Abstract
The development of a vaccine is still a priority in the fight against human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS). Since conventional vaccine strategies have failed to provide a highly immunoprotective effect, approaches based on the rational design of vaccines composed of multiple HIV neutralizing epitopes have been proposed as potential vaccines. The aim of this study is to design a multiepitopic protein (Multi-HIV) carrying several neutralizing epitopes from both gp120 and gp41 as an effort to develop a new broad immunization scheme against HIV. This Multi-HIV was initially produced in a recombinant Escherichia coli strain either as a single protein or fused to glutathione-S-transferase. These proteins were purified by immobilized metal ion affinity chromatography and shown to be antigenic by positive reactivity in Western blot analyses using sera from HIV-positive patients for labeling. Since global immunization strategies are often limited by costs, platforms that require minimal processing are the priority in this field. Therefore, we explored the possibility of using transplastomic tobacco plants as an experimental model of a low cost plant-based vaccine against HIV. Transplastomic tobacco plants carrying the multi-HIV gene were developed and verified by PCR analyses. The expected Multi-HIV recombinant protein was localized in the chloroplast as proven first by confocal microscopy and subsequently by Western blot analysis. Tobacco-derived Multi-HIV protein was clearly able to evoke humoral responses in mice when orally administered without adjuvants. This report constitutes an effort to explore a new low-cost candidate that could have future implications on the development of affordable HIV vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant cells constitute feasible hosts for the production of subunit vaccines. This concept has matured over the last two decades with many groups reporting models for the production of low-cost pharmaceuticals and vaccines. The impact derived from this technology is expected to be particularly high in developing countries where access to therapeutics is limited due to political, economical, and logistical factors. Among the immunogen expression modalities, transplastomic technologies offer an important approach with a number of singular advantages. First, the expression achieved by transplastomic technologies can be remarkably high, reaching 10- to 100-fold that of conventional nuclear-based technologies due to the lack of position effects since integration of the transgene is site-directed (Daniell et al. 2009; Ruhlman et al. 2010). Second, using transplastomic technologies it is possible to express multiple proteins from polycistronic mRNAs (Wang et al. 2009). Third, biosafety is favored by the fact that inheritance of the plastid genome is typically maternal (Corriveau and Coleman 1988), and thus transmission of plastids through pollen is a rare event (Ruf et al. 2007) and transfer of a chloroplast marker to the nucleus is a very rare event (Huang et al. 2003; Stegemann et al. 2003; Bock and Timmis 2008). Lastly, position effects are avoided as site-directed integration of the foreign DNA is achieved by double homologous recombination. These characteristics are in sharp contrast to those of nuclear expression-based systems where position effects and silencing derived from random integration and uncontrolled copy number take place. Inheritance of nuclear transgenes follow complex patterns, while chloroplasts characteristically contain multiple and homogeneous genomes that are typically inherited from one parent only, making transplastomic inheritance an approach of high stability and simplified analysis (Timmis et al. 2004).
Tobacco has been used in previous studies as initial model for exploring a number of vaccine candidates in the context of transplastomic approaches (Tiwari et al. 2009). The tobacco plant as a model system has main advantages, including (1) it is basically the only species in which plastid transformation is a routine technique; (2) it produces a huge number of seeds and thus offers a rapid scale-up, a high biomass yield; (3) a large-scale infrastructure for processing biomass is available. Therefore, tobacco is currently one of the prime candidate species in agricultural biotechnology for which numerous pharmaceutical-producing transgenic lines are already being grown in large-scale field trials. Although not edible, tobacco is considered an important platform for the synthesis of products intended to be subjected to purification processes (Kamenarova et al. 2005; Ruf et al. 2007).
Despite the achievements of highly active anti-retroviral therapy, the global spread of human deficiency virus/acquired immune deficiency syndrome (HIV/AIDS) remains at pandemic proportions, and the appearance of multiclass drug-resistance viruses represents a major concern in the field. Furthermore, the high cost of the therapy limits the possibility of distributing the drugs to the entire population (Gottlieb et al. 2009). Therefore, the development of prophylactic vaccines is the main goal in the fight against HIV. Since the emergence of AIDS, efforts to develop an effective vaccine have had only limited success.
Our group has been involved in the expression of chimeric proteins carrying sequences from HIV Env proteins which are considered primary targets for viral neutralization. In particular, C4 and V3 sequences from gp120 have been studied in the form of a chimeric protein, denoted C4(V3)6, which has been produced in both Escherichia coli and plant cells (Govea-Alonso et al. 2013; Rubio-Infante et al. 2012).
Since the use of chimeric proteins for immunization purposes is associated with several advantages, such as the inclusion of diverse protective epitopes, it is relevant to explore the development of new chimeric proteins carrying additional targets associated with the elicitation of neutralizing antibodies against this highly variable pathogen. Improvements in the response breadth are therefore envisioned using previous candidates, such as C4(V3)6 (Hemelaar 2012; Govea-Alonso et al. 2013).
In addition to the antigenic determinants from the V3 sequence, several epitopes have been explored as elicitors of neutralizing antibodies. For example, epitopes on the highly conserved membrane-proximal external region from gp41 comprise EQELLELDKWAS and LWNWFDITNWLWK, and they are recognized by neutralizing antibodies 2F5 and 4E10, respectively. It has been shown that the EQELLELDKWAS peptide induces neutralization against several HIV strains (Broliden et al. 1992; Vanini et al. 1993), and interestingly this sequence was found to be highly conserved in 72 % of the 79 isolates analyzed by Muster et al. (1993). These features make these epitopes promising targets for the design of HIV subunit vaccines. The design of chimeric proteins with this type of well-characterized epitope constitutes a relevant goal in the fight against HIV/AIDS. However, in order to assess the expression rate and immunogenic effect, each chimeric protein candidate must be evaluated individually.
In the study reported here we designed a multiepitopic protein comprising conformational and linear epitopes derived from HIV Env protein (gp120/gp41) associated with the elicitation of neutralizing antibodies. This multi-HIV protein (Multi-HIV) was produced in recombinant E. coli and tobacco chloroplasts in an effort to develop a low-cost production system.
Materials and methods
Design and construction of expression vectors
A recombinant Multi-HIV protein was designed that contained several epitopes from Env proteins along with a His tag. An in silico crystallographic model was built using the protein structure homology server SWISS-MODEL (Arnold et al. 2006). This analysis is based in QMEAN4 global scores (Benkert et al. 2011) that considers the Multi-HIV three-dimensional (3D) model, which is compared with the gp120 core (PDB 2b4c) obtained from the Protein Data Bank as a reference; thus, homologous structures are defined according to an algorithm. The cut-off parameters to model the target based on a BLASTp target–template alignment were an E-value of 0.0001, a minimum template size (amino acids) for ranking of 25, and a minimum sequence identity of 60. The retrieved structure from the crystallographic model was compared with the secondary structures predicted for the Multi-HIV based on the analysis performed with the following softwares: Porter, SOPMA, and GOR from the ExPASy (Expert Protein Analysis System) server.
The Multi-HIV encoding gene was flanked by EcoRV, XbaI, and XhoI restriction sites in order to facilitate cloning into expression vectors. The encoding gene for Multi-HIV was synthesized by GenScript (Piscataway, NJ) after optimizing codon usage for E. coli and the chloroplast as expression hosts (www.genscript.com).
For bacterial expression, the gene was cloned into the pET-28b(+) vector (Novagen Biosciences, Madison, WI), and the resulting expression vector was named pmulti-HIV. In order to express Multi-HIV as a glutathione-S-transferase-fusion (GST) protein, we constructed a second bacterial expression vector using the pGEX-4T1 vector (GE Healthcare Life Science, Piscataway, NJ: www.gelifesciences.com). This construct was obtained by modifying the gene with mutagenic PCR to introduce the restriction sites 5′EcoRI and 3′XhoI (underlined) using the primers: 5′-GAATTCATGGGATTAGTCAAACAGATCATC and 5′-CTCGAGCTGCAGTTAGTGGTG, respectively. The amplicon was cloned into pGEM® T-Easy vector System (Promega, Madison, WI: www.promega.com) and subsequently subcloned into pGEX-4T1 vector (Invitrogen, Carlsbad, CA: www.invitrogen.com) to obtain the pXmulti-HIV expression vector. A positive clone of both expression vectors was selected by restriction analysis and used for the protein expression assays.
To construct an expression vector suitable for chloroplast expression, we cloned the multi-HIV gene into the pKCZ-derived vector (kindly provided by Dr. Hans U. Koop; Zou et al. 2003) at the EcoRV site, located downstream of the Prrn promoter, in order to allow expression of the multi-HIV gene as a bicistron along with the aadA gene, the selectable marker gene providing spectinomycin resistance to the transformed cells.
Expression and purification of the E. coli-derived multi-HIV protein
Escherichia coli TOP10 (Invitrogen) or BL21 strains transformed with the pmulti-HIV or pXmulti-HIV expression vectors, were grown in LB media supplemented with 100 μg/mL of ampicillin or 100 μg/mL of kanamycin, respectively. Expression was induced by adding 1 mM of isopropyl-thiogalactoside (IPTG) to the media. Purification was performed using an Ni-charged resin in an immobilized metal ion affinity chromatography (Ni-IMAC) system as described by Varona-Santos et al. (2006), substituting the dialysis procedure for protein refolding with an on-column refolding procedure that reduced the ionic strength of the running solution. To ensure that protein refolding conditions were achieved, we continuously measured conductivity at the column outlet. The purified recombinant proteins were clarified by centrifugation and stored at −70 °C until further use. After the determination of protein concentration by the Bradford method (1976), we assayed the purity and molecular weight by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Transformation and analysis of tobacco plants
Chloroplast transformation
This procedure was performed according to the protocol described by Daniell et al. (2005). Briefly, Nicotiana tabacum cv. Petite Havana SR1 seeds were germinated in vitro on MS (Murashige and Skoog 1962) medium. Leaves from 6-week-old were bombarded with DNA-coated gold particles at 1,100 psi using the PDS-1000/He biolistic microprojectile gene gun (Bio-Rad, Hercules, CA). Following bombardment, leaf tissues were maintained in the dark for 48 h in RMOP media [MS salts and vitamins, 0.1 mg/L naphthaleneacetic acid, and 1 mg/L of 6-benzyladenine; Svab et al. 1990], then cut into several pieces (approx. 7 mm × 7 mm) and cultured on selection medium consisting of RMOP supplemented with 500 mg/L of spectinomycin. Explants were transferred to fresh selective medium once every 2 weeks and divided into 7-mm squares until shoots appeared. Spectinomycin-resistant shoots were used to conduct the second round of selection. Finally, a third round of selection was performed using leaf sections from shoots obtained in the second round of selection. After the third round of regeneration, shoots were rooted on free-hormone MS medium containing 100 mg/L of spectinomycin. When roots were 2 cm long, regenerated plants were transferred to pots containing Sunshine soil mix. Plants were grown at 24 °C under a 16/8-h (light/dark) photoperiod with light provided as an intensity of 100 μmol m−2 s−1.
PCR
Total DNA was isolated from leaves of both putative transformants and wild-type (WT) plants according to Dellaporta et al. (1983). The PCR reaction mixture (total volume 25 μL) contained 100 ng DNA, 1 × PCR buffer, 1.5 mM magnesium chloride, 2.5 U Taq DNA polymerase, 1 mM dNTPs, and 1 μM of primers [for Multi-HIV detection: 5′-ATGGGATTAGTCAAACAGATC (sense) and 5′-TGCAGTTAGTGGTG (anti-sense); for homoplasty assessment: 5′-AAGAATGGGTGAGGGTATTCTGCCTAAATA (sense) and 5′-GCATCTAAGTAGTAAGCCCACCCCAAGATG (anti-sense)]. Cycling conditions consisted of a setting temperature at 94 °C for 5 min (initial denaturation), 35 cycles at 95 °C for 30 s, maintained temperature at 56 °C for 30 s, 72 °C for 60 s (180 s for homoplasty assay), and a final extension at 72 °C for 10 min. PCR products were analyzed by electrophoresis on a 1 % agarose gel.
Immunohistofluorescence
Tobacco leaves were fixed in a freshly prepared ZSF solution (0.1 M Tris-base buffer with calcium acetate 0.05 %, pH 7–7.4, containing 0.5 % zinc acetate and 0.5 % zinc chloride) and the tissue was vacuum-infiltrated. Tissue slices were dehydrated in alcohol and indirect immunofluorescence was performed to localize Multi-HIV. Non-specific binding was reduced by treatment for 30 min with phosphate buffered saline (PBS) supplemented with 5 % bovine serum albumin and 0.05 % Triton X-100. Sections were incubated with rabbit serum anti-C4(V3)6 (1:1000 dilution in PBS + 0.07 % Triton X-100), followed by incubation with the secondary antibody goat anti-rabbit-fluorescein isothiocyanate (FITC; 1:1000 dilution in PBS + 0.07 % Triton X-100; Invitrogen). Control experiments for staining specificity included appropriate isotype-matched non-specific antibodies or replacement of primary antibodies with non-immune serum and omission of primary antibody from incubation protocols. Immunofluorescence images were acquired with using confocal laser scanning microscopy (TCS SP2; Leica Microsystems, Wetzlar, Germany: http://www.leica-microsystems.com/).
Antigenicity analysis
In order to identify the antigenic potential of Multi-HIV, we conducted an in silico analysis applying the Hopp and Woods method (Hoop and Woods 1981) and using the ExPASy database (http://web.expasy.org/protscale/). In addition, western blot analyses were performed. In brief, total soluble protein (TSP) fractions were obtained by grinding and re-suspending 10 mg of fresh tissue in 100 μL of 1× reducing loading buffer. Samples were denatured by boiling for 5 min at 95 °C, and debris were eliminated by centrifugation at 12,000g for 10 min. SDS-PAGE was then performed in a 4–12 % gradient acrylamide gel under denaturing conditions, and the gel was blotted onto BioTrace PVDF membranes (Pall Corp., Port Washington, NY: www.pall.com). After blocking with PBS-Tween 20 (PBST) + 4 % fat-free milk, the blots were incubated with either a mouse anti-His antibody (1:10,000 dilution; GenScript, Piscataway, NJ: www.genscript.com) or a pool of sera from HIV-positive patients. Horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000; Sigma, St. Louis, MO: www.sigmaaldrich.com) were applied for 1 h at 25 °C. Antibody binding was detected by incubation with the SuperSignal West Dura solution following the instructions of manufacturer (Thermo Scientific, Waltham, MA: www.thermoscientific.com). Signal detection was performed by means of an X-ray film following standard procedures.
Enzyme-linked immunosorbent assay analysis
Total soluble protein extracts were obtained by grinding approximately 50 mg of fresh leaf tissue in 400 μL of extraction buffer (50 mM Tris–HCl pH 8, 40 mM NaCl, 0.1 % Tween 20, 1 mM PMSF). Samples were kept on ice for 10 min and subsequently centrifuged for 5 min at 9,000g. Supernatants were separated from cell debris and used for coating enzyme-linked immunosorbent assay (ELISA) plates at dilutions prepared with carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3). After overnight incubation at 4 °C, plates were subjected to the following treatments that included a wash step between each procedure: (1) blocking with 5 % non-fat dry milk dissolved in PBS; (2) overnight incubation at 4 °C with a mice anti-serum (1:200 dilution) obtained in mice against the previously reported C4(V3)6 which recognizes the V3 and C4 epitopes present in Multi-HIV; (3) 1-h incubation at 25 °C with a rabbit anti-mice HRP conjugated immunoglobulin G (IgG; 1:2,000 dilution; R&D Systems, Minneapolis, MN); (4) 20-min incubation at 25 °C with an ABTS-based substrate solution [0.6 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), 0.1 M citric acid, pH 4.35]. A standard curve constructed with E. coli derived Multi-HIV was included in order to estimate expression levels in samples from transplastomic plants.
Immunogenicity analysis
Tobacco leaves from the THIV1 line were freeze-dried and ground in an analytical mill. Groups of 11-week old female BALB/c mice were established and handled according to the guidelines of the Federal Regulations for Animal Experimentation and Care (NOM-062-ZOO-1999, Ministry of Agriculture, México). Experimental groups (n = 5) received one of the following treatments via the oral route: (1) PBS; (2) 50 mg of freeze-dried tobacco leaves containing Multi-HIV protein (TT); (3) 50 mg of freeze-dried WT leaves. Four weekly doses were administered on days 0, 7, 14, and 28. Test animals were sacrificed on day 35 and sera collected to perform an ELISA assay to estimate antibody content. Polystyrene plates were coated overnight at 4 °C with 1 μg/well of E. coli-derived C4(V3)6, a recombinant protein carrying several V3 loop sequences and the C4 domain from gp120 reported by Govea-Alonso et al. (2013). After blocking with 5 % non-fat milk and washing, serum samples were added in a 1:20 dilution, and the plates were incubated overnight at 4 °C. A goat anti-mouse IgG–HRP-conjugated (1:1000; Pierce, Rockford, IL) was used as a secondary antibody, and the plates were incubated further for 1 h at 37 °C. After washing, the plates were incubated for 15 min with ABTS substrate (Sigma: www.sigmaaldrich.com). Specific antibody levels in serum samples were expressed as corresponding optical density values measured at 405 nm using a Multiskan Ascent microplate reader (Thermo Electron Corp., Waltham, MA). Data derived from antibody measurements were analyzed by a one-way analysis of variance with significance set at p < 0.05 using GraphPad Prism5 software (GraphPad Software, San Diego, CA).
Results
Multi-HIV as a novel multiepitopic protein
The description of the epitopes included into the sequence of the multi-HIV chimeric protein is presented in Table 1. The configuration comprises a region from the fourth conserved domain of gp120 (C4) which serves as the T helper and the CTL, and B cell epitopes and has been associated with adjuvant properties (Patterson et al. 2001), as well as a set of epitopes derived from the gp41 and gp120 sequences which have been associated with the elicitation of neutralizing antibodies against HIV.
A set of in silico analyses was performed to estimate the possible structural features of the Multi-HIV. The 3D structure of Multi-HIV was constructed by homology modeling using the crystal structure of gp120 as the template. This 3D model was able to successfully predict significant similitude, namely, 24 %, between PDB 2b4c and the region amino acids (aa) 8–232 (Fig. 1a). Significantly, the tridimensional architecture of the Multi-HIV reveals that epitopes from the V3 loop, reported as the principal immunodeterminant epitope of gp120, conserved their native structural features. Nonetheless, the structures of the other epitopes acquired different configurations depending on the adjacent residues. For example, the region comprising tandem repeats of the GPGRAF epitope from V3 acquired a possible β-sheet structure in combination with the NWFDITNW sequence. Interestingly, repeats of the ELDKWA epitope from gp160 took on a more coiled structure which, in theory, would allow for the possible interaction with B cells and the subsequent elicitation of antibodies. The V1/V2 tandem epitopes STSIRGKV acquired different configurations, including the α-helix, β-sheets, and coil structure. Interestingly, several strong peaks are present in the antigenicity plot derived from a Hoop and Woods hydrophilicity analysis which predicts potential antigenic peptides (Fig. 1b). Taken together, the results derived from the in silico analyses suggest that multi-HIV may be able to resemble the native epitopes, at least for some of the included epitopes. These data may account for the immunogenic properties of the Multi-HIV.
Predicted antigenic profile of multi-HIV protein (Multi-HIV). a Three-dimensional (3D) Multi-HIV structure obtained from the protein structure homology server SWISS-MODEL. Note the presence of helix and sheet structures. b Hoop and Woods hydrophilicity analysis. The hydrophilicity value (score) versus sequence position shows the peaks where the hydrophilic peaks corresponds to the antigenic peptides. Sequence of the Multi-HIV are shown above and below the corresponding peaks. Multi-HIV multiepitopic protein comprising conformational and linear epitopes derived from the human immunodeficiency virus (HIV) Env protein (gp120/gp41)
Multi-HIV expressed in E. coli and purified by IMAC
The multi-HIV gene was successfully cloned into vectors pET28b(+) and pGEX 4T1 (Fig. 2a, b), yielding the bacterial expression vectors pmulti-HIV and pXmulti-HIV, respectively. These were transferred to the appropriate E. coli strains, and the success of the transfer, i.e., detection of the multi-HIV gene, was confirmed by PCR. The expected 900-bp amplicons were present in both types of strains, while the amplicon was absent in the WT strain (data not shown). Expression induced by IPTG enabled detectable recombinant proteins to be produced in a SDS-PAGE gel stained with Coomassie blue. The molecular weight of these differential proteins was approximately 32 kDa for Multi-HIV and 53 kDa for GST:Multi-HIV protein (Fig. 2c, d, respectively). To assess the identity of these proteins, we carried out a western blot analysis using anti-His tag antibodies for labeling and observed that most of the tested clones showed a positive reactivity to those 32- and 53-kDa proteins, suggesting they correspond to the expected Multi-HIV (Fig. 3a, b, respectively). One clone was negative for the expression of GST:Multi-HIV protein, which may be attributed to protein instability when expressed in this bacterial host. Similar positive results were obtained when sera from HIV-infected patients were used for labeling, indicating that HIV antigenic determinants were present in the E. coli-derived Multi-HIV (Fig. 3c). Therefore, our results from both the immunodetection and in silico analyses support the antigenic potential of the Multi-HIV. Following IMAC purification and refolding, the presence of a band of the expected molecular weight after SDS-PAGE revealed that an acceptable grade of purity had been reached, although an additional band of lower molecular weight was also present which may correspond to degradation products (Fig. 3d).
Expression of Multi-HIV in E. coli. a Physical map of the transformation vector pmulti-HIV. The synthetic gene was cloned in pET-28(+) in the NcoI and XhoI restriction sites, and the T7 promoter drives the expression of the chimeric protein. The Lac operator (Lac O) and ribosome bonding site (RBS) were present, and termination was mediated by the T7 terminator (T7 Term). b Physical map of expression vector pXmulti-HIV. Multi-HIV is expressed as a glutathione-S-transferase (GST)–fusion protein; the multi-HIV gene was subcloned into the pGeX-4T1 vector in the EcoRI and XhoI restriction sites under the tac promoter (tac Prom). c Total protein patterns showing the expression of the 32-kDa heterologous protein using the vector pET28b(+). Lanes MW Molecular weight marker, 1 clone carrying pmultiHIV induced with isopropyl-thiogalactoside (IPTG), 2 clones carrying empty pET28b(+) vector. d Total protein patterns showing the expression of the 53-kDa heterologous protein using the vector pGEX 4T1. Lanes MW Molecular weight marker, 1 clone carrying pXmulti-HIV induced with IPTG, 2 clone carrying empty pGEX 4T1 vector
Immunodetection and purification of the E. coli-derived Multi-HIV. a Western blot analysis showing reactivity of the multi-HIV recombinant protein (32 kDa) with an anti-His antibody. Lanes 1 Clone carrying empty pET28b(+) vector, 2–4 clones carrying pmulti-HIV induced with IPTG. b Western blot analysis showing reactivity of the GST:Multi-HIV recombinant protein (53 kDa) with an anti-His antibody. Lanes MW Molecular weight marker, 1 clone carrying empty pGEX4T1 vector, 2–4 clones carrying pXmulti-HIV induced with IPTG. c Western blot analysis showing reactivity of the Multi-HIV recombinant protein (32 kDa) with sera from HIV-infected patients. Lanes 1 Clone carrying empty pET28b(+) vector, 2 clone carrying pmulti-HIV induced with IPTG. d Detection of the immobilized metal ion affinity chromatography (IMAC)-purified Multi-HIV in a SDS-PAGE analysis. Lanes MW Molecular weight marker, 1 IMAC-positive fraction concentrated by precipitation obtained from a clone carrying pmulti-HIV induced with IPTG
Multi-HIV is successfully expressed as an orally immunogenic protein into plant chloroplasts
The vector pClo used to develop a chloroplast-derived multi-HIV is schematically represented in Fig. 4a. After particle bombardment of five independent tobacco leaves, in vitro cultures were successfully maintained under aseptic conditions. Under selective pressure, calli and shoot development were observed in the transformed tobacco tissues 6 weeks after bombardment, whereas untransformed tissues died under the selective conditions (Fig. 4b, c). A total of five putative transformed lines were rescued, representing an average efficiency of one stable transformant per bombarded leaf. After completion of at least three selection rounds, four of these lines were selected to complete the regeneration and developmental processes to whole plants; these lines were designated THIV1–THIV4. An average of five shoots per line were rooted, and rooting was successful for all four independent lines. Plantlets were transferred to soil, acclimatized, and grown to maturity. During this process, no phenotypic alterations were observed (Fig. 4d–f). The presence of the multi-HIV transgene in total DNA preparations was confirmed by PCR analysis, which showed the expected 900-bp amplicon (Fig. 5a). No PCR product was present in reactions containing DNA from untransformed plants. PCR analysis to determine the homoplasmic state revealed the presence of 4.3-kb amplicons but not 2.3-kb amplicons, whereas the WT plant had only 2.3-kb amplicons (Fig. 5b). These findings confirm the site-specific insertion of the multi-HIV gene and the homoplastic state of the transplastomic lines.
Development of transplastomic tobacco plants. a Schematic representation for the integration of the multi-HIV expression cassette into the inverted repeat region of the chloroplast genome. Bold arrows Landing sites for oligonucleotides 1 and 2 used for multi-HIV detection, black box chloroplast genome near to the insertion site, thin arrows landing sites for oligonucleotides used to determine homoplasty. UTR Untranslated region. b Aspect of wild-type (WT) leaves under spectinomycin selective pressure at 4 weeks postbombardment. c Aspect of transformed leaf tissues under spectinomycin selective pressure, notice the presence of green shoots. d Aspect of plants from transplastomic line THIV1. e Aspect of plants from transplastomic line THIV2. f Aspect of WT plants
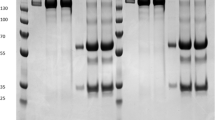
Characterization of transplastomic lines. a PCR analysis showing the presence of the multi-HIV gene in tobacco candidate lines. Lanes MW Molecular weight marker, 1 positive control (10 ng of plasmid carrying multi-HIV gene), 2–4 samples from THIV1, THIV2, THIV3, and THIV4 lines, respectively, 6 sample from a WT plant. Notice the presence of the expected amplicon of 900 bp. b PCR assay to assess homoplasty in tobacco lines. PCR was conducted to amplify the chloroplast genome at flanking points of the recombination regions. Lanes MW Molecular weight marker, 1, 2 samples from WT plant, 3–6 samples from THIV1–THIV4 lines, respectively. Since the inserted foreign DNA is approximately 2 kb in length, the presence of the 2.3-kb amplicon indicates the presence of the native plastome, while that of the 4.3-kb amplicon indicates a transplastomic state. Notice that no amplicons corresponding to the native genome are observed in transplastomic lines. c Anti-His tag western blot analysis showing reactivity of the tobacco-derived Multi-HIV (32 kDa) produced in the THIV lines. Lanes MW Molecular weight marker, 1 pure C4(V3)6 which is a His-tagged protein as positive control, 2–4 samples from THIV1, THIV2, and THIV3 lines, respectively, 5 sample from a WT plant. d Western blot analysis showing reactivity of sera from HIV+ patients with the tobacco-derived Multi-HIV protein (32 kDa) produced in THIV lines. Lanes MW molecular weight marker, 1 pure Multi-HIV protein as positive control, 2, 3 samples from THIV1 and THIV2 lines, respectively. Note that no reactivity was shown by samples from WT plants (lane 4)
To investigate the expression and antigenicity of the plant-derived Multi-HIV, a western-blot labeling assay with anti-His was performed which revealed the presence of an immunoreactive protein of 32 kDa; this indicates an appropriate production of the expected Multi-HIV in plant chloroplasts (Fig. 5c). However, some additional bands of 25 and 28 kDa were also observed, which may correspond to degradation products of Multi-HIV. Distinct positive signals at the expected molecular weight of 32 kDa developed when sera from HIV-positive patients were used for primary labeling (Fig. 5d). In both assays no signal was recorded for WT samples. The ELISA assays detected significantly higher OD readings in extracts from transplastomic plants than in those from WT plants. Quantification by this method estimated accumulation levels up to 16 μg of Multi-HIV/g of fresh tobacco biomass.
Chloroplast expression of Multi-HIV was further confirmed by confocal microscopy. Leaves from the transplastomic line THIV1 were subjected to an immunohistofluorescence assay using an anti-C4(V3)6 serum, which is expected to recognize the epitopes from the C4 and V3 sequences present in the Multi-HIV. Secondary labeling was performed with an FITC-conjugated antibody. As a marker for locating chloroplasts, samples were analyzed for chlorophyll fluorescence (Ex 488 nm, Em 687 nm). Confocal microscopy revealed strong FITC signals (Ex 488 nm, Em 520 nm) co-localized with the chlorophyll fluorescence. These findings indicate that regulatory sequences from the expression cassette allowed for the successful expression of multi-HIV in chloroplasts. No significant FITC signal was detected in leaves from WT plants (Fig. 6).
Detection of the Multi-HIV in tobacco chloroplasts by immunohistofluorescence. Tobacco leaves from either the WT or THIV1 line of transplastomic tobacco (TT) were labeled with rabbit anti-C4(V3)6 serum and then stained with FITC-conjugated secondary antibody. Laser confocal images showed chloroplasts accumulating Multi-HIV (green signal). Chlorophyll auto-fluorescence was also captured in order to localize chloroplasts (blue signal). The overlap of the two spectra (Merge) is shown in the set of photographs on the far right. Bars 14.83 μm
In order to obtain insight into the immunogenic properties of the tobacco-derived Multi-HIV protein, we performed oral immunization program in BALB/c mice. After antibody content measurement by ELISA, mice immunized with tobacco material from line THIV1 showed significant reactivity against the C4(V3)6 protein whereas a lower signal was attained by the group immunized with WT tobacco (Fig. 7). These findings prove the presence of antibodies recognizing the specific gp120 epitopes present in the C4 and V3 domains, therefore suggesting that the tobacco-derived Multi-HIV has a significant immunogenic activity when orally administered.
Oral immunogenicity of the tobacco-derived Multi-HIV. BALB/c mice experimental groups were orally immunized with phosphate buffered saline (PBS), WT tobacco material (WT) or transplastomic tobacco material from line THIV1 (TT). After three weekly doses, sera was obtained and levels of antibodies against C4 and V3 sequences of HIV gp120 were measured by ELISA analysis. Asterisk denotes significant differences versus the PBS group (p < 0.05)
Discussion
We have designed a Multi-HIV chimeric protein as an strategy to develop an immunogen targeting gp120 and gp41 of the HIV. Although a previously described gp120-based C4(V3)6 has been produced in tobacco by our group, the Multi-HIV proposed here is an effort to develop an improved tool targeting both gp120 and gp41, with the aim of refining the development of multi-target HIV vaccines. This candidate protein was produced in E. coli as a straightforward expression system. However, despite the use of an optimized gene, expression in this host was very low and required concentration by precipitation of the IMAC-positive fractions to obtain detectable amounts in an SDS-PAGE analysis. We hypothesize that the Multi-HIV is seriously degraded when expressed in this host, which is supported by the observation of a prominent band of lower molecular weight in the purified fractions. Since several factors are involved in the expression of heterologous proteins, several directions may be followed in this regard to improve the expression of Multi-HIV. Therefore, we choose to explore the fusion of Multi-HIV to GST as an strategy to improve expression. A remarkably improved production was observed applying this approach, suggesting that poor folding rates and degradation may take place when the single Multi-HIV version is expressed. Intrinsic toxicity of Multi-HIV in E. coli may also explain these observations, however further studies are required to verify this hypothesis.
A major priority of researchers in field of vaccinology is the development of immunization schemes for global immunization programs, especially in poor countries. Since downstream processing required for E. coli-derived recombinant proteins involves high costs for the production of biopharmaceutics, genetically engineered plants may serve as delivery vectors without the need of purification. As such, these plant-based vaccines would serve as economical platforms for the production of low-cost oral vaccines. Significant advances in this field have been made over the last two decades, with promising results obtained in terms of vaccine candidates for a wide variety of human diseases (Lössl and Waheed 2011). In this context, the Multi-HIV was also expressed in tobacco chloroplasts to assess the production potential for this particular antigen. The development of transplastomic tobacco plants is well established, and this technology has been successfully applied in several cases, including the production of functional antigens (Daniell et al. 2005). In our study, particle bombardment allowed us to rescue candidates for transplastomic lines in which the transgene was successfully detected by PCR. PCR analysis revealing the presence of 4.3-kb amplicons proved homoplasty in these lines; these amplicons represent the presence of the foreign DNA of approximately 2 kb in length, while the 2.3-kb amplicons expected for the WT genome were absent. Significantly, immunohistofluorescence provided evidence of chloroplast-specific expression with expression levels estimated as 16 μg of Multi-HIV/g of fresh tobacco biomass. Taken together, these findings indicate that plant chloroplasts can serve as a low-cost and efficient platform for the production of this particular multiepitopic protein.
In terms of antigenicity, the Hopp and Woods method allowed us to identify a number of putative antigenic regions in the Multi-HIV chimera based on the high hydrophilicity score of these regions. Although some regions with low scores were also detected it is important to note that these epitopes with a low degree of hydrophilicity, which are likely to be unexposed in the protein structure, still might be expected to elicit immune responses after being processed and presented to T helper cells via MHC II by the antigen-presenting cells. In addition to the theoretical antigenic potential of the Multi-HIV, western blot analysis, where a positive reaction was observed when labeled with sera from HIV-positive patients, provided evidence for the presence of specific antigenic determinants in the Multi-HIV.
These results indicate that the Multi-HIV is a promising candidate as oral vaccine since it is capable of eliciting humoral responses at the systemic level against V3 sequences without the need of co-administered adjuvants. Future evaluations will be conducted to evaluate the induction of IgA responses in different mucosal compartments.
We have previously produced in E. coli and plant cells two recombinant polypeptides, C4V3 and C4(V3)6, which are based on the C4 and V3 sequences from gp120, and showed that these are immunogenic: they induced systemic and mucosal responses in BALB/c mice without the need of adjuvants (Govea-Alonso et al. 2013; Varona-Santos et al. 2006). Although these chimeric proteins were shown to be a viable strategy to trigger immune responses against gp120, the inclusion of additional well-characterized Env epitopes would provide a broader range of immunity against the whole Env component. The Multi-HIV chimera produced in our study is intended to provide an expanded coverage which would constitute an important contribution to the field as plant-derived chimeric proteins targeting several HIV epitopes have not been extensively explored (Rosales-Mendoza et al. 2012).
In conclusion, our results provide evidence of the successful expression of a new multiepitopic HIV protein in a low-cost platform. This Multi-HIV showed a high antigenic and immunogenic potential when administered orally. Detailed characterization of the biological activity of Multi-HIV is the next logical parameter that must be analyzed to estimate the potential of inducing broad neutralizing antibodies. It is also envisioned that using this technology in edible crops, such as lettuce, which has been transformed efficiently at the chloroplast level (Kanagaraj et al. 2011), will provide new advances in the development of oral formulations obtained with minimal biomass processing. Therefore, this Multi-HIV candidate establishes interesting perspectives as a potential immunogen that could achieve broad neutralization potential and thus serve as an important tool in the field of HIV vaccine development.
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350
Bock R, Timmis JN (2008) Reconstructing evolution: gene transfer from plastids to the nucleus. BioEssays 30:556–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Broliden PA, von Gergerfelt A, Claphamm P, Rosen J, Fenyo EM, Wahren B, Broliden K (1992) Identification of human neutralization-inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc Natl Acad Sci USA 89:461–465
Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot 75:1443–1458
Daniell H, Ruiz ON, Dhingra A (2005) Chloroplast genetic engineering to improve agronomic traits. Methods Mol Biol 286:111–138
Daniell H, Singh ND, Mason H, Streatfield SJ (2009) Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci 14:669–679
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Gaudebout P, Zeliszewski D, Golvano JJ, Pignal C, Le Gac S, Borras-Cuesta F, Sterkers G (1997) Binding analysis of 95 HIV gp120 peptides to HLA-DR1101 and -DR0401 evidenced many HLA-class II binding regions on gp120 and suggested several promiscuous regions. J Acquir Immune Defic Syndr Hum Retrovirol 14:91–101
Geretti AM, Van Baalen CA, Borleffs JC, Van Els CA, Osterhaus AD (1994) Kinetics and specificities of the T helper-cell response to gp120 in the asymptomatic stage of HIV-1 infection. Scand J Immunol 39:355–362
Gottlieb GS, Badiane NM, Hawes SE, Fortes L, Toure M, Ndour CT, Starling AK, Traore F, Sall F, Wong KG, Cherne SL, Anderson DJ, Dye SA, Smith RA, Mullins JI, Kiviat NB, Sow PS (2009) Emergence of multiclass drug-resistance in HIV-2 in antiretroviral-treated individuals in Senegal: implications for HIV-2 treatment in resource-limited West Africa. Clin Infect Dis 48:476–483
Govea-Alonso DO, Gómez-Cardona EE, Rubio-Infante N, García-Hernández AL, Varona-Santos JT, Salgado-Bustamante M, Korban SS, Moreno-Fierros L, Rosales-Mendoza S (2013) Production of an antigenic C4(V3)6 multiepitopic HIV protein in bacterial and plant systems. Plant Cell Tissue Organ Cult 113:73–79
Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, Smith C, Ginsberg R, Eldridge J, Duerr A, Fast P, Haynes BF (2010) Immunization with cocktail of HIV-derived peptides in Montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS ONE 5:e11995
Haynes BF, Benjiang M, Montefiori DC, Wrin T, Petropoulos CJ, Sutherland LL, Scearce RM, Denton C, Xia SM, Kjober BT, Liao HX (2005) Analysis of HIV-1 subtype B third variable region peptide motifs for induction of neutralizing antibodies against HIV-1 primary isolates. Virology 345:44–55
Hemelaar J (2012) Implications of HIV diversity for the HIV-1 pandemic. J Infect 66:391–400
Hoop TP, Woods KR (1981) Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA 78:3824
Huang CY, Ayliffe MA, Timmis JN (2003) Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature 422:72–76
Kamenarova K, Abumhadi N, Gecheff K, Atanas Atanassov A (2005) Molecular farming in plants: an approach of agricultural biotechnology. J Cell Mol Biol 4:77–86
Kanagaraj AP, Verma D, Daniell H (2011) Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol 76:323–333
Kelker HC, Itri VR, Valentine FT (2010) A strategy for eliciting antibodies against cryptic, conserved, conformationally dependent epitopes of HIV envelope glycoprotein. PLoS ONE 5:e8555
Kent SJ, Greenberg PD, Hoffman MC, Akridge RE, McElrath MJ (1997) Antagonism of vaccine-induced HIV-1-specific CD4+ T cells by primary HIV-1 infection: potential mechanism of vaccine failure. J Immunol 158:807–815
Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659
Lössl AG, Waheed MT (2011) Chloroplast-derived vaccines against human diseases: achievements, challenges and scopes. Plant Biotechnol J 9:527–539
Malhotra U, Holte S, Zhu T, Delpit E, Huntsberry C, Sette A, Shankarappa R, Maenza J, Corey L, McElrath MJ (2003) Early induction and maintenance of Env-specific T-helper cells following human immunodeficiency virus type 1 infection. J Virol 77:2663–2674
Mehandru S, Wrin T, Galovich J, Stiegler G, Vcelar B, Hurley A, Hogan C, Vasan S, Katinger H, Petropoulos CJ, Markowitz M (2004) Neutralization profiles of newly transmitted human immunodeficiency virus type 1 by monoclonal antibodies 2G12, 2F5, and 4E10. J Virol 78:14039–14042
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H (1993) A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol 67:6642–6647
Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H (1994) Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol 68:4031–4034
Patterson LJ, Robey F, Muck A, Van Remoortere K, Aldrich K, Richardson E, Alvord WG, Markham PD, Cranage M, Robert-Guroff M (2001) A conformational C4 peptide polymer vaccine coupled with live recombinant vector priming is immunogenic but does not protect against rectal SIV challenge. AIDS Res Hum Retroviruses 17:837–849
Rosales-Mendoza S, Rubio-Infante N, Govea-Alonso DO, Moreno-Fierros L (2012) Current status and perspectives of plant-based candidate vaccines against the human immunodeficiency virus (HIV). Plant Cell Rep 31:495–511
Rubio-Infante N, Govea-Alonso DO, Alpuche-Solís ÁG, García-Hernández AL, Soria-Guerra RE, Paz-Maldonado LM, Ilhuicatzi-Alvarado D, Varona-Santos JT, Verdín-Terán L, Korban SS, Moreno-Fierros L, Rosales-Mendoza S (2012) A chloroplast-derived C4V3 polypeptide from the human immunodeficiency virus (HIV) is orally immunogenic in mice. Plant Mol Biol 78:337–349
Ruf S, Karcher D, Bock R (2007) Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci USA 104:6998–7002
Ruhlman T, Verma D, Samson N, Daniell H (2010) The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol 152:2088–2104
Stegemann S, Hartmann S, Ruf S, Bock R (2003) High-frequency gene transfer from the chloroplast genome to the nucleus. Proc Natl Acad Sci USA 100:8828–8833
Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87:8526–8530
Timmis JN, Ayliffe MA, Huang CY, Martin W (2004) Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet 5:123–135
Tiwari S, Verma PC, Singh PK, Tuli R (2009) Plants as bioreactors for the production of vaccine antigens. Biotechnol Adv 27:449–467
Vanini S, Longhi R, Lazzarin A, Vigo E, Siccardi AG, Viale G (1993) Discrete regions of HIV-1 gp41 defined by syncytia-inhibiting affinity-purified human antibodies. AIDS 7:167–174
Varona-Santos JT, Vazquez-Padrón RI, Moreno-Fierros L (2006) Production of a short recombinant C4V3 HIV-1 immunogen that induces strong anti-HIV responses by systemic and mucosal routes without the need of adjuvants. Viral Immunol 19:237–249
Wahren B, Rosen J, Sandström E, Mathiesen T, Modrow S, Wigzell H (1989) HIV-1 peptides induce a proliferative response in lymphocytes from infected persons. J Acquir Immune Defic Syndr 2:448–456
Wang HH, Yin WB, Hu ZM (2009) Advances in chloroplast engineering. J Genet Genomics 36:387–398
Warrier SV, Pinter A, Honnen WJ, Girard M, Muchmore E, Tilley SA (1994) A novel, glycan-dependent epitope in the V2 domain of human immunodeficiency virus type 1 gp120 is recognized by a highly potent, neutralizing chimpanzee monoclonal antibody. J Virol 68:4636–4642
Zou Z, Eibl C, Koop HU (2003) The stem-loop region of the tobacco psbA 5’UTR is an important determinant of mRNA stability and translation efficiency. Mol Genet Genomics 269:340–349
Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW (2001) Broadly neutralizing antibodies targeted to the membrane proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol 75:10892–10905
Acknowledgments
This research was funded by grants 102109 from CONACYT and 173858 from CIBIOGEM to SRM.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Sergio Rosales-Mendoza and Néstor Rubio-Infante contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rosales-Mendoza, S., Rubio-Infante, N., Monreal-Escalante, E. et al. Chloroplast expression of an HIV envelop-derived multiepitope protein: towards a multivalent plant-based vaccine. Plant Cell Tiss Organ Cult 116, 111–123 (2014). https://doi.org/10.1007/s11240-013-0387-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0387-y