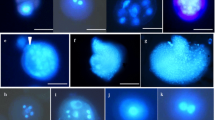
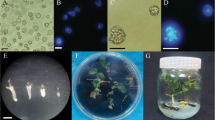
Abstract
An efficient protocol to improve microspore embryogenesis is established in an important oleiferous crop, Brassica juncea (Indian mustard). Colchicine was used for enhancing microspore embryogenesis and also to obtain doubled haploid embryos. Colchicine at high concentrations (>10 mg l−1), for 24 h, proved convenient for direct recovery of diploid embryos. Higher temperature treatment and an antiauxin PCIB (p-chlorophenoxyisobutyric acid) enhanced microspore embryogenesis significantly as compared to colchicine. An increase in temperature from 32°C to 35°C proved very efficient in increasing embryogenesis by 10-fold. The highest embryogenesis rate was obtained when PCIB was added at 35°C in the culture after 1 day of culture initiation. 20 μM PCIB could enhance microspore embryogenesis by 5-fold. Different abnormal shapes of embryos like lemon, banana, flask and fused cotyledons were observed. Both normal and fused cotyledonous embryos showed normal germination when transferred on the B5 basal medium.
Similar content being viewed by others
Abbreviations
- DAPI:
-
4,6-Diamidino-2-phenylindole
- PCIB:
-
p-Chlorophenoxyisobutyric acid
References
Agarwal PK, Bhojwani SS (1993) Enhanced pollen grain embryogenesis and plant regeneration in anther cultures of Brassica juncea cv. PR-45. Euphytica 70:191–196
Agarwal PK, Bhojwani SS (2004) Genetic variability in the progeny of androgenic dihaploid plants and selection of high agronomic performing lines in Brassica juncea. Biol Plant 48:503–508
Babbar SB, Agarwal PK, Sahay S, Bhojwani SS (2004) Isolated microspore culture of Brassica: an experimental tool for developmental studies and crop improvement. Ind J Plant Biotechnol 3:185–202
Binarova P, Straatman K, Hause B, Hause G, Lammeren AAM (1993) Nuclear DNA synthesis during the induction of embryogenesis in cultured microspores and pollen of Brassica napus L. Theor Appl Genet 87:9–16
Burström H (1950) Studies on growth and metabolism of roots: IV. Positive and negative auxin effects on cell elongation. Physiol Plant 3:277–292
Chanana NP, Dhawan V, Bhojwani SS (2005) Morphogenesis in isolated microspore cultures of Brassica juncea. Plant Cell Tissue Organ Cult 83:169–177
Chen ZZ, Snyder Z, Fan ZG, Loh WH (1994) Efficient production of doubled haploid plants through chromosome doubling of isolated microspores in Brassica napus. Plant Breed 113:217–221
da Silva Dias JC (1999) Effect of activated charcoal on Brassica oleracea microspore culture embryogenesis. Euphytica 108:65–69
Find J, Grace L, Krogstrup P (2002) Effect of antiauxins on maturation of embryogenic tissue cultures of Nordmanns fir (Abies nordmanniana). Physiol Plant 116:231–237
Foster RJ, McRae DH, Bonner J (1995) Auxin–antiauxin interaction at high auxin concentrations. Plant Physiol 30:323–327
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
George L, Rao PS (1982) In vitro induction of pollen embryos and plantlets in Brassica juncea through anther culture. Plant Sci Lett 26:111–116
Gland A, Lichter R, Schweiger HG (1988) Genetic and exogenous factors affecting embryogenesis in isolated microspore culture of Brassica napus L. J Plant Physiol 132:613–617
Gu HH, Hagberg P, Zhou WJ (2004) Cold pretreatment enhances microspore embryogenesis in oilseed rape (Brassica napus L.). Plant Growth Regul 42:137–143
Guha S, Maheshwari SC (1964) In vitro production of embryos from anthers of Datura. Nature 204:497
Guo YD, Pulli S (1996) High-frequency embryogenesis in Brassica campestris microspore culture. Plant Cell Tissue Organ Cult 46:219–225
Hansen M (2000) ABA treatment and desiccation of microspore-derived embryos of cabbage (Brassica oleracea ssp. capitata L.) improves plant development. J Plant Physiol 156:164–167
Hansen M, Svinnset K (1993) Microspore culture of Swede (Brassica napus ssp. rapifera) and the effects of fresh and conditioned media. Plant Cell Rep 12:496–500
Hadfi K, Speth V, Neuhaus G (1998) Auxin-induced developmental patterns in Brassica juncea embryos. Development 125:879–887
Heupel T, Stange L (1995) The auxin antagonist p-chlorophenoxyisobutyric acid abolishes polar distribution of DNA synthesizing cells within the meristem of Riella helicophylla. J Plant Physiol 146:757–759
Hiramatsu M, Odahara K, Matsue Y (1995) A survey of microspore embryogenesis in leaf mustard (Brassica juncea). Acta Hort 392:139–145
Katiyar RK (1994) Embryogenesis in isolated microspore cultures of Indian mustard (Brassica juncea L.). Plant Tissue Cult 4:117–121
Kim SK, Chang SC, Lee EJ, Chung W-S, Kim Y-S, Hwang S, Lee JS (2000) Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol 123:997–1004
Kott LS, Polsoni L, Beversdorf WD (1988a) Cytological aspects of isolated microspore culture of Brassica napus. Can J Bot 66:1658–1664
Kott LS, Polsoni L, Ellis B, Beversdorf WD (1988b) Autotoxicity in isolated microspore cultures of Brassica napus. Can J Bot 66:1665–1670
Lanteri S, Portis E, Bergervoet HW, Groot SPC (2000) Molecular markers for the priming of pepper seeds (Capsicum annum L.). J Hort Sci Biotechnol 75:607–611
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
Lichter R (1989) Efficient yield of embryoids by culture of isolated microspores of different Brassicaceae species. Plant Breed 103:119–123
Lionneton E, Beuret W, Delaitre C, Ochatt S, Rancillac M (2001) Improved microspore culture and doubled-haploid plant regeneration in the brown condiment mustard (Brassica juncea). Plant Cell Rep 20:126–130
Liu CM, Xu ZH, Chua NH (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5:621–630
MacRae DH, Bonner J (1953) Chemical structure and antiauxin activity. Physiol Plant 6:485–510
Malik MR, Rangaswamy NS, Shivanna KR (2001) Induction of microspore embryos in a CMS line of Brassica juncea and formation of the androgenic plantlets. Euphytica 120:195–203
Michalczuk L, Cooke TJ, Cohen JD (1992) Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry 31:1097–1103
Pechan PM, Bartels D, Brown DCW, Schell J (1991) Messenger-RNA and protein changes associated with induction of Brassica microspore embryogenesis. Planta 184:161–165
Purnima, Rawat S (1997) Regeneration of Brassica juncea plants from in vitro microspore culture. Cruciferae Newslett 19:47–48
Senaratna T, Kott L, Beversdorf WD, McKersie BC (1991) Desiccation of microspore derived embryos of oilseed rape (Brassica napus L.). Plant Cell Rep 10:342–344
Sharma KK, Bhojwani SS (1985) Microspore embryogenesis in anther cultures of two Indian cultivars of Brassica juncea (L.) Czern. Plant Cell Tissue Organ Cult 4:235–239
Stead AD (1992) Pollination induced flower senescence: a review. Plant Growth Regul 11:13–20
Swanson EB, Herrgesell MJ, Arnoldo M, Sippell D, Wong RSC (1989) Microspore mutagenesis and selection: Canola plants with field tolerance to the imidazolinones. Theor Appl Genet 78:525–530
Takahata Y, Brown DCW, Keller WA, Kaizuma N (1993) Dry artificial seeds and desiccation tolerance induction in microspore derived embryos of broccoli. Plant Cell Tissue Organ Cult 35:121–129
Telmer CA, Newcomb W, Simmonds DH (1993) Microspore development in Brassica napus and the effect of high temperature on division in vivo and in vitro. Protoplasma 172:154–165
Telmer CA, Newcomb W, Simmonds DH (1995) Cellular changes during heat shock induction and embryo development of cultured microspores of Brassica napus cv. Topas. Protoplasma 185:106–112
Telmer CA, Simmonds DH, Newcomb W (1992) Determination of developmental stage to obtain high frequencies of embryogenic microspores in Brassica napus. Physiol Plant 84:417–424
Wakui K, Takahata Y, Kaizuma N (1994) Effect of abscisic acid and high osmoticum concentration on the induction of desiccation tolerance in microspore derived embryos of Chinese cabbage (Brassica campestris L.). Breed Sci 44:29–34
Wong RSC, Zee SY, Swanson EB (1996) Isolated microspore culture of Chinese flowering cabbage (Brassica campestris ssp. para chinensis). Plant Cell Rep 15:396–400
Xie Q, Frugis G, Colgan D, Chua NH (2000) Arabidopsis NAC1 transduced auxin signal downstream of TIR1 to promote lateral root development. Genes Dev 14:3024–3036
Zaki M, Dickinson H (1995) Modification of cell development in vitro: the effect of colchicine on anther and isolated microspore culture in Brassica napus. Plant Cell Tissue Organ Cult 40:255–270
Zhang GQ, Zhang DQ, Tang GX, He Y, Zhou WJ (2006) Plant development from microspore-derived embryos in oilseed rape as affected by chilling, desiccation and cotyledon excision. Biol Plant 50:180–186
Zhao JP, Simmonds DH, Newcomb W (1996) High frequency production of doubled haploid plants of Brassica napus cv. Topas derived from colchicine-induced embryogenesis without heat shock. Plant Cell Rep 15:668–671
Zhou WJ, Hagberg P, Tang GX (2002a) Increasing embryogenesis and doubling efficiency by immediate colchicine treatment of isolated microspores in spring Brassica napus. Euphytica 128:27–34
Zhou WJ, Tang GX, Hagberg P (2002b) Efficient production of doubled haploid plants by immediate colchicine treatment of isolated microspores in winter Brassica napus. Plant Growth Regul 37:185–192
Zhou YM, Scarch Y (1995) Microspore culture of hybrids between Brassica napus and B. campestris. Acta Bot Sin 37:848–855
Acknowledgement
The authors thank European commission for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agarwal, P.K., Agarwal, P., Custers, J.B.M. et al. PCIB an Antiauxin Enhances Microspore Embryogenesis in Microspore Culture of Brassica juncea . Plant Cell Tiss Organ Cult 86, 201–210 (2006). https://doi.org/10.1007/s11240-006-9108-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-006-9108-0