Abstract
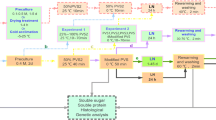
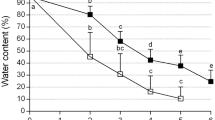
Protocorm-like bodies (PLBs) of Dendrobium candidum Wall. ex Lindl., orchid, were successfully cryopreserved using an encapsulation vitrification method. PLBs were precultured in liquid Murashige and Skoog (MS) medium containing 0.2 mg l−1 α-naphthalene acetic acid and 0.5 mg l−1 6-benzyladenine enriched with 0.75 M sucrose, and grown under continuous light (36 μmol m−2 s−1) at 25 ± 1°C for 5 days. PLBs were osmoprotected with a mixture of 2 M glycerol and 1 M sucrose for 80 min at 25°C and dripped in a 0.5 M CaCl2 solution containing 0.5 M sucrose at 25 ± 1°C and left for 15 min to form Ca-alginate beads (about 4 mm in diameter). Then, these were dehydrated with a plant vitrification solution 2 (PVS2) consisting of 30% (w/v) glycerol, 15% (w/v) ethylene glycol, and 15% (w/v) dimethyl sulfoxide in 0.5 M sucrose, pH 5.8, for 150 min at 0°C. Encapsulated and dehydrated PLBs were plunged directly into liquid nitrogen for 1 h. Cryopreserved PLBs were then rapidly re-warmed in a water bath at 40°C for 3 min and then washed with MS medium containing 1.2 M sucrose for three times at 10 min intervals. Within 60 days, plantlets with the cryopreserved PLBs developed normal shoots and roots, and without any observed morphological abnormalities, were obtained. The survival rate of encapsulated-vitrified PLBs was above 85%. Thus, this encapsulation-vitrification method was deemed promising for cryopreservation of PLBs of D. candidum.






Similar content being viewed by others
Abbreviations
- MS:
-
Tissue culture medium from Murashige and Skoog (1962)
- NAA:
-
α-Naphthalene acetic acid
- BA:
-
6-Benzyladenine
- PLBs:
-
Protocorm-like bodies
- LN:
-
Liquid nitrogen
- PVS2 :
-
Plant vitrification solution 2
References
Ai PF, Luo ZR (2003) Cryopreservation of dormant shoot-tips of persimmon by vitrification and plant regeneration. Sci Agric Sin 36:553–556
Bao XS, Shun QS, Chen LZ (2001) The medicinal plants of Dendrobium (Shi-Hu) in China, a coloured atlas. Fudan University Press, Shanghai
Bian HW, Wang JH, Lin WQ, Han N, Zhu MY (2002) Accumulation of soluble sugars, heat-stable proteins and dehydrins in cryopreservation of protocorm-like bodies of Dendrobium candidum by the air-drying method. J Plant Physiol 159:1139–1145. doi:10.1078/0176-1617-00824
Bouafia S, Jelti N, Lairy G, Blanc A, Bonnel E, Dereuddre J (1996) Cryopreserveation of potato shoot tips by encapsulation-dehydration. Potato Res 39:69–78. doi:10.1007/BF02358208
Chen Y (2000) Protoplasm of Dendrobium candidum cryopreserved by vitrification. J Wenzhou Teachers Coll (Nat Sci) 21:40–41
Chen Y, Wan JH, Huan CN (2001) Germplasm cryopreservation of Dendrobium candidum by vitrification. J Zhejiang Univ (Agric & Life Sci) 27:436–438
Engelmann F (1997) In vitro conservation methods. In: Callow CA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources. CAB International, Oxford, pp 119–161
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of mint (Mentha spicata L.) by encapsulation vitrification. Plant Cell Rep 19:150–155. doi:10.1007/s002990050725
Hong SR, Yin MH, Shao XH, Wang AP, Xu WH (2009) Cryopreservation of embryogenic callus of Dioscorea bulbifera by vitrification. Cryo Lett 30:64–75
Ishikawa K, Harata K, Mii M, Sakai A, Yoshimatsu K, Shimomura K (1997) Cryopreservation of zygotic embryos of a Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep 16:754–757. doi:10.1007/s002990050314
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (1997) Ultrastructural study on mechanism of increased freezing tolerance due to extracelluar glucose in cabbage leaf cells. Cryo Lett 18:33–44
Liu YG, Wang XY (2002) Study of cryopreservation technique of apple germplasm by vitrification. J Shandong Agric Univ (Nat Sci) 33:32–36
Luo JP, Wang Y, Zha XQ, Huang L (2008) Micropropagation of Dendrobium densiflorum Lindl. ex Wall. through protocorm-like bodies: effects of plant growth regulators and lanthanoids. Plant Cell Tissue Organ Cult 93:333–340. doi:10.1007/s11240-008-9381-1
Lurswijidjarus W, Thammasiri K (2004) Cryopreservation of shoot tips of Dendrobium Walter Oumae by encapsulation/dehydration. Sci Asia 30:293–299. doi:10.2306/scienceasia1513-1874.2004.30.293
Maneerattanarungroj P, Bunnag S, Monthatong M (2007) In vitro conservation of Cleisostoma areitinum (Rchb.f.) Garay, rare Thai orchid species by an encapsulation-dehydration method. Asian J Plant Sci 6:1235–1240. doi:10.3923/ajps.2007.1235.1240
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479. doi:10.1111/j.1399-3054.1962.tb08052.x
Na HY, Kondo K (1996) Cryopreservation of tissue cultured shoot primordia from shoot apices of cultured protocorms in Vanda pumila following ABA preculture and desiccation. Plant Sci 118:195–201. doi:10.1016/0168-9452(96)04438-X
Nikishina TV, Popova EV, Vakhrameeva MG, Varlygina TI, Kolomeitseva GL, Burov AV, Popovich EA, Shirokov AI, Shumilov VY, Popov AS (2007) Cryopreservation of seeds and protocorms of rare temperate orchids. Russ J Plant Physiol 54:121–127. doi:10.1134/S1021443707010189
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73. doi:10.1016/0168-9452(93)90189-7
Pornchuti W, Thammasiri K (2008) Cryopreservation of protocorms of Dendrobium virgineum Rchb.f. Acta Hortic 788:63–68
Puchooa D (2004) Comparison of different culture media for the in vitro culture of Dendrobium (Orchidaceae). Int J Agric Biol 6:884–888
Qin TH (2008) Rapid propagation of Dendrobium candidum Wall. ex Lindl. in vitro. Chin J Trop Agric 28:25–29
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33. doi:10.1007/BF00232130
Shiau Y-J, Nalawade SM, Hsia C-N, Mulabagal V, Tsay H-S (2005) In vitro propagation of the Chinese medicinal plant, Dendrobium candidum wall. ex lindl., from axenic nodal segments. In Vitro Cell Dev Biol Plant 41:666–670. doi:10.1079/IVP2005685
Shibli RA, Haagenson DM, Cunningham SM, Berg WK, Volenec JJ (2001) Cryopreservation of alfalfa (Medicago sativa L.) cells by encapsulation-dehydration. Plant Cell Rep 20:445–450. doi:10.1007/s002990100347
Thammasiri K (2000) Cryopreservation of seeds of a Thai orchid by vitrification. Cryo Lett 21:237–244
Thammasiri K (2008) Cryopreservation of some Thai orchid species. Acta Hortic 788:53–62
Vacin EF, Went FW (1949) Some pH changes in nutrient solutions. Bot Gaz 110:605–613
Vaillant V, Bade P, Constant C (2005) Photoperiod affects the growth and development of yam plantlets obtained by in vitro propagation. Biol Plant 49:355–359. doi:10.1007/s10535-005-0007-8
Wang Y, Liu Y (2006) Cryopreservation of ornamental plant germplasm. Plant Physiol Commun 42:559–566
Wang JH, Ge JG, Liu F, Bian HW, Huang CN (1998) Cryopreservation of seeds and protocorms of Dendrobium candidum. Cryo Lett 19:123–128
Wang QC, Batuman O, Li P, Bar-Joseph M, Gafny R (2002a) Cryopreservation of in vitro-grown shoot tips of ‘Troyer’ citrange [Poncirus trifoliata Raf. × Citrus sinensis (L.) Osbeck.] by encapsulation-dehydration. Plant Cell Rep 20:901–906. doi:10.1007/s00299-001-0425-9
Wang QC, Gafny R, Sahar N, Sela I, Mawassi M, Tanne E, Perl A (2002b) Cryopreservation of grapevine (Vitis vinifera L.) embryogenic cell suspensions and subsequent plant regeneration by encapsulation-dehydration. Plant Sci 162:551–558. doi:10.1016/S0168-9452(01)00594-5
Wu XM, Tang HR (2005) Research advances in cryopreservation of plant germplasm by encapsulation-vitrification method. Chin Bull Bot 22:238–245
Xu H, Wang ZT, Ding XY, Zhou KY, Xu LS (2006) Differentiation of Dendrobium species used as ‘‘Huangcao Shihu’’ by rDNA ITS sequence analysis. Planta Med 72:89–92. doi:10.1055/s-2005-916228
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Organ Cult 92:251–260. doi:10.1007/s11240-007-9329-x
Yang L, Wang ZT, Xu LS (2006) Simultaneous determination of phenols (bibenzyl, phenanthrene, and fluorenone) in Dendrobium species by high-performance liquid chromatography with diode array detection. J Chromatogr A 1104:230–237. doi:10.1016/j.chroma.2005.12.012
Zhao P, Wang W, Feng FS, Wu F, Yang ZQ, Wang WJ (2007) High-frequency shoot regeneration through transverse thin cell layer culture in Dendrobium candidum Wall Ex Lindl. Plant Cell Tissue Organ Cult 90:131–139. doi:10.1007/s11240-006-9181-4
Zhao P, Wu F, Feng FS, Wang WJ (2008) Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum Wall ex Lindl. In Vitro Cell Dev Biol Plant 44:178–185. doi:10.1007/s11627-007-9101-2
Acknowledgments
This study was supported by Science and Technology Foundation of the Education Department of Jiangxi province (No. GJJ09617) and key scientific and technological plan of Shangrao Normal College in 2009–2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, M., Hong, S. Cryopreservation of Dendrobium candidum Wall. ex Lindl. protocorm-like bodies by encapsulation-vitrification. Plant Cell Tiss Organ Cult 98, 179–185 (2009). https://doi.org/10.1007/s11240-009-9550-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9550-x