Abstract
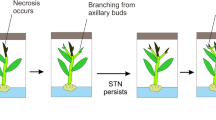
Plant tissue culture plays an important role in the production and conservation of plant species. Its application, however, is hindered by some growth abnormalities such as shoot-tip necrosis (STN) caused by the culture conditions. This review article summarizes the literature published on the causes of in vitro STN in plants such as medium type, plant growth regulators, calcium, boron, medium additives, the culture environment, their interaction and physiological effects.
Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- B:
-
Boron
- BA:
-
[6-benzylaminopurine]
- Ca:
-
Calcium
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- iP:
-
N6-isopentenyladenine
- MS medium:
-
Murashige and Skoog (1962) basal medium
- mT:
-
meta-Topolin
- mTR:
-
meta-Topolin riboside
- PGR:
-
Plant growth regulators
- STN:
-
Shoot-tip necrosis
- TDZ:
-
Thidiazuron
- WPM:
-
Woody plant medium
References
Abdulnour JE, Donnelley DJ, Barthakur NN (2000) The effect of boron on calcium uptake and growth in micropropagated potato plantlets. Potato Res 43:287–295
Abousalim A, Mantell SH (1994) A practical method for alleviating shoot-tip necrosis symptoms in in vitro shoot cultures of Pistacia vera cv. Matheur. J Hortic Sci 69:357–365
Apostol KG, Zwiazek JJ (2004) Boron and water uptake in jack pine (Pinus banksiana) seedlings. Environ Exp Bot 51:145–153
Bairu MW, Jain N, Stirk WA, Dolezal K, van Staden J (2009) Solving the problem of shoot-tip necrosis in Harpagophytum procumbens by changing the cytokinin types, calcium and boron concentrations in the medium. S Afr J Bot 75:122–127
Barghchi M, Alderson PG (1996) The control of shoot tip necrosis in Pistacia vera L. in vitro. Plant Growth Regul 20:31–35
Bhalla PL, Mulwa RMS (2003) Tissue culture and macadamia propagation. Acta Hortic 616:343–346
Biddulph O, Nakayama FS, Cory R (1961) Transpiration stream and ascension of calcium. Plant Physiol 36:429–436
Bohnsack CW, Albert LS (1977) Early effects of boron deficiency on indoleacetic acid oxidase levels of squash root tips. Plant Physiol 59:1047–1050
Borchert R (1986) Calcium acetate-induced calcium uptake and formation of calcium-oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 168:571–578
Bornman CH, Vogelmann TC (1984) Effect of rigidity of gel medium on benzyladenine induced adventitious bud formation and hyperhydricity in vitro in Picea abies. Physiol Plant 61:505–512
Bowen JE (1972) Effect of environmental factors on water utilization and boron accumulation and translocation in sugar cane. Plant Cell Physiol 13:703–714
Brown PH, Hu H (1996) Phloem mobility of boron is species dependent: evidence of phloem mobility in sorbitol-rich species. Ann Bot 77:497–505
Brown PH, Shelp BJ (1997) Boron mobility in plants. Plant Soil 193:85–101
Brown PH, Bellaloui N, Hu H, Dandekar A (1999) Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency. Plant Physiol 119:17–20
Cassells AC, Walsh C (1994) The influence of gas-permeability of the culture lid on calcium uptake and stomatal function in Dianthus microplants. Plant Cell Tissue Organ Cult 37:171–178
Chang Y-C, Miller WB (2005) The development of upper leaf necrosis in Lilium ‘Star Gazer’. J Am Soc Hortic Sci 130:759–766
De Block M (1990) Factors influencing the tissue culture and the Agrobacterium tumefaciens-mediated transformation of hybrid Aspen and Poplar clones. Plant Physiol 93:1110–1116
Debergh PC (1983) Effect of agar brand and concentration on the tissue culture medium. Physiol Plant 59:270–276
Dunwell JM (1979) Anther culture in Nicotiana tabacum: the role of the culture vessel atmosphere in pollen embryo induction and growth. J Exp Bot 30:419–428
Dyson PW, Digby J (1975) Effect of calcium on sprout growth and sub-apical necrosis in Majestic potatoes. Potato Res 18:290–305
Grigoriadou K, Leventakis N, Vasilakakis M (2000) Effects of various culture conditions on proliferation and shoot tip necrosis in the pear cultivars ‘William’s’ and ‘Highland’ grown in vitro. Acta Hortic 520:103–108
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155
Hepler PK, Wayne RO (1985) Calcium and plant development. Annu Rev Plant Physiol 36:397–439
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Hu H, Brown PH (1994) Localization of boron in cell walls of squash and tobacco and its association with pectin: evidence for a structural role of boron in the cell wall. Plant Physiol 105:681–689
Hu H, Penn SC, Lebrilla CB, Brown PH (1997) Isolation and characterization of soluble boron complexes in higher plants: the mechanism of phloem mobility of boron. Plant Physiol 113:649–655
Jain N, Bairu MW, Stirk WA, Dolezal K, van Staden J (2009) The effect of medium, carbon source and explant on regeneration and control of shoot-tip necrosis in Harpagophytum procumbens. S Afr J Bot 75:117–121
Karhu ST (1997) Axillary shoot proliferation of blue honeysuckle. Plant Cell Tissue Organ cult 48:195–201
Kataeva NV, Alexandrova IG, Butenko RG, Dragavtceva EV (1991) Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tissue Organ Cult 27:149–154
Kintzios S, Stravropoulous ER, Skamneli S (2004) Accumulation of selected macronutrients and carbohydrates in melon tissue cultures: association with pathways of in vitro dedifferentiation and differentiation (organogenesis, somatic embryogenesis). Plant Sci 167:655–664
Kohl HC, Oertli JJ (1961) Distribution of boron in leaves. Plant Physiol 36:420–424
Kulkarni K, D’Souza L (2000) Control of in vitro shoot tip necrosis in Butea monosperma. Curr Sci 78:125–126
Lakshmi SG, Raghava SBV (1993) Regeneration of plantlets from leaf disc cultures of rosewood: control of leaf abscission and shoot tip necrosis. Plant Sci 88:107–112
Lehto T, Kallio E, Aphalo PJ (2000) Boron mobility in two coniferous species. Ann Bot 86:547–550
Mackay WA, Tipton JL, Thompson GA (1995) Micropropagation of Mexican redbud, Cercis canadensis var. mexicana. Plant Cell Tissue Organ Cult 43:295–299
Marin JA (2003) High survival rates during acclimatization of fruit tree rootstocks by increasing exposure to low relative humidity. Acta Hortic 616:139–142
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, Boston
Martin KP, Zhang C-L, Slater A, Madasser YJ (2007) Control of shoot necrosis and plant death during micropropagation of banana and plantains (Musa spp.). Plant Cell Tissue Organ Cult 88:51–59
McCown BH, Sellmer JC (1987) General media and vessels suitable for woody plant culture. In: Bonga JM, Durzan L (eds) Cell and tissue culture in forestry. General principles and biotechnology, vol 1. Martinus Nijhoff, Dordrecht, pp 4–16
Misra P, Chakrabarty D (2009) Clonal propagation of Rosa clinophylla Thory. through axillary bud culture. Sci Hortic 119:212–216
Nhut DT, Thi NN, Khiet BLT, Luan VQ (2008) Peptone stimulates in vitro shoot and root regeneration of avocado (Persea americana Mill.). Sci Hortic 115:124–128
Oertli JJ (1993) The mobility of boron in plants. Plant Soil 155–156:301–304
Ogasawara N (2003) Ventilation and light intensity during in vitro culture affect relative growth rate and photosynthate partitioning of Caladium plantlets after transplanting to ex vitro. Acta Hortic 616:143–149
Ongaro V, Leyser O (2008) Hormonal control of shoot branching. J Exp Bot 59:67–74
Pan MJ, Van Staden J (1998) The use of charcoal in in vitro culture. Plant Growth Regul 26:155–163
Pasqua G, Manes F, Monacelli B, Natale L, Anselmi S (2002) Effects of the culture medium pH and ion uptake in in vitro vegetative organogenesis in thin cell layers of tobacco. Plant Sci 162:947–955
Perěz C, Rodrĭguez R, Taměs R (1985) In vitro filbert (Corylus avellana L.) micropropagation from shoots and cotyledonary segments. Plant Cell Rep 4:137–139
Perez-Tornero O, Burgos L (2000) Different media requirements for micropropagation of apricot cultivars. Plant Cell Tissue Organ Cult 63:133–141
Piagnani C, Zocchi G, Mignani I (1996) Influence of Ca2+ and 6-benzyladenine on chestnut (Castanea sative Mill.) in vitro shoot-tip necrosis. Plant Sci 118:89–95
Raven JA (1977) H+ and Ca2+ in phloem and symplast: relation of relative immobility of the ions to the cytoplasmic nature of the transport paths. New Phytol 79:465–480
Redondo-Nieto M, Wilmot AR, EL-Hamdaoui A, Bonilla I, Bolaňos L (2003) Relationship between boron and calcium in the N2-fixing legume-rhizobia symbiosis. Plant Cell Environ 26:1905–1915
Rugini M (1984) In vitro propagation of some olive (Olea europaea sativa L.) cultivars with different root-ability, and medium development using analytical data from developing shoots and embryos. Sci Hortic 24:123–134
Sakakibara H (2004) Cytokinin biosynthesis and metabolism. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action, 3rd edn. Kluwer, London, pp 95–114
Sha L, McCown BH, Peterson LA (1985) Occurrence and cause of shoot-tip necrosis in shoot cultures. J Am Soc Hortic Sci 110:631–634
Shear CB (1975) Ca-related disorders of fruits and vegetables. HortScience 10:361–365
Shelp BJ, Marentes E, Kitheka AM, Vivekanandan P (1995) Boron mobility in plants. Physiol Plant 94:356–361
Singha S, Townsend EC, Oberly GH (1990) Relationship between calcium and agar on vitrification and shoot-tip necrosis of quince (Cydonia oblonga Mill.) shoots in vitro. Plant Cell Tissue Organ Cult 23:135–142
Tang MP, Dela Fuente RK (1986a) The transport of indole-3-acetic acid in boron- and calcium-deficient sunflower hypocotyl segments. Plant Physiol 81:646–650
Tang MP, Dela Fuente RK (1986b) Boron and calcium sites involved in indole-3-acetic acid transport in sunflower hypocotyl segments. Plant Physiol 81:651–655
Thomas P (2000) Microcutting leaf area, weight and position on stock shoot influence root vigour, shoot growth and incidence of shoot tip necrosis in grape plants in vitro. Plant Cell Tissue Organ Cult 61:189–198
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Vieitez AM, Sanchez C, San-Jose C (1989) Prevention of shoot tip necrosis in shoot cultures of chestnut and oak. Sci Hortic 41:101–109
Wang H, van Staden J (2001) Establishment of in vitro cultures of tree peonies. S Afr J Bot 67:358–361
Wang G, Römheld V, Li C, Bangerth F (2006) Involvement of auxin and CKs in boron deficiency induced changes in apical dominance of pea plants (Pisum sativum L.). J Plant Physiol 163:591–600
Werbrouck SPO, van Der Jeugt B, Dewitte W, Prinsen E, van Onckelen HA, Debergh PC (1995) The metabolism of benzyladenine in S. floribundum schott ‘petite’ in relation to acclimatization problems. Plant Cell Rep 14:662–665
Wojcik P, Wojcik M (2003) Effects of boron fertilization on ‘Conference’ pear tree vigour, nutrition and fruit yield and storability. Plant Soil 256:413–421
Xing Z, Satchwell MF, Powell WA, Maynard C (1997) Micropropagation of American chestnut: increasing rooting rate and preventing shoot-tip necrosis. In Vitro Cell Dev Biol 33:43–48
Zocchi G, Mignani I (1995) Calcium physiology and metabolism in fruit trees. Acta Hortic 383:15–23
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bairu, M.W., Stirk, W.A. & Van Staden, J. Factors contributing to in vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tiss Organ Cult 98, 239–248 (2009). https://doi.org/10.1007/s11240-009-9560-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9560-8