Abstract
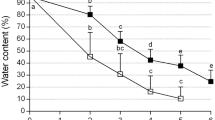
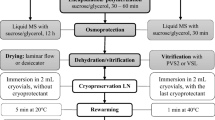
Embryogenic calli of Dioscorea bulbifera L. were successfully cryopreserved using an encapsulation-vitrification method. Embryogenic calli were cooled at 6°C for 5 days on solid MS medium (Murashige and Skoog 1962) containing 2 mg L−1 Kinetin (Kn), 0.5 mg L−1 α-naphthalene acetic acid (NAA) and 0.5 mg L−1 2,4-dichlorophenoxy-acetic acid (2,4-D). These were prior precultured on liquid basal MS medium enriched with 0.75 M sucrose at 25 ± 1°C for 7 days. Embryogenic calli were osmoprotected with a mixture of 2 M glycerol and 1 M sucrose for 80 min at 25°C and dropped in a 0.1 M CaCl2 solution containing 0.4 M sucrose at 25 ± 1°C. After 15 min of polymerization, Ca-alginate beads (about 4 mm in diameter) were dehydrated for 150 min at 0°C in a PVS2 solution [30% glycerol, 15% ethylene glycol, and 15% dimethyl sulfoxide (w/v)] containing 0.5 M sucrose. The encapsulated embryogenic calli were then plunged directly into LN (liquid nitrogen) for 1 h. After rapid thawing in a water bath (37°C; 2 min), the beads were washed 3 times at 10-min intervals in liquid basal MS medium containing 1.2 M sucrose. Following thawing, the embryogenic calli were transferred to fresh solid basal MS media supplemented with Kn 2 mg L−1, 0.09 M sucrose and 0.75% (w/v) agar (embryoid induction medium) and cultured under light conditions of 12-h photoperiod with a light intensity of 36 μmol m−2 s−1 provided by white cool fluorescent tubes after a 2-day dark period at 25 ± 1°C. After 30 days, the embryoids developed from embryogenic calli were transferred to fresh solid basal MS media supplemented with Kn 2 mg L−1, NAA 0.5 mg L−1, 3% (w/v) sucrose and 0.75% (w/v) agar (regeneration medium). After 60 days, the embryogenic calli developed normal shoots and roots. No morphological abnormalities were observed after plating on the regeneration medium. The survival rate of encapsulated vitrified embryogenic callus reached over 70%. This encapsulation-vitrification method appears promising as a routine and simple method for the cryopreservation of Dioscorea bulbifera embryogenic callus.







Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog
- NAA:
-
α-naphthalene acetic acid
- Kn:
-
Kinetin
- LN:
-
Liquid nitrogen
- 2,4-D:
-
2,4-dichlorophenoxy-acetic acid
References
Adriana S, Mirta F, Ricardo M, Sof′ıa O, Luis M (2004) Plant recovery of cryopreserved apical meristem-tips of Melia azedarach L. using encapsulation/dehydration and assessment of their genetic stability. Euphytica 135:29–38
Alka N, Sanjeev K, Srivastava PS (2005) Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep 24:250–254
Alka N, Sanjeev K, Srivastava PS (2007) Genetic fidelity of in vitro regenerants, encapsulation of shoot tips and high diosgenin content in Dioscorea bulbifera L., a potential alternative source of diosgenin. Biotechnol Lett 29:623–629
Bachiri Y, Gazeau C, Hansz J, Morisset C, Dereuddre J (1995) Successful crypreservation of suspension cells by encapsulation-dehydration. Plant Cell Tiss Organ Cult 43:241–248
Dixit S, Mandal BB, Ahuja S, Srivastava PS (2003) Genetic stability assessment of plants regenerated from cryopreserved embryogenic tissues of Dioscorea bulbifera l. using RAPD, biochemical and morphological analysis. Cryoletters 24:77–84
Engelmann F (2000) Importance of crop for conservation of plant genetic resources. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. JIRCAS, Tsukuba, Japan, pp 8–20
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of mint (Mentha spicata L.) by encapsulation vitrification. Plant Cell Rep 19:150–155
Hong SR, Yin MH (2009) High-efficiency vitrification protocols for cryopreservation of in vitro grown shoot tips of rare and endangered plant Emmenopterys henryi Oliv. Plant Cell Tiss Organ Cult 99:217–226
Hong S, Yin M, Shao X, Wang A, Xu W (2009) Cryopreservation of embryogenic callus of Dioscorea bulbifera by vitrification. CryoLetters 30:64–75
Inoue M, Akiko T (2009) Secondary dispersal of Dioscorea japonica (Dioscoreaceae) bulbils by rodents. J For Res 14:95–100
Kazumasa H, Misuzu M, Saeko G, Masumi IK, Kenji Y, Sakai A, Kazuhisa M (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376
Leunufna S, Keller ERJ (2003) Investigating a new cryopreservation protocol for yams (Dioscorea spp.). Plant Cell Rep 21:1159–1166
Mandal BB, Sangeeta AG (2007) Regeneration of Dioscorea floribunda plants from cryopreserved encapsulated shoot tips: effect of plant growth regulators. CryoLetters 28:329–336
Mandal BB, Sonali DS (2007) Cryopreservation of in vitro shoot tips of Dioscorea deltoidea Wall., an endangered medicinal plant: effect of cryogenic procedure and storage duration. CryoLetters 28:461–470
Mandal BB, Dwivedi S, Chandel KPS (1996) Cryopreservation of yam (Dioscorea spp) shoot apices by encapsulation-dehydration. CryoLetters 17:165–174
Marc S, Anne-Claire MO, Marie-Aleth LD (2007) The Dioscorea genus: a review of bioactive steroid saponins. J Nat Med 61:91–101
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol 15:473–497
Reaney MJ, Gusta LV (1987) Factors influencing the induction of freezing tolerance by abscisic acid in cell suspension cultures of Bromus inermis Leyss and Medicago sativa L. Plant Physiol 83:423–427
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Škrlep K, Bergant M, Winter GMD, Bohanec B, Žel J, Verpoorte R, Van Iren F, Camloh M (2008) Cryopreservation of cell suspension cultures of Taxus × media and Taxus floridana. Biol Plant 52:329–333
Sonali DS, Ahuja GS, Mandal BB, Srivastava PS (2005) Metabolic stability of plants regenerated from cryopreserved shoot tips of Dioscorea deltoidea—an endangered medicinal plant. Sci Hortic 105:513–517
Steponkus PL, Langis LR, Fujikawa S (1992) Cryopreservation of plant tissues by vitrification. In: Steponkus PL (ed) Advances in low-temperature biology, vol 1. JAI Press, London, pp 1–61
Suzuki M, Tandon P, Ishikawa M, Toyomasu T (2008) Development of a new vitrification solution, VSL, and its application to the cryopreservation of gentian axillary buds. Plant Biotechnol Rep 2:123–131
Tang ZX, Zhou Y, Zeng YK, Zang SL, He PG, Fang YZ (2006) Capillary electrophoresis of the active ingredients of Dioscorea bulbifera L. and its medicinal preparations. Chromatographia 63:617–622
Wang QC, Munir M, Nachman S, Li P, Colova-Tsolova V, Ron G, Ilan S, Edna T, Avihai P (2004) Cryopreservation of grapevine (Vitis spp.) embryogenic cell suspensions by encapsulation–vitrification. Plant Cell Tiss Organ Cult 77:267–275
Wheeler GS, Pemberton RW, Raz L (2007) A biological control feasibility study of the invasive weed-air potato, Dioscorea bulbifera L. (Dioscoreaceae): an effort to increase biological control transparency and safety. Nat Areas J 27:269–279
Wolfgang W, Hyeon-Yong P (2004) The apparent loss of tissue culture competence during leaf differentiation in yams (Dioscorea bulbifera L.). Plant Cell Tiss Organ Cult 34:101–105
Yin MH, Hong SR (2009a) Establishment of regeneration system of leaves and stems of Dioscorea bulbifera L. virus-free plantlets. Bulletin of Botanical Res 29:492–499
Yin MH, Hong SR (2009b) Cryopreservation of Dendrobium candidum wall. ex Lindl. protocorm-like bodies by encapsulation-vitrification. Plant Cell Tiss Organ Cult 98:179–185
Acknowledgments
This research was supported by Science and Technology Foundation of the Education Department of Jiangxi province (No. GJJ09374) and key scientific and technological plan of Shangrao Normal University in 2010-2011 (SR1014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ming-Hua, Y., Sen-Rong, H. A simple cryopreservation protocol of Dioscorea bulbifera L. embryogenic calli by encapsulation-vitrification. Plant Cell Tiss Organ Cult 101, 349–358 (2010). https://doi.org/10.1007/s11240-010-9695-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9695-7