Abstract
Alstroemeria is multiplied in vitro by forced outgrowth of lateral rhizomes from rhizome explants. The multiplication rate is very low because of strong apical dominance and poor rhizome growth. We report here that moderate abiotic stresses stimulate both rhizome growth and outgrowth of lateral rhizomes, and accordingly increase multiplication. Rhizome explants were exposed to heat by a hot-water treatment (HWT) or by a hot-air treatment. Both increased rhizome growth when applied for 1 or 2 h in the range of 30–40 °C. The maximal enhancement was 75 %. Other abiotic stresses were also examined. Cold (0 °C) and partial anaerobiosis increased rhizome growth significantly. The increases brought about by drought and salinity were not statistically significant. Because underground storage tissues like rhizomes are adaptations to survive climatic stresses, we presume that the increased sink-strength of rhizomes induced by moderate stress is related to stress adaptation. Moderate heat stress (38 °C HWT, 1 h) also resulted in protection of Alstroemeria plantlets from severe heat stress (45 °C HWT, 1–2 h) a few hours after the moderate stress. All abiotic stresses also increased the outgrowth of lateral rhizomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alstroemeria hybrids are grown as cut flowers and bedding plants, and more recently also for pot plant production (Bridgen 1997). Alstroemeria occupies the 7th position in the top ten of cut flowers sold worldwide (Flower Magazine 2011). For large-scale vegetative propagation of Alstroemeria micropropagation is the preferred method (Pedersen et al. 1996) and Alstroemeria ranks at the 2nd position of micropropagated cut flowers (Pierik and Ruibing 1997). Propagation in vitro is achieved by stimulating the outgrowth of axillary buds to lateral rhizomes. Even though propagation in vitro is much faster than in the greenhouse, the propagation factor in vitro is still very low and on average each rhizome produces per cycle of 4 weeks less than one lateral rhizome that is sufficiently large to separate from the main rhizome (Pumisutapon et al. 2011). This is caused by strong apical dominance and poor rhizome growth.
According to Alstroemeria growers, rhizome growth strongly increases in greenhouse plants after abiotic stress, e.g., a change of temperature or fertilizer (R. Veenhof, Könst Alsroemeria personal communication). Storage of resources in a “safe” organ to enable fast growth when the stress has ended is a widespread adaptation to stress (Chapin III et al. 1990).
In a previous paper, we examined apical dominance in Alstroemeria cultured in vitro (Pumisutapon et al. 2011). In the present article, we show the positive effects of moderate stresses (heat, cold, drought, anaerobiosis and salt) on rhizome growth and multiplication of Alstroemeria cultured in vitro.
Materials and methods
Plant material and growth conditions
Cultures of the Alstroemeria hybrid ‘4962 2G’ were obtained from Könst Alstroemeria (Nieuwveen, the Netherlands) and were used in most experiments. Two other cultivars, ‘24098 2B’ (Könst Alstroemeria, Nieuwveen, The Netherlands) and ‘Sara’ (Van Zanten, Rijsenhout, The Netherlands), were used to examine applicability of the results in other Alstroemeria cultivars. The medium used for propagation of the stock and for all experiments comprised MS salts and vitamins (Murashige and Skoog 1962), 9 μM 6-benzyladenine (BA), 4 % sucrose and 0.2 % (w/v) gelrite. The pH was adjusted to 5.8 before autoclaving (121 °C for 20 min). The cultures were grown at 19 °C and 16 h light per day (30 μmol m−2 s−1, Phillips TL 33). In all experiments, standard explants consisting of an intact rhizome with two decapitated shoots were used (Pumisutapon et al. 2011).
Heat treatments
For hot-water treatment (HWT), the explants were transferred to a polystyrene jar (ø 66 mm) with sterile water (5 explants in 25 ml; water level ca. 7 mm) and incubated in a water bath (Lauda Bath Circulator C12). The HWT-temperatures were held within 0.3 °C. For hot-air treatment (HAT), the explants were placed in jars with standard solidified medium (5 explants on 25 ml) or in jars without medium, and the jars with explants were placed in an incubator (Labcon LTIM 10). The HAT-temperatures were held within 1.0 °C. The explants were cultivated on standard medium after the heat treatments.
The influence of pretreatments with HWT was investigated using the procedure for Arabidopsis seedlings (De Klerk and Pumisutapon 2008). Explants were pretreated at 38 °C for 0 and 1 h. After that they were kept on standard medium at room temperature for 4 h before giving severe heat stress at 45 °C for 0, 1, 2 and 3 h.
Other stresses
Other abiotic stresses included cold, anaerobiosis, drought and salinity. For cold stress, the explants were submerged in sterile water (5 explants in 25 ml) and incubated in the dark at 0 °C in a foam box containing ice for 0, 4, 8 or 24 h. For partial anaerobiosis, the explants were submerged in sterile water (5 explants in 25 ml) and kept in the dark at 19 °C for 0, 8, 24, 72 or 216 h. Drought stress was given by keeping the explants on dry filter paper in the laminar air flow cabinet for 0, 0.5, 1 or 2 h. Salinity stress was carried out by submerging the explants in a NaCl solution (500 mM; 5 explants in 25 ml) for 0, 1, 2 or 4 h and rinsing with sterile water before transfer to standard medium.
Statistical analysis
In each determination, three jars (ø 66 mm) containing 25 ml medium with five explants each were used. Rhizome growth (fresh weight increase) and multiplication (the number of new lateral rhizomes; see Pumisutapon et al. 2011) were determined after 4 weeks. After severe stress treatments some explants died. In these treatments, the means refer to the surviving explants only. In the figures, means of 15 determinations ± SEs are shown. For rhizome growth, dry weight increase was also determined and the results were the same as for fresh weight. The means were evaluated with the Student t test.
Results
Effect of abiotic stresses on rhizome growth
All explants survived a HWT when treated for 1 or 2 h at temperatures up to 42.5 °C. However, after 2 h at 42.5 °C rhizome growth decreased indicating persistent damage (Fig. 1a). Temperatures between 36 and 39 °C increased rhizome growth maximally by ca. 50 % compared to the control. The 2 h HWT gave a better result than the 1 h HWT (Fig. 1a).
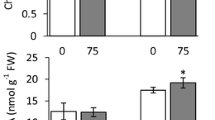
Rhizome growth after a HWT of rhizome explants at increasing temperatures for 1 and 2 h in 25 ml water (a). Rhizome growth after a HAT of rhizome explants at different temperatures for 1 h. HAT was given to explants in jars without or with medium (b). After the HAT and HWT, the explants were grown on standard medium for 4 weeks
Since explants were submerged during the HWT, they may have suffered from anaerobiosis. To examine this, the explants were submerged in increasing water volumes during the HWT. In 5 ml, the lowest volume tested (water level ca. 1.5 mm), the explants were only partially submerged and the highest increase of rhizome growth was achieved. In 25 ml (water level ca. 7 mm), the explants were just submerged and rhizome growth was only a little more than in the control (P < 0.1). In 100 ml water, the explants were completely submerged (water level ca. 30 mm); the rhizomes did grow significantly less than the control (P < 0.01). Moreover, many explants died (data not shown).
For HAT, explants were incubated at a range of temperatures for 1 h either without or with solid medium in the jars. Figure 1b shows that HAT between 30 and 40 °C increased rhizome growth. When the plants were given a HAT in a jar without medium, rhizome growth increased less. Maximum growth promotion (75 %) was reached for the 1 h treatment at 35 °C with solid medium in the jar. When the temperature was increased up to 50 °C, many explants died (data not shown) and the surviving rhizomes decreased in weight (Fig. 1b).
To check whether the positive effect of mild heat stress was limited to the cultivar used in the present examinations (‘4962 2G’), two other cultivars, ‘24098 2B’ and ‘Sara’, were tested. They showed similar results (data not shown).
We also examined other moderate abiotic stresses, namely cold (0 °C), partial anaerobiosis, drought and salinity. All resulted in increased rhizome growth. Under the stress conditions used, cold stress gave the highest promotion of growth (97 %; P < 0.0005). Partial anaerobiosis promoted rhizome growth by maximally 38 % (P < 0.005), drought stress by 35 % (not significant; P = 0.30), and salinity stress by 14 % (not significant; P = 0.39). Protracted stress treatments resulted in less growth promotion and often in death. In Fig. 2, the effects of cold stress and anaerobiosis stress are shown.
After all moderate stresses, shoot growth did not increase but actually often decreased (data not shown).
Moderate HWT protects against severe heat stress
We tested whether a moderate heat treatment also renders rhizome explants resistant to a severe stress given briefly after the moderate stress. The explants were given a 1 h HWT at 38 °C and 4 h later a severe HWT at 45 °C for 1, 2 or 3 h. Figure 3 shows survival after 1 week. Almost all rhizomes that received the HWT at 38 °C survived a 1 h HWT at 45 °C, whereas almost all control rhizomes (no preceding HWT at 38 °C) died (P < 0.0001). After 2 h HWT at 45 °C, the control rhizomes died for 100 %, whereas 40 % (P < 0.005) of the rhizomes pretreated with a HWT at 38 °C survived. Only after 3 h at 45 °C, also 100 % of the pretreated rhizomes died.
Effect of abiotic stresses on rhizome propagation
Besides enhancing rhizome growth, abiotic stresses also increased the outgrowth of lateral rhizomes, but the promotive effect was in most cases smaller. The optimal conditions for the different stresses were a 1 h HWT at 38 °C (50 % increase), a 1 h HAT at 35 °C (52 % increase), a 0.5 h drought treatment (23 % increase), an 8 h cold treatment at 0 °C (23 % increase), and a 1 h salinity treatment (67 % increase) (for all P < 0.05). Only anaerobiosis did not result in a significant increase. As an example, the effect of HWT is shown (Fig. 4). It should be noted that whereas the effect of stresses on rhizome growth was relatively stable in consecutive experiments, the effect on lateral rhizome outgrowth was more variable.
Discussion
Improvement of rhizome growth
When plants are exposed to a moderate stress, they may initiate a response that renders them resistant to a future severe stress. This has been observed for, e.g., moderate drought (Villar-Salvador et al. 2004) and heat stress (De Klerk and Pumisutapon 2008) and occurs both in vitro and in vivo. Protection is instantly but short-lived. Plants have also protection strategies for protracted adverse periods. Thus, they develop dormancy and form storage organs to survive an unfavorable season and to ascertain fast regrowth after that. Many plants that grow in stressful habitats have increased mass allocation to the storage organs (Chapin III et al. 1990; Ryser and Urbas 2000; Hutchings and John 2004; Puijalon et al. 2005; Puijalon et al. 2008). Alstroemeria responds to moderate heat stress similarly by increasing rhizome growth. Since shoot growth did not increase but actually often decreased after the moderate stress, the moderate stress did cause a specific increase of the sink activity of the rhizome. To the best of our knowledge, there are no other reports on enhancement of storage organ growth (tubers, bulbs, corms or rhizomes) by a moderate stress. It has been reported that growth of storage organs is reduced by severe stress. Heat stress lowers potato tuber yields by reduced partitioning to the tubers and by reduced photosynthesis (Ewings 1981). Growth of onion (Allium cepa L.) bulbs is reduced by salinity stress with increasing NaCl concentration up to 100 mM (Chang and Randle 2004).
The mechanism by which the moderate stress enhances sink activity of rhizomes is not known. Signaling molecules which have been reported to regulate plant responses to heat stress include abscisic acid, salicylic acid, ethylene and hydrogen peroxide (Larkindale and Huang 2005), calcium ions (Knight 2000), and jasmonic acid (Clarke et al. 2009). Among these signaling molecules, abscisic acid (Kim et al. 1994), ethylene and jasmonic acid (Jásik and De Klerk 2006; Rayirath et al. 2011) play an important role in plant storage organ formation.
Hot-water and hot-air stresses
Heat stress was applied by HWT or by HAT. For HWT, the explants were transferred to a small jar with water and the jar was placed in a water bath. For HAT, the tissue culture container was transferred into an incubator. Both efficiently improved growth and multiplication of Alstroemeria rhizomes (Figs. 1, 4) and a HWT gave a slightly better effect (data not shown). Possibly better heat conduction of water and additional moderate stress by partial anaerobiosis during HWT are involved (Jackson 1985; Dolferus et al. 2003). In this respect it should also be noted that it is difficult to control the temperature within an incubator and that an incubator should be used with sufficient air circulation. Accurate temperature control is highly important, because the effect of temperature may depend on a few °C (in Arabidopsis seedlings, a rise of 1 °C for 2 h leads to an increase of the death rate from 10 to 20 % to almost 100 %; R. Stolker and G. J. De Klerk, unp. results).
When the tissues are submerged during the HWT, gas exchange is inhibited (Jackson 1985) whereas the need for gas exchange has enhanced because the high temperature increases the metabolic rate and consequently the production of CO2 and the requirement for O2. Moreover, the solubility of gases decreases with temperature (by ca. 1/3 when the temperature increases from 20 to 40 °C, Anonymus 2012). The importance of adequate uptake and emission of gases by submerged plant tissue during a HWT, is demonstrated by a sharp decrease of survival of Arabidopsis seedlings when the number of seedlings per tube increases (De Klerk and Pumisutapon 2008). In Alstroemeria, increasing the water volume resulted in a reduction of rhizome growth and in increased incidence of death (data not shown). Explants were totally submerged in the highest water volume (100 ml), but only partially in the lowest water volume (5 ml).
Enhancement of lateral rhizome outgrowth
Abiotic stresses also increased rhizome multiplication. In bulbous plants, abiotic stress such as heat increases the formation of bulbs and roots of chives (Fölster and Krug 1977). A short period of heat stress (32/25 °C day/night for five consecutive days) given to greenhouse grown potatoes decreases apical dominance and results in the development of many branches (Hammer et al. 1989). In pineapple, a HWT (6 or 8 min at 60 °C) of dormant axillary buds before transfer to nutrient medium increases the percentage bud-responsiveness and shoot emergence (Broomes and McEwan 1994). In the tropical fruit tree Hancornia speciosa, the multiplication rate of shoot cultures is low because of strong apical dominance that cannot be overcome by cytokinin treatment. However, an increase in culture temperature from 31 to 35 °C over a 4-week period suppresses elongation and induces branching of all shoots, and a two-week period at 35 °C followed by culture at 31 °C leads to vigorous branching (De Pereira-Netto and McCown 1999). In red raspberry (Rubus idaeus L.), endodormancy is released after a HWT at 45 °C for 1 h (Rantanen and Palonen 2010). A heat treatment is also used to break dormancy in seeds (Farooq et al. 2008). These observations indicate that a heat treatment induces bud outgrowth and can overcome apical dominance. Abiotic stresses may cause an increase in endogenous cytokinin level in plants accompanied by down-regulation of the activity of the main cytokinin degrading enzyme cytokinin oxidase/dehydrogenase (Rivero et al. 2009; Dobra et al. 2010). Abiotic stresses may also reduce the activity of the apical bud thereby reducing its ability to inhibit outgrowth of the axillary bud. The outgrowth of daughter bulbs in tulip after a heat treatment is an extreme example as in this case the apical bud often deceases (Rees 1971).
Conclusion
Moderate stress is a usable method to enhance the growth of Alstroemeria rhizomes in vitro and an increase of 70 % may be obtained. The increase of growth is likely based on a protective adaptation by the rhizomes. The outgrowth of axillary buds into lateral rhizomes is promoted as well by moderate stress. Before commercial use, though, possible after-effects of the treatments should be examined first.
Abbreviations
- BA:
-
6-Benzyladenine
- HAT:
-
Hot-air treatment
- HWT:
-
Hot-water treatment
- MS:
-
Murashige and Skoog
References
Anonymus (2012) Solubility of gases in water. http://www.engineeringtoolbox.com/gases-solubility-water-d_1148.html. Accessed 16 Mar 2012
Bridgen MP (1997) Alstroemeria. In: Ball V (ed) Ball red book, 16th edn. Geoge J. Ball Publishing Company, West Chicago, pp 341–348
Broomes VFA, McEwan FA (1994) Heat treatment for enhanced responsiveness of dormant axillary buds of pineapples. Turrialba 44:117–121
Chang PT, Randle WM (2004) Sodium chloride in nutrient solutions can affect onion growth and flavor development. HortScience 39:1416–1420
Chapin FS III, Schulze E-D, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Clarke SM, Cristescu SM, Miersch O, Harren FJM, Wasternack C, Mur LAJ (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187. doi:10.1111/j.1469-8137.2008.02735.x
De Klerk G-J, Pumisutapon P (2008) Protection of in vitro grown Arabidopsis seedlings against abiotic stresses. Plant Cell Tissue Organ Cult 95:149–154. doi:10.1007/s11240-008-9426-5
De Pereira-Netto AB, McCown BH (1999) Thermally induced changes in shoot morphology of Hancornia speciosa microcultures: evidence of mediation by ethylene. Tree Physiol 19:733–740
Dobra J, Motyka V, Dobrev P, Malbeck J, Prasil IT, Haisel D, Gaudinova A, Havlova M, Gubis J, Vankova R (2010) Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J Plant Physiol 167:1360–1370. doi:10.1016/j.jplph.2010.05.013
Dolferus R, Klok EJ, Delessert C, Wilson S, Ismond KP, Good AG, Peacock WJ, Dennis ES (2003) Enhancing the anaerobic response. Ann Bot 91:111–117. doi:10.1093/aob/mcf048
Ewing EE (1981) Heat stress and the tuberization stimulus. Am Potato J 58:31–49
Farooq M, Basra SMA, Saleem BA, Nafees M (2008) Germination, seedling vigor and electrical conductivity of seed leachates as affected by dry heat treatment of tomato seeds. Acta Hortic 771:43–50
Flower Magazine (2011) Top ten cut flowers and their meaning. http://www.flowers-magzine.com/top_ten_cut_flowers. Accessed 16 Mar 2012
Fölster E, Krug H (1977) Influence of the environment on growth and development of chives (Allium schoenoprasum L.). II. Breaking of the rest period and forcing. Sci Hortic 7:213–224
Hammer PS, Beyers EA, Birnbaum H (1989) The effect of short periods of heat stress on the growth and yield of potatoes. S Afr J Plant Soil 6:215–217
Hutchings MJ, John EA (2004) The effects of environmental heterogeneity on root growth and root/shoot partitioning. Ann Bot 94:1–8. doi:10.1093/aob/mch111
Jackson MB (1985) Ethylene and responses of plants to soil waterlogging and submergence. Annu Rev Plant Physiol 36:145–174
Jásik J, De Klerk G-J (2006) Effect of methyl jasmonate on morphology and dormancy development in lily bulblets regenerated in vitro. J Plant Growth Regul 25:45–51. doi:10.1007/s00344-005-0048-4
Kim K-S, Davelaar E, De Klerk G-J (1994) Abscisic acid controls dormancy development and bulb formation in lily plantlets regenerated in vitro. Physiol Plant 90:59–64
Knight H (2000) Calcium signaling during abiotic stress in plants. Int Rev Cytol 195:269–324
Larkindale J, Huang B (2005) Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul 47:17–28. doi:10.1007/s10725-005-1536-z
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Pedersen C, Hansen CW, Brandt K, Kristiansen K (1996) Alstroemeria plantlets can be induced to flowering by cold treatment during in vitro culture. Sci Hortic 66:217–228
Pierik RLM, Ruibing MA (1997) Developments in the micropropagation industry in the Netherlands. Plant Tissue Cult Biotechnol 3:152–156
Puijalon S, Bornette G, Sagnes P (2005) Adaptations to increasing hydraulic stress: morphology, hydrodynamics and fitness of two higher aquatic plant species. J Exp Bot 56:777–786. doi:10.1093/jxb/eri063
Puijalon S, Piola F, Bornette G (2008) Abiotic stresses increase plant regeneration ability. Evol Ecol 22:493–506. doi:10.1007/s10682-007-9177-5
Pumisutapon P, Visser RGF, De Klerk GJ (2011) Hormonal control of the outgrowth of axillary buds in Alstroemeria cultured in vitro. Biol Plant 55:664–668. doi:10.1007/s10535-011-0165-9
Rantanen M, Palonen P (2010) Hot water treatment released endodormancy but reduced number of flowers in potted red raspberry plants. HortScience 45:894–898
Rayirath UP, Lada RR, Caldwell CD, Asiedu SK, Sibley KJ (2011) Role of ethylene and jasmonic acid on rhizome induction and growth in rhubarb (Rheum rhabarbarum L.). Plant Cell, Tissue Organ Cult 105:253–263. doi:10.1007/s11240-010-9861-y
Rees AR (1971) Factors affecting the growth of daughter bulbs in tulip. Ann Bot 35:43–55
Rivero RM, Shulaev V, Blumwald E (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150:1530–1540. doi:10.1104/pp.109.139378
Ryser P, Urbas P (2000) Ecological significance of leaf life span among Central European grass species. Oikos 91:41–50
Villar-Salvador P, Planelles R, Oliet J, Peñuelas-Rubira JL, Jacobs DF, González M (2004) Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol 24:1147–1155
Acknowledgments
We thank the Royal Thai Government and the Productchap Tuinbouw (The Netherlands) for financial support, and Könst Alstroemeria (Nieuwveen, The Netherlands) and Van Zanten Plants (Rijsenhout, the Netherlands) for the plant material. We thank Yanru Song and Monica Du for their assistance in some experiments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pumisutapon, P., Visser, R.G.F. & de Klerk, GJ. Moderate abiotic stresses increase rhizome growth and outgrowth of axillary buds in Alstroemeria cultured in vitro. Plant Cell Tiss Organ Cult 110, 395–400 (2012). https://doi.org/10.1007/s11240-012-0160-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0160-7