, Rigo A (2008) Fast and simple method for the simultaneous evaluation of the capacity and efficiency of food antioxidants in trapping peroxyl radicals in an intestinal model system. J Agric Food Chem 56:3486–3492
Sajc L, Grubisic D, Vunjak-Novakovic G (2000) Bioreactors for plant engineering: an outlook for further research. Biochem Eng J 4:89–99
Schaffer S, Schmitt-Schillig S, Muller W, Eckert G (2005) Antioxidant properties of Mediterranean food plant extracts: geographical differences. J Physiol Pharmacol 56:115–124
Shohael AM, Ali MB, Yu K-W, Hahn E-J, Paek K-Y (2006) Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell Tissue Organ Cult 85:219–228
Simopoulos AP (2004) Omega-3 fatty acids and antioxidants in edible wild plants. Biol Res 37:263–278
St John-Sweeting RS (2011) Dispersal and genetic variability of Sonchus oleraceus L. in relation to its resistance to ALS—inhibiting herbicides. Doctoral dissertation, School of Agriculture, Food and Wine, The University of Adelaide, Australia
Thomson B, Shaw I (2002) Comparison of risk and protective factors for colorectal cancer in the diet of New Zealand Māori and non-Māori. Asian Pac J Cancer Prev 3:319–324
Thygesen L, Thulin J, Mortensen A, Skibsted LH, Molgaard P (2007) Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem 101:74–81
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Waterhouse AL (2002) Determination of total phenolics. In: Wrolstad RE, Decker EA, Schwartz SJ, Sporns P (eds) Current protocols in food analytical chemistry. Wiley, New Jersey, pp I1.1.1–I1.1.8
Wilson SA, Roberts SC (2012) Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J 10:249–268
Wu C-H, Murthy HN, Hahn E-J, Paek K-Y (2007) Enhanced production of caftaric acid, chlorogenic acid and cichoric acid in suspension cultures of Echinacea purpurea by the manipulation of incubation temperature and photoperiod. Biochem Eng J 36:301–303
Zhang Y-H, Zhong J-J, Yu J-T (1995) Effect of osmotic pressure on cell growth and production of ginseng saponin and polysaccharide in suspension cultures of Panax notoginseng. Biotechnol Lett 17:1347–1350
Zhang W, Seki M, Furusaki S (1997) Effect of temperature and its shift on growth and anthocyanin production in suspension cultures of strawberry cells. Plant Sci 127:207–214
Zhang W, Wei J, Hu Z, Zhong G (2008) Study on extraction and purification of chlorogenic acid from Flos lonicerae flowers and its antioxidation properties. Food Sci 3:1–7
Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in Thellungiella and Arabidopsis callus. Plant Cell Tissue and Organ Cult 98:97–103
Zhao J-L, Zhou L-G, Wu J-Y (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol 87:137–144
Acknowledgments
Work was funded by a New Zealand International Doctoral Research Scholarship (NZIDRS) to SMMRM.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mawalagedera, S.M.M.R., Gould, K.S. Chilling and salinity increase extractable antioxidants in cell suspension cultures of the sow thistle, Sonchus oleraceus L.. Plant Cell Tiss Organ Cult 121, 35–44 (2015). https://doi.org/10.1007/s11240-014-0676-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0676-0