Abstract
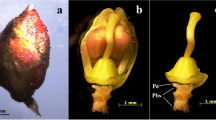
Inflorescence induction and morphogenesis of regenerated flowers were investigated in vitro in Dioscorea zingiberensis C. H. Wright. Inflorescence induction was influenced by the type and concentration of phytohormones. When floral bud explants were incubated on a Murashige and Skoog medium containing a combination of 2.0 mg l−1 6-benzyladenine and 0.5 mg l−1 indole-3-butyric acid, the highest frequency of inflorescence induction was observed. However, in the presence of gibberellic acid, induction efficiency was reduced although node length of inflorescence was increased. Ontogenetic studies revealed that the inflorescence primordia originated directly from axillary epidermal cells of the perianth and bract of the explants after 7 days. In vitro, male flowers developed normally and blossomed after 90–100 days. In addition, some bisexual flowers were observed. These results demonstrated that there were differences in sexual differentiation of floral buds in vitro compared with that in vivo.


Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- GA3 :
-
Gibberellic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- KT:
-
Kinetin
- MS:
-
Medium Murashige and Skoog medium
- NAA:
-
Naphthaleneacetic acid
References
Chang C, Chang WC (2003) Cytokinins promotion of flowering in Cymbidium ensifolium var. misericors in vitro. Plant Growth Regul 39:217–221
Isabel B, Caroline D (2006) The timing of developmental transitions in plants. Cell 125:655–664
Kostenyuk I, Oh BJ, So IS (1999) Induction of early flowering in Cymbidium niveo-marginatum Mak in vitro. Plant Cell Rep 19:1–5
Lin CS, Lin CC, Hsiao HW et al (2003) In vitro flowering of Bambusa edulis and subsequent plantlet survival. Plant Cell Tissue Organ Cult 72:71–78
Lin CS, Lin CC, Chang WC (2004) Effect of thidiazuron on vegetative tissue-derived somatic embryogenesis and flowering of bamboo Bambusa edulis. Plant Cell Tissue Organ Cult 76:75–82
Lin CS, Lin CC, Hsiao HW et al (2005a) Effects of growth regulators on direct flowering of isolated ginseng buds in vitro. Plant Cell Tissue Organ Cult 83:241–244
Lin CS, Lin CC, Chang WC (2005b) Shoot regeneration, re-flowering and post flowering survival in bamboo inflorescence culture. Plant Cell Tissue Organ Cult 82:243–249
Lu WL (2003) Control of in vitro regeneration of individual reproductive and vegetative organs in Dracaena fragrans cv. massangeana hort.—regularities of the direct regeneration of individual organs in vitro. Acta Bot Sin 45(12):1453–1464
Lu WL, Bai SN, Zhang XS (1999) Induction of continuous tepal differentiation from in vitro regenerated flower buds of Hyacinthus orientalis. Acta Bot Sin 41(9):921–926
Men L, Zhu HT, Lui XK et al (2000) Micro propagation of Dioscorea zingiberensis. Nat Prod Res Dev 12(6):17–21
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Plant Physiol 15:473–497
Naor V, Kigel K, Ziv M (2004) Hormonal control of inflorescence development in plantlets of calla lily (Zantedeschia spp.) grown in vitro. Plant Growth Regul 42:7–14
Narasimhulu SB, Reddy GM (1984) In vitro flowering and pod formation from cotyledons of groundnut (Arachis hypogaea L.). Theor Appl Genet 69:87–91
Pang JL, Wang LL, Hu JQ et al (2006) Morphological studies on direct regeneration of floral buds from in vitro cultures of sepal segments in Sinningia Speciosa Hiern. J Mol Cell Biol 39(4):383–389
Peeters AJM, Gerarda W, Barendse GWM et al (1991) In vitro flower bud formation in tobacco: interaction of hormones. Plant Physiol 97:402–408
Peter MC (1999) Genetic analysis of hormone signaling. Annu Rev Plant Physiol Plant Mol Biol 50:219–243
Roberts NJ, Luckman GA, Menary RC (1993) In vitro flowering of Boronia megastigma Nees. and the effect of 6-benzylaminopurine. J Plant Growth Regul 12:117–122
Saritha KV, Naidu CV (2007) In vitro flowering of Withania somnifera Dunal.—an important antitumor medicinal plant. Plant Sci 172:847–851
Scorza R (1982) In vitro flowering. Hortic Rev 4:106–127
Song FJ (2002) Research and production status of diosgenin from dioscorea plants of steroidal druggery fountainhead. Nat Prod Res Dev 14(3):89–93
Sun CH, Deng XJ, Fang J et al (2007) An overview of flowering transition in higher plants. Hereditas 29(10):1182–1190
Tang W (2000) High-frequency plant regeneration via somatic embryogenesis and organogenesis and in vitro flowering of regenerated plantlets in Panax ginseng. Plant Cell Rep 19:727–732
Taylor NJ, Light ME, Staden JV et al (2005) In vitro flowering of Kniphofia leucocephala: influence of cytokinins. Plant Cell Tissue Organ Cult 83:327–333
Wang S, Tang L, Chen F et al (2001) In vitro flowering of bitter melon. Plant Cell Rep 20:393–397
Weigel D (1995) The genetics of flower development: from floral induction to ovule morpho- genesis. Annu Rev Genet 29:19–39
Wu W, Jia C, Liu XJ et al (2002) Tissue culture and plant regeneration of Dioscorea zingiberensis Wright. Chin Acad Med Mag Organ 1:41–43
Wu CY, You CG, Li CS et al (2008) RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA 105(35):12915–12920
Xu Xl, Liu XM, Zhou PH et al (2000) In vitro tissue culture and tuberization in Dioscorea zingiberensis CH Wright. J Hunan Agric Univ 26(4):282–285
Yan LX, Hu CG, Yao JL (2007) Haploid callus and plantlets regenerated from anther culture of Dioscorea zingiberensis (Dioscoreaceae). Acta Bot Yunnanica 29(1):33–37
Yang Bo, Huang XL, Hu CG et al (2009) Factors influencing flower inducing of Dioscorea zingberensis CH Wright in vitro. J Wuhan Bot Res 27(3):318–322
Acknowledgments
We greatly appreciate the help of Dr. Jihong Liu in revising this paper. This work was supported by grants from the Ph.D. Programs Foundation of Ministry of Education of China (No. 2005050416).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, XL., Yang, B., Hu, CG. et al. In vitro induction of inflorescence in Dioscorea zingiberensis . Plant Cell Tiss Organ Cult 99, 209–215 (2009). https://doi.org/10.1007/s11240-009-9595-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9595-x