Abstract
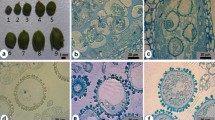
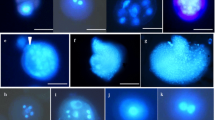
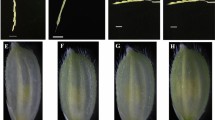
Barley microspores from five field-grown breeding lines were isolated using an ultra-speed blender and the effect of co-culture with young florets was investigated. Floret co-culture in the induction stage increased the formation of MCS, ELS and green plant regeneration. The florets of teraploid plant were more effective than ones of diploid plant. For line S23, co-culture with florets from tetraploid plants gave rise to 2.6 and 7.8 times more MCS and ELS, respectively, than non-co-culture control, whereas co-culture with florets from diploid plants resulted in 1.8 and 6.1 times more MCS and ELS, respectively, than non-co-culture control (Table 2). Florets subjected to cold treatment for 10–20 days induced a greater response than fresh ones, and florets with uninucleate microspores surpassed binucleate microspores. For microspores culture from 15-day cold pre-treated spikes, 93A floret co-culture gave rise to 3.6 and 6.8 times more MCS and ELS, respectively, than the non-co-cultured control, while SD1 floret co-culture resulted in 1.9 and 4.0 times more, respectively. Similarly, for microspore culture from 20-day cold pre-treated spikes, 93A floret co-culture gave rise to 2.6 and 5.1 times more MCS and ELS, respectively, than non-co-cultured control, while SD1 floret co-culture resulted in 1.5 and 3.0 times more, respectively (Table 3). Some microspores formed dense MCS that did not develop further. Compared with the control, floret co-culture resulted in less dense MCS formation, indicating that the isolated florets were beneficial to the normal development of MCS. Floret co-culture was only effective when the spikes were cold pre-treated before microspore isolation. Spike cold pre-treatment before microspore preparation was crucial for dedifferentiation of cultured isolated microspores, and this could not be replaced by floret co-culture. It is postulated that the florets provided essential substances for in vitro cultured isolated microspores to undergo dedifferentiation and embryogenesis. Both the genotype selection and the physiological status (developmental status and cold treatment) adjustment of the florets for co-culture could improve barley microspore culture. Compared with ovary co-culture, floret co-culture is more efficient. The technique is of simple application in breeding programs and can be a solution for coping with recalcitrant genotypes and or plant donor condition.

Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- KT:
-
Kinetin
- NAA:
-
Naphthaleneacetic acid
- 6-BA:
-
6-Benzyladenine
- FDA:
-
Fluorescein diacetate
- MCS:
-
Multicellular structures
- ELS:
-
Embryo-like structures
Reference
Bakos F, Darkó É, Pónya Zs, Barnabás B (2003) Regeneration of fertile wheat (Triticum aestivum) plants from isolated zygotes using wheat microspore culture as nurse cells. Plant Cell Tiss Org Cult 74:243–247
Bruins MBM, Rakoczy-Trojanowska M, Snijders CHA (1996) Isolated microspore culture in wheat (Triticum aestivum L.): the effect of co-culture of wheat or barley ovaries on embryogenesis. Cereal Res Commun 24:401–408
Chu CC, Hill RD (1988) An improved anther culture method for obtaining higher frequency of pollen embyroids in Triticum aestivum L. Plant Sci 55:175–181
Cistué L, Soriano M, Castillo AM, Vallés MP, Sanz JM, Echávarri B (2006) Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep 25:257–264
Datta SK, Wenzel G (1987) Isolated microspore derived plant formation via embryogenesis in Triticum aestivum L. Plant Sci 48:49–54
Hu H (1997) In vitro induced haploids in wheat, vol 4. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, pp 73–97. Kluwer Academic Publishers, Dordrecht
Hu T, Kasha KJ (1997) Improvement of isolated microspore culture of wheat (Triticum aestivum L.) through ovary co-culture. Plant Cell Rep 16:520–525
Jähne A, Lörz H (1995) Cereal microspore culture. Plant Sci 109:1–12
Khosrowchahli M, Naghilou MH, Moghaddam M (2001) Effect of ovary-microspore co-culture on androgenesis in barley. Abstracts of the XVIth EUCARPIA congress, Edinburgh, Scotland, pp 10–14
Kohler F, Wenzel G (1985) Regeneration of isolated barley microspores in conditioned media and trials to characterize the responsible factor. J Plant Physiol 121:181–191
Li H, Devaux P (2001) Enhancement of microspore culture efficiency of recalcitrant barley genotypes. Plant Cell Rep 20:475–481
Mejza SJ, Morgant V, DiBona DE, Wong JR (1993) Plant regeneration from isolated microspores of Triticum aestivum L. Plant Cell Rep 12:149–153
Minesh P, Norman LD, Donald RM, James OB (2004) Optimization of culture conditions for improved plant regeneration efficiency from wheat microspore culture. Euphytica 140:197–204
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Oleszczuk S, Sowa S, Zimny J (2006) Androgenic response to preculture stress in microspore cultures of barley. Protoplasma 228:95–100
Puolimatka M, Laine S, Pauk J (1996) Effect of ovary co-cultivation and culture medium on embryogenesis of directly isolated microspores of wheat. Cereal Res Commun 24:393–400
Puolimatka M, Pauk J (1999) Impact of explant type, duration and initiation time on the co-culture effect in isolated microspore culture of wheat (Triticum aestivum L.). J Plant Physiol 154:367–373
Ritala A, Mannonen L, Oksman-Caldentey K-M (2001) Factors affecting the regeneration capacity of isolated barley microspores (Hordeum vulgare L.). Plant Cell Rep 20:403–405
Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996) Efficient microspore embryogenesis in wheat (Triticum aestivum L.), induced by starvation at high temperature. Sex Plant Reprod 9:209–215
Xu ZH, Huang B (1984) Anther factor(s) in barley anther culture. Acta Botanica Sinica 26:1–10
Zheng MY, Weng Y, Liu W, Konzak CF (2002) The effect of ovary-conditioned medium on microspore embryogenesis in common wheat (Triticum aestivum L.). Plant Cell Rep 20:802–807
Acknowledgments
The work was supported by China National Projects No. 2006BAD02B04 and No. nyhzx 07-001-barley. We would like to thank Dr. Yuhua Zhang at Rothamsted Research for his critical reading of and his comments on the Chinese version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, R., Wang, Y., Sun, Y. et al. Improvement of isolated microspore culture of barley (Hordeum vulgare L.): the effect of floret co-culture. Plant Cell Tiss Organ Cult 93, 21–27 (2008). https://doi.org/10.1007/s11240-008-9338-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9338-4