Abstract
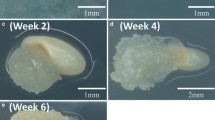
To optimize Agrobacterium tumefaciens-mediated transformation, factors influencing gene delivery, selection of transformed cells, and plant regeneration were investigated using two major switchgrass cultivars, including a lowland tetraploid cultivar Alamo and an upland octoploid cultivar Cave-in-Rock (CIR). Transient expression studies monitored by histochemical β-glucuronidase assay in seedling segments indicated that A. tumefaciens strain EHA105 was more effective in gene delivery than LBA4404 or GV3101. Of three major selectable genes, the bialaphos resistance (bar) gene and the hygromycin phosphotransferase (hpt) allowed effective selection of transformed cells using 2 mg l−1 glufosinate ammonium herbicide and 50 mg l−1 hygromycin, respectively; whereas the neomycin phosphotransferase II gene did not yield effective selection using 100 mg l−1 kanamycin. Herbicide- or hygromycin-resistant calluses were induced from seedling segments after 2–3 months of selection. Transformants of ‘Alamo’ with the bar or hpt were obtained 3–4 weeks after the resistant calluses were transferred onto regeneration medium; in contrast, no regenerant was produced from the calluses of ‘CIR’. Most of transformants showed normal growth in the greenhouse. Low percentages of mature seeds ranging from 1.7 to 8.7% of husks were obtained from open pollinated plants. Southern blot analysis confirmed stable integration of the bar in selected T0 transformants. Reverse transcription PCR and herbicide/hygromycin tolerance tests indicated expression of transgenes. The optimized transformation protocol using basal parts of seedling as explants shortened the process by 4–5 weeks, and it has potential use for transformation of other switchgrass cultivars.





Similar content being viewed by others
References
Bouton JH (2007) Molecular breeding of switchgrass for use as a biofuel crop. Curr Opin Genet Dev 17:553–558
Bregitzer P, Campbell RD (2001) Genetic markers associated with green and albino plant regeneration from embryogenic barley callus. Crop Sci 41:173–179
Burris JN, Mann DGJ, Joyce BL, Stewart CN Jr (2009) An improved tissue culture system for embyrogenic callus production and plant regeneration in switchgrass (Panicum virgatum L.). BioEnergy Res 2:267–274
Cabral GB, Carneiro VTC, Lacerda AL, do Valle CB, Martinelli AP, Dusi DMA (2011) Somatic embryogenesis and organogenesis in apomictic and sexual Brachiaria brizantha. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-011-9978-7
Chen X, Equi R, Baxter H, Berk K, Han J, Agarwal S, Zale J (2010) RA high-throughput transient gene expression system for switchgrass (Panicum virgatum L.) seedlings. Biotechnol Biofuels 3:9
Creemers-Molenaar J, Loeffen JPM, van Rossum M, Colijn-Hooymans CM (1992) The effect of genotype, cold storage and ploidy level on the morphogenic response of perennial ryegrass (Lolium perenne L.) suspension cultures. Plant Sci 83:87–94
Day A, Ellis T (1985) Deleted forms of plastid DNA in albino plants from cereal anther culture. Current Genet 9:671–678
Denchev PD, Conger BV (1995) In vitro culture of switchgrass: influence of 2, 4-D and picloram in combination with benzyladenine on callus initiation and regeneration. Plant Cell Tiss Org Cult 40:43–48
Doğramacı-Altuntepe M, Peterson TS, Jauhar PP (2001) Anther culture-derived regenerants of durum wheat and their cytological characterization. J Hered 92:56–64
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Elbersen HW, Christian DG, El Bassen N, Bacher W, Sauerbeck G, Aleopoulou E, Sharma N, Piscioneri I, De Visser P, Van Den Berg D (2001) Switchgrass variety choice in Europe. Aspects Appl Biol 65:21–28
Fike JH, Parrish DJ, Wolf DD, Balasko JA, Green JT Jr, Rasnake M, Reynolds JH (2006) Switchgrass production for the upper southeastern USA: influence of cultivar and cutting frequency on biomass yields. Biomass Bioenergy 30:207–213
Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, Rodriguez M, Chen F, Foston M, Ragauskas A, Bouton J, Dixon R, Wang Z-Y (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. PNAS 108:3803–3808
Gressel J (2008) Transgenics are imperative for biofuel crops. Plant Sci 174:246–263
Hoekema A, Hirsch PR, Hooykaas JJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Hopkins AA, Vogel KP, Moore KJ, Johnson KD, Carlson IT (1995) Genotypic effects and genotype environment interactions for traits of elite switchgrass populations. Crop Sci 35:125–131
Jefferson R (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Bio Rep 5:387–405
Jha P, Shashi S, Rustagi A, Agnihotri P, Kulkarni V, Bhat V (2011) Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explant source. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-011-0001-0
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol General Genet 204:383–396
Kumar V, Campbell LM, Rathore KS (2011) Rapid recovery- and characterization of transformants following Agrobacterium-mediated T-DNA transfer to sorghum. Plant Cell Tissue Organ Cult 104:137–1466
Li R, Qu R (2010) High throughput Agrobacterium-mediated switchgrass transformation. Biomass Bioenergy 35:1046–1054
McLaughlin SB, Kiniry JR, Taliaferro CM, De La Torre Ugarte D, Donald LS (2006) Projecting yield and utilization potential of switchgrass as an energy crop. Adv Agron 90:267–297
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Ni M, Cui D, Einstein J, Narasimhulu S, Vergara CE, Gelvin SB (1995) Strength and tissue specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J 7:661–676
Okubara PA, Blechl AE, McCormick SP, Alexander NJ, Dill-Macky R, Hohn TM (2002) Engineering deoxynivalenol metabolism in wheat through the expression of a fungal trichothecene acetyltransferase gene. Theor Appl Genet 106:74–83
Odjakova MK, Conger BV (1999) The influence of osmotic pretreatment and inoculum age on the initiation and regenerability of switchgrass suspension cultures. In Vitro Cell Biol-Plant 35:442–444
Richards HA, Rudas VA, Sun H, McDaniel JK, Tomaszewski Z, Conger BV (2001) Construction of a GFP-BAR plasmid and its use for switchgrass transformation. Plant Cell Rep 20:48–54
Seo M, Takahara M, Takamizo T (2010) Optimization of culture conditions for plant regeneration of Panicum spp. through somatic embryogenesis. Grassl Sci 56:6–12
Seo MS,Takahashi S, Kadowaki KI, Kawamukai M, Takahara M, Takamizo T (2011) Expression of CoQ10-producing ddsA transgene by efficient Agrobacterium-mediated transformation in Panicum meyerianum. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-011-9984-9
Sharma M, Chajer AK, Chugh SJ, Kothari SL (2011) Factors influencing Agrobacterium tumefaciens-mediated genetic transformation of Eleusine coracana (L.) Gaertn. Plant Cell Tissue Organ Cult 105:93–104
Somleva MN (2006) Switchgrass (Panicum virgatum L.). In: Wang K (ed) Agrobacterium protocols. Humana Press, Totowa, pp 65–74
Somleva MN, Tomaszewski Z, Conger BV (2002) Agrobacterium- mediated genetic transformation of switchgrass. Crop Sci 42:2080–2087
Somleva MN, Snell KD, Beaulieu JJ, Peoples OP, Garrison BR, Patterson NA (2008) Production of polyhydroxybutyrate in switchgrass, a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnol J 6:663–678
Vogel KP (2004) Switchgrass. In: Moser LE, Burson BL, Sollenberger LE (eds) Warm-season (C4) grasses. American Society of Agronomy, Washington, pp 561–588
Xi Y, Fu C, Ge Y, Nandakumar R, Hisano H, Bouton J, Wang Z (2009) Agrobacterium-mediated transformation of switchgrass and inheritance of the transgenes. Bioenerg Res 2:275–283
Zhang K, Wang J, Hu X, Yang A, Zhang J (2010) Agrobacterium-mediated transformation of shoot apices of Kentucky bluegrass(Poa pratensis L.) and production of transgenic plants carrying a betA gene. Plant Cell Tissue Organ Cult 102:135–143
Acknowledgments
This research was partly supported by MSU Project GREEEN (Generating Research and Extension to Meet Economic and Environmental Needs).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Gq., Walworth, A. & Hancock, J.F. Factors influencing Agrobacterium-mediated transformation of switchgrass cultivars. Plant Cell Tiss Organ Cult 108, 445–453 (2012). https://doi.org/10.1007/s11240-011-0056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-0056-y