Summary
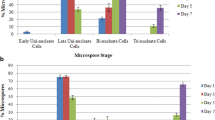
Brassica napus cv. Topas microspores isolated and cultured near the first pollen mitosis and subjected to a heat treatment develop into haploid embryos at a frequency of about 20%. In order to obtain a greater understanding of the induction process and embryogenesis, transmission electron microscopy was used to study the development of pollen from the mid-uninucleate to the bicellular microspore stage. The effect of 24 h of high temperature (32.5 °C) on microspore development was examined by heat treating microspore cultures or entire plants. Mid-uninucleate microspores contained small vacuoles. Late-uninucleate vacuolate microspores contained a large vacuole. The large vacuole of the vacuolate stage was fragmented into numerous small vacuoles in the late-uninucleate stage. The late-uninucleate stage contained an increased number of ribosomes, a pollen coat covering the exine and a laterally positioned nucleus. Prior to the first pollen mitosis the nucleus of the lateuninucleate microspore appeared to be appressed to the plasma membrane; numerous perinuclear microtubules were observed. Microspores developing into pollen divided asymmetrically to form a large vegetative cell with amyloplasts and a small generative cell without plastids. The cells were separated by a lens-shaped cell wall which later diminished. At the late-bicellular stage the generative cell was observed within the vegetative cell. Starch and lipid reserves were present in the vegetative cell and the rough endoplasmic reticulum and Golgi were abundant. The microspore isolation procedure removed the pollen coat, but did not redistribute or alter the morphology of the organelles. Microspores cultured at 25 °C for 24 h resembled late-bicellular microspores except more starch and a thicker intine were present. A more equal division of microspores occurred during the 24 h heat treatment (32.5 °C) of the entire plant or of cultures. A planar wall separated the cells of the bicellular microspores. Both daughter cells contained plastids and the nuclei were of similar size. Cultured embryogenie microspores contained electron-dense deposits at the plasma membrane/cell wall interface, vesicle-like structures in the cell walls and organelle-free regions in the cytoplasm. The results are related to embryogenesis and a possible mechanism of induction is discussed.
Similar content being viewed by others
Abbreviations
- B:
-
binucleate
- LU:
-
late uninucleate
- LUV:
-
late uninucleate vacuolate
- M:
-
mitotic
- MU:
-
mid-uninucleate
- RER:
-
rough endoplasmic reticulum
- TEM:
-
transmission electron micrograph
References
Evans DE, Taylor PE, Singh MB, Knox RB (1992) The interrelationship between the accumulation of lipids, protein and the level of acyl carrier protein during the development ofBrassica napus L. pollen. Planta 186: 343–354
Fan Z, Armstrong KC, Keller WA (1988) Development of microspores in vivo and in vitro inBrassica napus L. Protoplasma 147: 191–199
Fife CA, Newcomb W, Lefebvre DL (1990) The effect of phosphate deprivation on protein synthesis and fixed carbon storage reserves inBrassica nigra suspension cells. Can J Bot 68: 1840–1847
Fry SC (1990) Roles of primary cell wall in morphogenesis. In: Ninkamp HJJ, van der Plas LHW, van Aartrijk J (eds) Progress in plant cellular and molecular biology. Kluwer, Dordrecht, pp 504–513
Grant I, Beversdorf WD, Peterson RL (1986) A comparative light and electron microscopic study of microspore and tapetal development in male fertile and cytoplasmic male sterile oilseed rape (Brassica napus). Can J Bot 64: 1055–1068
Gunning BES, Steer MW (1975) Plant cell biology and ultrastructural approach. Edward Arnold, London
Heberle-Bors E (1989) Isolated pollen culture in tobacco: plant reproductive development in a nutshell. Sex Plant Reprod 2: 1–10
Kapil RN, Bhatnagar AK (1981) Female gametophyte in flowering plants. Int Rev Cytol. 291–341
Kott LS, Polsoni L, Beversdorf WD (1988) Cytological aspects of isolated microspore culture ofBrassica napus. Can J Bot 66: 1658–1664
Kutschera U, Bergfeld R, Schopfer P (1987) Cooperation of epidermis and inner tissues in auxin-mediated growth of maize coleoptiles. Planta 170: 168–180
Mascarenhas JP (1989) The male gametophyte of flowering plants. Plant Cell 1: 657–664
Mineyuki Y, Palevitz BA (1990) Relationship between preprophase band organization. F-actin and the division site inAllium. J Cell Sci 97: 283–295
Murgia M, Detchepare S, Van Went JL, Cresti M (1991)Brassica napus pollen development during generative cell and sperm cell formation. Sex Plant Reprod 4: 176–181
Palevitz BA (1987) Actin in the preprophase band ofAllium cepa. J Cell Biol 104: 1515–1519
Pechan PM, Keller WA (1988) Identification of potentially embryogenie microspores ofBrassica napus L. Physiol Plant 74: 377–384
—, Bartels D, Brown DCW, Schell J (1991) Messenger-RNA and protein changes associated with induction ofBrassica microspore embryogenesis. Planta 184: 161–165
Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K (1991) Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3: 1317–1326
Polowick PI, Sawhney VK (1990) Microsporogenesis in a normal line and in theogu cytoplasmic male-sterile line ofBrassica napus I. The influence of high temperature. Sex Plant Reprod 3: 263–276
Sangwan RS, Sangwan-Norreel BS (1987) Ultrastructural cytology of plastids in pollen grains of certain androgenic and nonandrogenic plants. Protoplasma 138: 11–22
Scott R, Dagless E, Hodge R, Paul W, Soufleri I, Draper J (1991) Patterns of gene expression in developing anthers ofBrassica napus. Plant Mol Biol 17: 195–207
Simmonds DH, Gervais C, Keller WA (1991) Embryogenesis from microspores of embryogenie and non-embryogenic lines ofBrassica napus. In: McGregor DI (ed) Proceedings GCIRC 8th International Rapeseed Congress, Saskatoon, Sask, Canada. Groupe Consultatif International de Recherche sur la Colza (GCIRC) and the Canola Council of Canada, pp 306–311
Tanaka I, Ito M (1981) Control of division patterns in explanted microspores ofTulipa gesneriana. Protoplasma 108: 329–340
Telmer CA, Simmonds DH, Newcomb W (1992) Determination of developmental stage to obtain high frequencies of embryogenie microspores inBrassica napus. Physiol Plant 84: 417–424
Terasaka O, Niitsu T (1990) Unequal cell division and chromatin differentiation in pollen grain cells II. Microtubule dynamics associated with the unequal cell division. Bot Mag Tokyo 103: 133–142
Van Lammeren AAM, Keijzer CJ, Willemse MTM, Kieft H (1985) Structure and function of the microtubular cytoskeleton during pollen development inGasteria verrucosa (Mill.) H. Duval. Planta 165: 1–11
Venable JH, Coggleshall R (1965) A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25: 407–408
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42: 579–620
Zaki MAM, Dickinson HG (1990) Structural changes during the first divisions of embryos resulting from anther and free microspore culture inBrassica napus. Protoplasma 156: 149–162
— — (1991) Microspore-derived embryos inBrassica: the significance of division symmetry in pollen mitosis I to embryogenie development. Sex Plant Reprod 4: 48–55
Zimmerman JL, Apuya N, Darwish K, O'Carroll C (1989) Novel regulation of heat shock genes during carrot somatic embryo development. Plant Cell 1: 1137–1146
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Telmer, C.A., Newcomb, W. & Simmonds, D.H. Microspore development inBrassica napus and the effect of high temperature on division in vivo and in vitro. Protoplasma 172, 154–165 (1993). https://doi.org/10.1007/BF01379373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01379373