Abstract
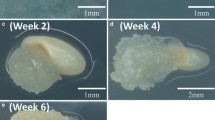
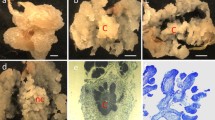
The increased emphasis on research of dedicated biomass and biofuel crops begs for biotechnology method improvements. For switchgrass (Panicum virgatum L.), one limitation is inefficient tissue culture and transformation systems. The objectives of this study were to investigate the utility of a new medium described here, LP9, for the production and maintenance of switchgrass callus and its regeneration, which also enables genetic transformation. LP9 medium is not based on Murashige and Skoog (MS) medium, the basal medium that all published switchgrass transformation has been performed. We demonstrate an efficient tissue culture system for switchgrass Alamo 2, which yields increased viability of callus and the ability to maintain callus for a duration of over 6 months. This longevity gives a greater useful callus lifetime than for published switchgrass MS-based media. This increased longevity enables greater potential efficiency and throughput for a transformation pipeline. Callus produced on LP9 is categorized as type II callus, which is more friable and easier to multiply, maintain and transfer than type I callus obtained from previously described tissue culture systems.





Similar content being viewed by others
Abbreviations
- BA:
-
Benzyladenine
- BAP:
-
6-Benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- SEM:
-
Scanning electron microscopy
- TDZ:
-
Thidiazuron
References
Alexandrova KS, Denchev PD, Conger BV (1996) In vitro development of inflorescences from switchgrass nodal segments. Crop Sci 36:175–178
Alexandrova KS, Denchev PD, Conger BV (1996) Micropropagation of switchgrass by node culture. Crop Sci 36:1709–1711
Denchev PD, Conger BV (1994) Plant regeneration from callus cultures of switchgrass. Crop Sci 34:1623–1627
Denchev PD, Conger BV (1995) In vitro culture of switchgrass: influence of 2,4-D and picloram in combination with benzyladenine on callus initiation and regeneration. Plant Cell Tissue Organ Cult 40:43–48
Dutta Gupta S, Conger BV (1999) Somatic embryogenesis and plant regeneration from suspension cultures of switchgrass. Crop Sci 39:243–247
Dutta Gupta S, Conger BV (1998) In vitro differentiation of multiple shoot clumps from intact seedlings of switchgrass. In Vitro Cell Dev Biol-Plant 34:196–202
Seo M-S, Takahara M, Ebina M, Takamizo T (2008) Evaluation of tissue culture response from mature seeds of Panicum spp. Grassland Sci 54(3):125–130
Armstrong CI, Green CE (1985) Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 164:207–214
Assam SK (2001) Callus production and plant regeneration in Egyptian maize genotypes. Arab J Biotechnol 4:247–256
Frame BR, Zhang H, Cocciolone SM, Sidorenko LV, Dietrich CR, Pegg SE et al (2000) Production of transgenic maize from bombarded type II callus: effect of gold particle size and callus morphology on transformation efficiency. In Vitro Cell Dev Biol-Plant 36:21–29
Lu C, Vasil IK, Ozias-Akins P (1982) Somatic embryogenesis in Zea mays L. Theor Appl Genet 62:109–112
McCain JW, Kamo KK, Hodges TK (1988) Characterization of somatic embryo development and plant regeneration from friable maize callus cultures. Bot Gazette 149:16–20
Songstad DD, Petersen WL, Armstrong CL (1992) Establishment of friable embryogenic (type II) callus from immature tassels of Zea mays (Poaceae). Am J Bot 79:761–764
Welter ME, Clayton DS, Miller MA, Petolino JF (1995) Morphotypes of friable embryogenic maize callus. Plant Cell Rep 14:725–729
Chen T-S, Lam L, Chen S-C (1985) Somatic embryogenesis and plant regeneration from cultured young inflorescences of Oryza sativa L. (rice). Plant Cell Tissue Organ Cult 4:51–54
Nakamura T, Maeda E (1989) A scanning electron microscope study on Japonica type rice callus cultures, with emphasis on plantlet initiation. Japan J Crop Sci 58:395–403
Rueb S, Leneman R, Schilperoort RA, Hesngens LAM (1994) Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.). Plant Cell Tissue Organ Cult 36:259–264
Jeoung JM, Krishnaveni S, Muthukrishnan S, Trick HN, Liang GH (2002) Optimization of sorghum transformation parameters using genes for green fluorescent protein and β-glucuronidase as visual markers. Hereditas 137:20–28
Guiderdoni E, Demarly Y (1988) Histology of somatic embryogenesis in cultured leaf segments of sugarcane plantlets. Plant Cell Tissue Organ Cult 14:71–88
Redway FA, Vasil V, Lu D, Vasil IK (1990) Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.). Theor Appl Genet 79:609–617
Bajaj YPS, Sidhu BS, Dubey VK (1981) Regeneration of genetically diverse plants from tissue cultures of forage grass—Panicum spp. Euphytica 30:135–140
Chaudhury A, Qu R (2000) Somatic embryogenesis and plant regeneration of turf-type bermudagrass: effects of 6-benzyladenine in callus induction medium. Plant Cell Tissue Organ Cult 60:113–120
Lu C, Vasil IK (1981) Somatic embryogenesis and plant regeneration from leaf tissue of Panicum maximum Jacq. Theor Appl Genet 59:275–280
Lu C-Y, Vasil IK (1981) Somatic embryogenesis and plant regeneration from freely-suspended cells and cell groups of Panicum maximum Jacq. Ann Bot 48:543–548
Lu C-Y, Vasil IK (1985) Histology of somatic embryogenesis in Panicum maximum (guinea grass). Amer J Bot 72:1908–1913
Zhang S, Hanna W, Ozias-Akins P (2007) Comparison of callus induction and plant regeneration from different explants in triploid and tetraploid turf-type bermudagrass. Plant Cell Tissue Organ Cult 90:71–78
Vasil IK, Vasil V (1994) In vitro cultures of cereals and grasses. In: Vasil IK, Thorpe TA (eds) Plant cell and tissue culture. Kluwer Academic Publishers, Dordrecht, p 293
Vasil V, Vasil IK (1986) Plant regeneration from friable embryogenic callus and cell suspension cultures of Zea mays L. J Plant Physiol 123:211–227
Vasil V, Vasil IK, Lu C (1984) Somatic embryogenesis in long-term callus cultures of Zea mays. Am J Bot 71:158–161
Moore KJ, Moser LE, Vogel KP, Waller SS, Johnson BE, Pedersen JF (1991) Describing and quantifying growth stages of perennial forage grasses. Agron J 83:1073–1077
Lu S, Wang Z, Peng X, Guo Z, Zhang G, Han L (2006) An efficient callus suspension culture system for triploid bermudagass (Cynodon transvaalensis × C. dactylon) and somaclonal variations. Plant Cell Tissue Organ Cult 87:77–84
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K et al (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45(4):616–629
Covert SF, Kapoor P, M-h L, Briley A, Nairn CJ (2001) Agrobacterium tumefaciens-mediated transformation of Fusarium circinatum. Mycol Res 105(3):259–264
Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, Beltran-Ramirez V et al (2008) Diversity and evolution of coral fluorescent proteins. PLoS ONE 3(7):e2680
Somleva MN, Snell KD, Beaulieu JJ, Peoples OP, Garrison BR, Patterson NA (2008) Production of polyhydroxybutyrate in switchgrass a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnol J 6:663–678
Shaeffer WI (1990) Terminology associated with cell, tissue and organ culture, molecular biology and molecular genetics. In Vitro Cell Dev Biol 26:97–101
Richards HA, Rudas VA, Sun V, McDaniel JK, Tomaszewski Z, Conger BV (2001) Construction of a GFP-BAR plasmid and its use for switchgrass transformation. Plant Cell Rep 20:48–54
Somleva MN, Tomaszewski Z, Conger BV (2002) Agrobacterium-mediated genetic transformation of switchgrass. Crop Sci 42:2080–2087
Acknowledgments
This work was funded by grants obtained from the Bioenergy Science Center. The BioEnergy Science Center is a US Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burris, J.N., Mann, D.G.J., Joyce, B.L. et al. An Improved Tissue Culture System for Embryogenic Callus Production and Plant Regeneration in Switchgrass (Panicum virgatum L.). Bioenerg. Res. 2, 267–274 (2009). https://doi.org/10.1007/s12155-009-9048-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-009-9048-8