Abstract
Source preference in which equally distributed risks may be valued differently has been receiving increasing attention. Using subjects recruited in Berkeley, Fox and Tversky (1995) demonstrate a familiarity bias in source preference—betting on a less than even-chance event based on San Francisco temperature is valued more than betting on a better than even-chance event based on Istanbul temperature. Neophobia is associated with the amygdala which is GABA-rich and is known to be modulated by benzodiazepines as anxiolytic agents that enhance the activity of the GABAA receptor in processing anxiety and fear. This leads to our hypothesis that familiarity bias in decision making may be explained by polymorphic variations in this receptor mediated by anxiety regulation in the amygdala. In two companion studies involving Beijing-based subjects, we examine 10 single nucleotide polymorphisms (SNPs) of GABRB2 (coding for GABAA receptor, beta 2 subunit) and find 7 SNPs each showing negative association between familiarity bias—preference for betting on parity of Beijing temperature over Tokyo temperature—and having at least one minor allele (less than 50% prevalence). In an imaging genetics study of a subsample of subjects based on the SNP with the most balanced allelic distribution, we find that subjects’ familiarity bias in terms of risk aversion towards bets on the parity of the temperature of 20 Chinese cities is negatively associated with their post-scanning familiarity ratings of the cities only for those with no minor allele in this SNP. Moreover, familiarity bias is positively associated with activation in the right amygdala along with the brain’s attention networks. Overall, our findings help discriminate between ambiguity aversion and familiarity bias in source preference and supports our gene–brain–behavior hypothesis of GABAergic modulation of amygdala activation in response to familiarity towards the source of uncertainty.






Similar content being viewed by others
Notes
Subjects each completed a five-minute survey (including unrelated items) and received a California lottery ticket.
Source preference is closely related to Heath and Tversky’s (1991) competence hypothesis in which betting on one’s own judgment—holding judged probability constant—when they consider themselves knowledgeable is preferred and that our feeling of competence is enhanced by general knowledge, familiarity, and experience. Tversky and Kahneman (1992) followed by Tversky and Wakker (1995) made use of cumulative prospect theory to describe this phenomenon.
Chew et al. (2008) investigate familiarity bias in a fMRI study using parity of the trailing digit of the price of a stock to construct even-chance bets of varying levels of familiarity and extend the earlier finding from ambiguity aversion to familiarity bias.
Chew, Ebstein, and Zhong (2012) find associations between ambiguity aversion and familiarity bias with candidate serotonin genes related to anxiety and fear.
We note that insects, animals, and people often show the opposite trait, neophilia. Indeed, novelty seeking, openness to experience, and neophilia along with neophobia, are all human personality traits that clearly impact economic behavior (see, e.g., Zhao and Seibert, 2006).
While Tokyo as a label is known to the Chinese subjects, the city remains foreign and unfamiliar, not unlike the case of Istanbul to the Berkeley subjects in Fox and Tversky (1995).
Subjects decide whether to bet against an opponent. Should their choices of color match, both receive the certainty payoff. Otherwise, the subject wins only if his or her choice of color is realized. In the case of an informed opponent, subjects are disadvantaged by betting against an opponent who can see a sample of up to nine cards (with replacement) before choosing his or her color.
GABRB2 contains single-nucleotide polymorphisms (SNPs) in non-coding sequences in the intron 8—intron 9 region that have been shown to be quantitatively correlated with mRNA expression and alternative splicing.
Trautmann and Van De Kuilen (2015) offer a thorough review of the experimental literature on ambiguity aversion which also touches on source preference though not familiarity bias particularly.
Contemporaneously, Coval and Moskowitz (1999) found that people invest disproportionately more in geographically proximate firms within the US.
In this regard, it is notable that the neuropeptide S (NPS) system which plays an important role in fear and fear memory processing has become hypofunctional in the course of human evolution (Reinscheid, Mafessoni, Lüttjohann, Jüngling, Pape, and Schulz (2021)). Moreover, neuropeptide S mediated fear and its extinction is dependent on intercalated GABA neurons in the amygdala (Jüngling, Seidenbecher, Sosulina, Lesting, Sangha, Clark, Okamura, Duangdao, Xu, and Reinscheid (2008)).
References
Abdellaoui, M., Baillon, A., Placido, L., & Wakker, P. P. (2011). The rich domain of uncertainty: Source functions and their experimental implementation. American Economic Review, 101, 695–723.
Amaral, D. G. (2002). The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biological Psychiatry, 51, 11–17.
Arce, E., Miller, D. A., Feinstein, J. S., Stein, M. B., & Paulus, M. P. (2006). Lorazepam dose-dependently decreases risk-taking related activation in limbic areas. Psychopharmacology (berl), 189, 105–116.
Asplund, C. L., Todd, J. J., Snyder, A. P., & Marois, R. (2010). A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience, 13, 507–512.
Aupperle, R. L., Ravindran, L., Tankersley, D., Flagan, T., Stein, N. R., Simmons, A. N., Stein, M. B., & Paulus, M. P. (2011). Pregabalin influences insula and amygdala activation during anticipation of emotional images. Neuropsychopharmacology, 36, 1466–1477.
Bach, D. R., Hulme, O., Penny, W. D., & Dolan, R. J. (2011). The known unknowns: Neural representation of second-order uncertainty, and ambiguity. Journal of Neuroscience, 30, 4811–4820.
Bach, D. R., Seymour, B., & Dolan, R. J. (2009). Neural activity associated with the passive prediction of ambiguity and risk for aversive events. Journal of Neuroscience, 29, 1648–1656.
Barrett, J. C., Fry, B., Maller, J., & Daly, M. J. (2005). Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics, 21, 263–265.
Bechara, A., & Damasio, A. R. (2005). The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior, 52, 336–372.
Behrmann, M., Geng, J. J., & Shomstein, S. (2004). Parietal cortex and attention. Current Opinion in Neurobiology, 14, 212–217.
Benjamin, D. J., Cesarini, D., van der Loos, M. J. H. M., Dawes, C. T., Koellinger, P. D., Magnusson, P. K. E., et al. (2012). The genetic architecture of economic and political preferences. PNAS, 109(21), 8026–8031.
Blasi, G., Lo Bianco, L., Taurisano, P., Gelao, B., Romano, R., Fazio, L., Papazacharias, A., Di Giorgio, A., Caforio, G., & Rampino, A. (2009). Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. Journal of Neuroscience, 29, 14812–14819.
Bocchio, M., Nabavi, S., & Capogna, M. (2017). Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron, 94, 731–743.
Brett, M., Anton, J.-L., Valabregue, R., & Poline, J.-B. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. NeuroImage, 16, S497.
Breuer, R., Hamshere, M. L., Strohmaier, J., Mattheisen, M., Degenhardt, F., Meier, S., Paul, T., O’Donovan, M. C., Mühleisen, T. W., & Schulze, T. G. (2011). Independent evidence for the selective influence of GABA A receptors on one component of the bipolar disorder phenotype. Molecular Psychiatry, 16, 587–589.
Broniarczyk, S. M., & Alba, J. W. (1994). The importance of the brand in brand extension. Journal of Marketing Research, 31, 214–228.
Bzdok, D., Laird, A. R., Zilles, K., Fox, P. T., & Eickhoff, S. B. (2013). An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping, 34, 3247–3266.
Camerer, C. F., Dreber, A., Forsell, E., Ho, T.-H., Huber, J., Johannesson, M., Kirchler, M., Almenberg, J., Altmejd, A., & Chan, T. (2016). Evaluating replicability of laboratory experiments in economics. Science, 351, 1433–1436.
Campbell, M. C., & Keller, K. L. (2003). Brand familiarity and advertising repetition effects. Journal of Consumer Research, 30, 292–304.
Chabris, C. F., Lee, J. J., Cesarini, D., Benjamin, D. J., & Laibson, D. I. (2015). The fourth law of behavior genetics. Current Directions in Psychological Science, 24, 304–312.
Chen, Y. and Zhong, S. (2021): “Source Preference in Gift Exchange,” Working paper2.
Chew, S. H., K. K. Li, R. Chark, and S. Zhong. (2008): Source Preference and Ambiguity Aversion: Models and Evidence from Behavioral and Neuroimaging Experiments, in Neuroeconomics, Emerald Group Publishing Limited.
Chew, S. H., K. K. Li, and J. S. Sagi. (2021): Experimental Evidence of Source Preference: Familiarity and Home Bias, Available at SSRN 3870716.
Chew, S. H., Ebstein, R. P., Zhong, S., Hong, C. S., Ebstein, R. P., & Songfa, Z. (2012). Ambiguity aversion and familiarity bias: Evidence from behavioral and gene association studies. Journal of Risk and Uncertainty, 44, 1–18.
Chew, S. H., & Sagi, J. S. (2008). Small worlds: Modeling attitudes toward sources of uncertainty. Journal of Economic Theory, 139, 1–24.
Christopoulos, G. I., Tobler, P. N., Bossaerts, P., Dolan, R. J., & Schultz, W. (2009). Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience, 29, 12574–12583.
Cronqvist, H., and S. Siegel. (2012): Why do individuals exhibit investment biases, Claremont McKenna College Robert Day School of Economics and Finance Research Paper, 1.
Cronqvist, H., Münkel, F., & Siegel, S. (2014). Genetics, homeownership, and home location choice. The Journal of Real Estate Finance and Economics, 48, 79–111.
Damasio, A. R. (1994). Descartes’ error: Emotion, rationality and the human brain. Grosset/Putnam.
Dammhahn, M., & Almeling, L. (2012). Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Animal Behaviour, 84, 1131–1139.
Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35, 105–135.
Davis, M., & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13–34.
Deakin, J. B., Aitken, M. R. F., Dowson, J. H., Robbins, T. W., & Sahakian, B. J. (2004). Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (berl), 173, 88–97.
Ellsberg, D. (1961): Risk, ambiguity, and the Savage axioms, The quarterly journal of economics, 643–69.
Fecteau, S., Knoch, D., Fregni, F., Sultani, N., Boggio, P., & Pascual-Leone, A. (2007). Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: A direct current stimulation study. Journal of Neuroscience, 27, 12500–12505.
Forrest, J., & Thomson, J. D. (2009). Pollinator experience, neophobia and the evolution of flowering time. Proceedings of the Royal Society b: Biological Sciences, 276, 935–943.
Fox, C. R., & Tversky, A. (1995). Ambiguity aversion and comparative ignorance. Quarterly Journal of Economics, 110, 585–603.
French, K. R., & Poterba, J. M. (1991). Investor diversification and international equity markets. The American Economic Review, 81, 222–226.
Galef, B. G. (1987). Social influences on the identification of toxic foods by Norway rats. Animal Learning & Behavior, 15, 327–332.
Gospic, K., E. Mohlin, P. Fransson, P. Petrovic, M. Johannesson, and M. Ingvar. (2011): Limbic justice—amygdala involvement in immediate rejection in the ultimatum game, PLoS Biology, 9, e1001054.
Gray, J. A., Mellanby, J., & Buckland, C. (1984). Behavioural studies of the role of GABA in anxiolytic drug action. Neuropharmacology, 23, 827.
Greco, V., & Roger, D. (2003). Uncertainty, stress, and health. Personality & Individual Differences, 34, 1057–1068.
Haefly, M., Schoch, P., Martin, J. R., Bonetti, E. P., Moreau, J. L., Jenck, F., & Richards, J. G. (1992). Partial agonists of benzodiazepine receptors for the treatment of epilepsy, sleep, and anxiety disorders. Advances in Biochemical Psychopharmacology, 47, 379–394.
Hariri, A. R., & Weinberger, D. R. (2003). Imaging Genomics. British Medical Bulletin, 65, 259–270.
Hsu, M., Bhatt, M., Adolphs, R., Tranel, D., & Camerer, C. F. (2005). Neural systems responding to degrees of uncertainty in human decision-making. Science, 310, 1680–1683.
Huberman, G. (2001). Familiarity breeds investment. The Review of Financial Studies, 14, 659–680.
Huettel, S. A., Stowe, C. J., Gordon, E. M., Warner, B. T., & Platt, M. L. (2006). Neural signatures of economic preferences for risk and ambiguity. Neuron, 49, 765–775.
Jakobsdottir, J., M.B. Gorin, Y.P. Conley, R.E. Ferrell, and D.E. Weeks. (2009), Interpretation of genetic association studies: markers with replicated highly significant odds ratios may be poor classifiers, PLoS Genetics, 5, e1000337.
Kalueff, A., & Nutt, D. J. (1996). Role of GABA in memory and anxiety. Depression and Anxiety, 4, 100–110.
Kastner, S., & Ungerleider, L. G. (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–341.
Kent, R. J., & Allen, C. T. (1994). Competitive interference effects in consumer memory for advertising: The role of brand familiarity. Journal of Marketing, 58, 97–105.
Khaw, M. W., Z. Li, and M. Woodford. (2017): Risk Aversion as a Perceptual Bias, NBER Working Paper Series.
Knoch, D., Gianotti, L. R. R., Pascual-Leone, A., Treyer, V., Regard, M., Hohmann, M., & Brugger, P. (2006). Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. Journal of Neuroscience, 26, 6469–6472.
Lane, S. D., E. Yechiam, and J. R. Busemeyer. (2006): Application of a computational decision model to examine acute drug effects on human risk taking., Experimental and Clinical Psychopharmacology, 14, 254.
Lane, S. D., Tcheremissine, O. V., Lieving, L. M., Nouvion, S., & Cherek, D. R. (2005). Acute effects of alprazolam on risky decision making in humans. Psychopharmacology (berl), 181, 364–373.
LeDoux, J. E. (1996). Emotion: Clues from the brain. Annual Review of Psychology, 46, 209–235.
LeDoux, J. E. (2013). The slippery slope of fear. Trends in Cognitive Sciences, 17, 155–156.
Levy, I., Snell, J., Nelson, A. J., Rustichini, A., & Glimcher, P. W. (2010). Neural representation of subjective value under risk and ambiguity. Journal of Neurophysiology, 103, 1036–1047.
Lewis, D. A., & Gonzalez-Burgos, G. (2006). Pathophysiologically based treatment interventions in schizophrenia. Nature Medicine, 12, 1016–1022.
Lo, W. S., Lau, C. F., Xuan, Z., Chan, C. F., Feng, G. Y., He, L., Cao, Z. C., Liu, H., Luan, Q. M., & Xue, H. (2004). Association of SNPs and haplotypes in GABA A receptor β 2 gene with schizophrenia. Molecular Psychiatry, 9, 603–608.
Lo, W.-S., Xu, Z., Yu, Z., Pun, F. W., Ng, S.-K., Chen, J., Tong, K.-L., Zhao, C., Xu, X., & Tsang, S.-Y. (2007). Positive selection within the schizophrenia-associated GABAA receptor β2 gene. PLoS ONE, 2, e462.
Loewenstein, G. F., E. U. Weber, C. K. Hsee, and N. Welch. (2001): Risk as feelings., Psychological bulletin, 127, 267.
Meyer-Lindenberg, A., B. Kolachana, B. Gold, A. Olsh, K. K. Nicodemus, V. Mattay, M. Dean, and D. R. Weinberger. (2009): Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans, Molecular Psychiatry.
Muller, H., Grossman, H., & Chittka, L. (2010). ‘Personality’ in bumblebees: Individual consistency in responses to novel colours? Animal Behaviour, 80(6), 1065–1074.
Muthukrishnan, A. V., Wathieu, L., & Xu, A. J. (2009). Ambiguity aversion and the preference for established brands. Management Science, 55, 1933–1941.
Obstfeld, M., & Rogoff, K. (2000). The six major puzzles in international macroeconomics: Is there a common cause? NBER Macroeconomics Annual, 15, 339–390.
Okbay, A., & Benjamin, D. (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533, 539–542.
Parr, T., & Friston, K. J. (2019). Attention or salience? Current Opinion in Psychology, 29, 1–5.
Paulus, M. P., Feinstein, J. S., Castillo, G., Simmons, A. N., & Stein, M. B. (2005). Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry, 62, 282–288.
Posner, M. I., & Petersen, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42.
Rasch, B., Spalek, K., Buholzer, S., Luechinger, R., Boesiger, P., Papassotiropoulos, A., & de Quervain, D.-F. (2009). A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proceedings of the National Academy of Sciences, 106, 19191–19196.
Roiser, J. P., de Martino, B., Tan, G. C. Y., Kumaran, D., Seymour, B., Wood, N. W., & Dolan, R. J. (2009). A genetically mediated bias in decision making driven by failure of amygdala control. Journal of Neuroscience, 29, 5985–5991.
Schultze, T., Huber, J., Kirchler, M., & Mojzisch, A. (2019). Replications in economic psychology and behavioral economics. Journal of Economic Psychology, 75(5), 102199.
Schwarz, N., and G. L. Clore. (1983): Mood, misattribution, and judgments of well-being: informative and directive functions of affective states., Journal of personality and social psychology, 45, 513.
Shackman, A. J., McMenamin, B. W., Maxwell, J. S., Greischar, L. L., & Davidson, R. J. (2009). Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science, 20, 1500–1506.
Tellis, G. J. (1988). Advertising exposure, loyalty, and brand purchase: A two-stage model of choice. Journal of Marketing Research, 25, 134–144.
Tversky, A., & Kahneman, D. (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty, 5, 297–323.
Tversky, A., & Wakker, P. (1996). Risk attitudes and decision weights. Econometrica, 63, 1255–1280.
Vuilleumier, P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–594.
Vuilleumier, P., & Huang, Y.-M. (2009). Emotional attention: Uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Science, 18, 148–152.
Weaver, M., Hutton, P., & McGraw, K. J. (2019). Urban house finches (Haemorhous mexicanus) are less averse to novel noises, but not other novel environmental stimuli, than rural birds. Behaviour, 156, 1393–1417.
Whalen, P. J. (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–188.
Williams, M. A., McGlone, F., Abbott, D. F., & Mattingley, J. B. (2005). Differential amygdala responses to happy and fearful facial expressions depend on selective attention. NeuroImage, 24, 417–425.
Zajonc, R. B. (1968): Attitudinal effects of mere exposure., Journal of personality and social psychology, 9, 1.
Zhao, C., Xu, Z., Chen, J., Yu, Z., Tong, K. L., Lo, W. S., Pun, F. W., Ng, S. K., Tsang, S. Y., & Xue, H. (2006). Two isoforms of GABA A receptor β 2 subunit with different electrophysiological properties: Differential expression and genotypical correlations in schizophrenia. Molecular Psychiatry, 11, 1092–1105.
Zhao, C., Xu, Z., Wang, F., Chen, J., Ng, S. L., Wong, P. W., Yu, Z., et al. (2009). Alternative-splicing in the exon-10 region of gabaa receptor β2 subunit gene: relationships between novel isoforms and psychotic disorders. PLoS ONE, 4, e6977.
Zhong, S., Israel, S., Xue, H., Sham, P. C., Ebstein, R. P., & Chew, S. H. (2009). A neurochemical approach to valuation sensitivity over gains and losses. Proceedings of the Royal Society b: Biological Sciences, 276, 4181–4188.
Zhou, M., Liu, Z., Melin, M. D., Ng, Y. H., Xu, W., & Südhof, T. C. (2018). A central amygdala to zona incerta projection is required for acquisition and remote recall of conditioned fear memory. Nature Neuroscience, 21, 1515–1519.
Acknowledgment
This work was funded by Hong Kong University of Science and Technology. We acknowledge the partial support of the National Science Foundation of China (NSFC) under key project (No. 72033006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Materials and methods
1.1.1 Study 1
Participants 350 participants were recruited in Beijing through internet, posters, and word of mouth. The first group (123 non-student participants) was recruited in July 2007 and the second group (227 student participants) was recruited in February 2008. Demographics of the participants are summarized as follows: mean age 28.2 ± 10.8 (s.d.); 162 males; 67 participants with high school education, 194 participants with college education, 89 participants with postgraduate education; 325 Han Chinese, 25 non-Han Chinese. Only the 325 individuals of Han Chinese descent (150 males) were included in analysis for Study 1 to have a better control of population (see Table 3 for other demographic information). This study was approved by the Internal Review Board of the Hong Kong University of Science and Technology (HKUST). Prior to running the experiment, participants were each given a written informed consent form for donation of blood samples and for participation in the behavioral experiment. Subsequently, participants participated in the behavioral experiment as described below. After the experiment, participants donated 10 ml of blood each for genotyping, taken by nurses and doctors from hospitals in Beijing (Tables 4, 5, 6, 7,8, 9, 10,11, 12, 13, 14, 15, 16, 17,18,19 and 20).
Experimental paradigm We adapted the Fox and Tversky’s paradigm on familiarity bias. In Fox and Tversky’s original study, their participants, recruited in Berkeley, California, were asked to value four lotteries based on the next day’s temperature of San Francisco and Istanbul are above 60 degrees Fahrenheit or not. For each city, one lottery pays $100 when the next day temperature is above and $0 otherwise. The other is the exact opposite paying $100 when the next day temperature is not above 60 degrees Fahrenheit and $0 otherwise. In this setting, valuing a bet more than its opposite suggests that the subjective likelihood of the underlying event exceeds ½. In most models of decision making, the valuation of a lottery is fully determined by its payoffs and associated probabilities. As such, when comparing two binary lotteries with the same payoffs, the one with a higher likelihood of delivering the higher payoff will be valued more. Fox and Tversky reported the paradoxical finding that the higher valued of the Istanbul bets was worth less than the lower valued of the San Francisco bets. This suggests that their subjects have an intrinsic preference for betting on the temperature of the more familiar city of San Francisco than on the less familiar city of Istanbul. In our experiment, participants chose between bets on whether the temperature at a specific historical day of Beijing vs. Tokyo would be odd or even (see below for instructions). This procedure induces the same unambiguous probability of one half for odd versus even regardless of the city chosen.
Candidate gene GABA is the major inhibitory neurotransmitter in the vertebrate nervous system, where a fast synaptic inhibition is mediated by the opening of a chloride channel formed by the GABAA receptor. Subtypes of GABAA receptors are assembled from pentameric combinations of eight classes of subunits. Among them, β1, β2 and β3 subunits constitute the principal components of most native GABAA receptors. GABAergic neurons contribute to the orchestration of pyramidal neuron firing and GABAergic cortical deficits are implicated in neurodevelopmental abnormality and neuropsychiatric disease pathophysiology, including that of schizophrenia (Lo et al., 2004) and bipolar disorder (Breuer et al., 2011). GABRB2, which encodes the β2 subunit of GABAA receptors, contains SNPs in non-coding sequences in the intron 8—intron 9 region that have been under positive selection (Lo et al., 2007) and quantitatively correlated with mRNA expression and alternative splicing. Variations across this gene may modulate traits in healthy participants. In particular, the minor allele of SNP rs1816072 correlated with reduced expression of short form which is less prone to current rundown and thus is less dependent on the ATP levels.
Genotyping To sequence the 3,551-bp segment flanked by SNPs rs6556547 and rs187269 in GABRB2, two genomic DNA regions were PCR-amplified to serve as first PCR templates for subsequent nested-PCR. Nested-PCR and SNP genotyping by resequencing of the nested PCR products were carried out as described in Lo et al. (2004) which classified the homozygous major (MM) participants as the Major Group and the rest (Mm + mm) as the Minor Group (Lo et al., 2007). When the odd-ratios of choosing the Beijing bet vs. the Tokyo bet is broken down by the three genotypes (MM, Mm and mm), six out of seven SNPs found to be significantly associated with familiarity bias also display this dominance pattern (see Table 5).
Analysis We adopted the case–control study approach commonly used in genetic association studies (Zhong et al., 2009). The effect of each of ten candidate SNPs on familiarity bias is evaluated by logistic regressions. For each SNP, the association is evaluated using logistic regression with the choice of the Beijing lottery as the dependent variable (i.e., the dependent variable equals one when participants choose the Beijing lottery and zero otherwise). The independent variable is the genotype which equals one when the participant carries the homozygous major genotype (M/M) at the SNP being examined and zero when the participant carries either the heterozygous (M/m) or homozygous minor (m/m) genotype. Logistic regressions with robust standard error are performed using Stata 11.0. Markers with the strongest effect on the phenotype examined are those accompanied by the smallest p values and most extreme odds ratios were tested for statistical association see Table S1).
Mutational load analysis We further examine if the effect of the seven SNPs found significantly individually (p < 0.05, uncorrected) display a dose-dependent manner by running another genetic load analysis. A logistic regression with the choice of Beijing as dependent variable and the instances of minor allele as a continuous, independent variable is performed (Barrett et al., 2005). This mutational load analysis reveals a statistically significant predisposition (z = 2.64, p < 0.008; see Fig. 1B and Table 6) for the degree of familiarity bias to decrease with an increased presence of minor alleles at the ten GABRB2 SNPs.
Instructions of Study 1—English translation
You have the following two options:
T1: Guess whether the high temperature recorded in Beijing on ____ 2008 was odd or even. You will receive RMB11 if your guess is correct; and nothing if your guess is wrong.
T2: Guess whether the high temperature recorded in Tokyo on ____ 2008 was odd or even. You will receive RMB13 if your guess is correct; and nothing if your guess is wrong.
Put a tick “√” to indicate your choice. Pick one lottery only. You will be paid based on your decision.

Study 2
Participants Study 2 was conducted in April 2009. From the 325 student participants analyzed in Study 1, we selected forty healthy and right-handed participants (16 males, age = 21.95 ± 2.75) for the present study. Participants were selected to accomplish an even distribution of the GABRB2 gene (see Table S5 for the distributions of genotypes). All participants had a bachelor or postgraduate degree or were in the process of obtaining one. Each participant’s informed consent was obtained via consent form approved by the Internal Review Board at the HKUST and the Beijing Normal University. Three participants (two are heterozygous and one is homozygous minor) were excluded from our analysis since there were no variations in their reported degrees of familiarity of the cities.
Candidate Gene Of the ten SNPs tested, SNP rs1816072 is chosen in our follow up imaging genetics analyses since it has the highest minor allele frequency (40.4%) and achieved second highest significance in its association with familiarity bias. The three genotypes at SNP rs1816072 were TT for the homozygous major, TC for the heterozygous, and CC for the homozygous minor. For our behavioral genetics and imaging genetics association analyses, subjects with TT was defined as being in the Major Group while those with TC or CC belong to the Minor Group who were found to display less familiarity bias. In this sample, the hypothesis that SNP rs1816072 is in Hardy–Weinberg Equilibrium is not rejected (p > 0.99). We also tested if the demographics of the participants (see Table 3) for a summary) are correlated with the genotype. Gender and education level are regressed against genotype separately using logistic regressions. Both are not significant (p > 0.5). A two-sample t-test with unequal variance also reveals that age does not differ across the two genotypes (p > 0.05).
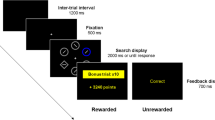
Design The experiment consists of three parts: instruction phase, scanning phase during which participants performed the task, and post-scan questionnaire. Participants familiarized themselves with the decision-making tasks during the instruction phase. The scanning phase lasted for about twenty minutes. There were 80 trials. In each trial (see the timeline of a trial in Fig. 2 in the main text), participant chose between an even chance lottery and receiving a specific amount of money for sure. The even chance lotteries were about a bet on the odd or even of the trailing digit of the temperature of one of the twenty cities on a date randomly drawn from the past twelve months. The prizes of a correct guess ranged between RMB150 and RMB250. An incorrect guess would yield RMB0. Each trial lasts for about 15 s, after a 1.5–2.5 s random jitter fixation, participant saw an even-chance gamble for 6 s (perceptual epoch) followed by another 1.5–2.5 s random second jitter before the participant saw an amount of money being displayed. At this point, the participant also made a decision within 5 s whether to take the lottery or to take a sure amount which ranged from 25 to 60% of the prize (decision epoch). At the time when they were completing the task in the scanner, no feedback about the outcome of the lotteries was given. Each participant received the payoff of one of the eighty decisions made. On top of the payoff from this particular decision, participants also received a show-up fee of RMB120. In the post-scan questionnaire, participants reported their degrees of familiarity towards the 20 cities on a scale of –5 (not familiar at all) to 5 (very familiar). Table 7 summarizes the familiarity ratings by the 37 participants.
Behavioral data analysis We take advantage of participants’ incentivized choices to pin down their differences in familiarity bias. Significantly, this design enables us to distinguish among individuals’ attitudes towards risk from their degree of familiarity bias.
We estimated the implied risk attitudes from the 80 choices made during the fMRI study with a power utility function \(u\left(x\right)={x}^{\alpha }\) where 1–α represents the Arrow–Pratt index of relative risk aversion (Khaw, Li, and Woodford, 2017). The decision maker exhibits risk aversion (neutrality, tolerance) when \(\alpha \) < (=, >) 1. To study familiarity bias, we use a source-dependent utility function (23) of the form, \(u\left(x\right)={x}^{\alpha+\gamma F}\) with its Arrow–Pratt index being dependent on the normalized familiarity rating F reported by the participants. The probability of choosing a lottery over a sure amount is given by Φ(\({\frac{1}{2}x}^{\alpha +\gamma F}\) –\({c}^{\alpha +\gamma F}\))], where Φ(·) is the logit cumulative density function. The MLE estimation is done in Stata 11.0 with program code developed in (Harrison and Rutstrom, 2008). Variances are clustered by participant.
To test for the effect of the SNP rs1816072 of GABRB2 on familiarity bias, we introduce a genotype dummy G. The gene-specific source-dependent power utility function now takes this form
where G equals one when the participant is classified as being homozygous major (MM, n = 15), and zero otherwise (Mm + mm, n = 22). Here, \({\alpha }_{0}\) captures the baseline risk aversion inferred from the lottery of the least unfamiliar city for the Minor Group (TC + CC) and \({\gamma }_{0}\) is the corresponding measure of sensitivity to familiarity representing the decrease in relative risk aversion in response to a point increase in normalized familiarity. Thus, \({\alpha }_{1}\) and \({\gamma }_{1}\) pick up the marginal effect of being in the Major Group of SNP rs1816072 on baseline degree of risk aversion and sensitivity towards familiarity respectively. Alternatively, these dummies may be interpreted as a way to stratify the risk attitude and the behavioral sensitivity to familiarity. We expect α1 not to be different from zero since the risk attitudes of the major and minor groups are expected to be similar when they have the same degree of familiarity bias while \({\gamma }_{1}\) is expected to be significantly greater than zero. To generate individual estimates of the parameter of behavioral sensitivity to familiarity (\({\gamma }_{1}\)), we run a fixed effects model in which both parameters in the group level model, \(u\left(x\right)={x}^{\alpha+\gamma F}\), are individual specific.
Results In the first estimation, we find that \(\alpha \) = 0.7426 (p < 0.0001) and \(\gamma \) = − 0.0018 (p > 0.4). Thus there is no support for familiarity bias for the group as a whole. When the dummy variable G is introduced, we find that \({\alpha }_{0}\) = 0.7560 (p < 0.0001) and \({\gamma }_{0}\) = − 0.0065 (p > 0.1), \({\alpha }_{1}\) = − 0.0351 (p > 0.3) and \({\gamma }_{1}\) = 0.0107 (p < 0.03). These estimates show that participants are on average risk averse and their degrees of risk aversion are consistent with past reports (Tversky & Kahneman, 1992). Consistent with the notion of risk attitude and familiarity bias being distinct, we find that a single GABRB2 SNP rs1816072 stratifies people in their degrees of familiarity bias but not in risk aversion.
fMRI Data Acquisition fMRI was performed on a Siemens 3 T MRI at the Beijing Normal University, BJ, PRC, with an 8 channel quadrature birdcage head coil. A sagittal spin echo localizer image was acquired initially. fMRI was performed in the transverse plane, parallel to the anterior–posterior commissure (AC-PC) line. A 35-slice set of fMRI images was acquired with the following scan parameters: TR = 2000 ms; TE = 30 ms; flip angle = 90°; matrix = 64 × 64; field of view = 22 cm × 22 cm; functional 3.4 × 3.4 × 4 mm3 voxels, without inter-slice gap. Anatomical whole brain MRI was acquired using a T1-weighted turbo spin echo (TSE) sequence with TR 2000 ms and TE 10 ms with IR delay 800 ms. Around 600 fMRI volume images were collected during each run. The first four fMRI volume images of each run were discarded to insure steady state magnetization. Subjects lay supine with their heads in the scanner bore and observed the rear-projected computer screen via a 45° mirror mounted above subjects’ faces on the head coil. Participants’ choices were registered using two MRI-compatible button boxes. High-resolution T1-weighted scans (1.3 × 1.0 × 1.3 mm) were acquired on Siemens 3 T scanners.
fMRI Data Processing The imaging data were processed and analyzed using SPM2 (Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, UK), running on Matlab (Version 7.1; Math Works Inc., Natick, MA, USA). Functional images were realigned using a six-parameter rigid-body transformation. Each individual’s structural T1 image was co-registered to the average of the motion-corrected images using 12-parameter affine transformation. None of our 37 participants had motion artifacts of more than 2 mm in translation or 2 degrees in rotation. The time series volumes were then registered to the brain template adopted by the International Consortium for Brain Mapping (Mazziotta, 1995); spatial normalization into Montreal Neurological Institutes coordinates (resampled 3 mm × 3 mm × 3 mm). Functional images were then slice-timing artifact corrected, normalized into MNI space by applying the transformation matrix adopted from previous T1 warping. Images were then smoothed with an 8 mm isotropic Gaussian kernel and high-pass filtered in the temporal domain (filter width of 128 s). Physiological noise was filtered using a window function that corresponds to a homodynamic impulse response function (HRF).
fMRI Data Analysis There are two aims in our analysis: (1) to identify brain regions whose sensitivity to unfamiliarity correlates with behavioral sensitivity of familiarity, and (2) to test if these differences in brain sensitivity may also explain the difference in behavioral sensitivity of familiarity across the two genotype groups. To achieve these, our study makes use of a parametric design. First, participants’ self-reported degrees of familiarity of twenty Chinese cities are used to elicit their brain sensitivity of familiarity. Second, their brain sensitivity of familiarity enters our analysis directly at the group level. These enable greater precision in comparing familiarity ratings across individuals.
The perceptual epoch is modeled as a box car function with duration of the onset of the presentation of the gamble and the decision epoch is modeled as an event with the onset when participants press the button. To identify neural correlates for decisions involving the choice of less familiar sources over those of more familiar sources, we use a general linear model with parametric modulation of the average reported familiarity rating and the EV of the gamble in both epochs. Autocorrelation of the hemodynamic responses was modeled as an AR (Zajonc, 1968) process. The contrast value is calculated as the sensitivity towards unfamiliarity to the city in each trial at the individual level. To identify regions in which activity in the response to unfamiliarity correlated with individuals’ tendency of familiarity bias, we enter the estimated sensitivity coefficient γ as the regressor in the group level analysis. All results presented in the main text are threshold at p < 0.001 (uncorrected).
ROI analysis is performed by extracting the estimated value from the activated cluster in the right amygdala (peaked at x = 18, y = − 3, z = − 24; k = 10) showing the correlation with familiarity bias. Individual estimated values of activated cluster in the right amygdala are extracted by MarsBar (Brett et al., 2002). The estimated values are regressed against the individual sensitivity coefficient γ, the genotype dummy G, and their interaction.
Since we use self-reported rating of familiarity, the two regressors, familiarity rating and the EV, may be correlated. To test for the orthogonality of these regressors, we conduct correlation analysis between familiarity rating and the EV of the gambles on each participant. We find the mean correlation across all 37 participants to be − 0.024. The largest absolute value of the correlation is not bigger than 0.2. The smallest p value of either Spearman’s or Kendall’s rank correlation is not lower than 0.1. We further test for the possibility that the difference in behavior or brain activation between the two genotype groups is driven by the difference in demography. In a logistic regression, none of several demographic variables, namely gender, age and education level (see Table 4 for a summary) correlate significantly with genotype (p > 0.1).
Brain imagining results To examine the relationship between brain activation and familiarity bias, we first estimate the brain sensitivity to unfamiliarity by running a general linear model with parametric modulation on the familiarity rating of the cities for each trial. The perceptual epoch is modeled as a box car function with duration of the presentation of the gamble and the decision epoch is modeled as an event with the onset when participants press the button. The mean centered individual estimates of behavioral sensitivity to familiarity γ (= γ0 + γ1G) are then entered as the regressor for conducting a whole brain search for the correlates with the brain sensitivity to unfamiliarity.
To examine differences due to genotype, the estimated values of the activation of the right amygdala responding to unfamiliarity are regressed against the individual sensitivity coefficient γ, the genotype dummy G, and their interaction. Both the sensitivity coefficient γ (p < 0.002) and the genotype dummy (p < 0.03) are significant, but the interaction term is not (p > 0.9). The lack of significance of the interaction term suggests that the relationship between the right amygdala activation and familiarity bias does not differ across the two groups, despite the Major Group having on average higher activation at the right amygdala. A post-hoc t test reveals a significant difference in the estimated value of the activation in the right amygdala between the major group and the minor group (t = 2.71, p < 0.006), corroborating the significance of the Genotype dummy.
This result is further confirmed by a whole brain search. To identify brain regions showing both relationships—correlation with the degree of familiarity bias and differential activation between the two genotypes—the statistical map of the correlation with individual degree of familiarity bias (p < 0.001, uncorrected; k ≥ 10) is inclusively masked with both the group level and gene difference (p < 0.05, uncorrected). Only two brain regions—amygdala and dlPFC—display both relationships concurrently (Fig. 4). The dlPFC appears to be involved in the GABA deficits observed in psychopathology (Lewis and Gonzalez-Burgos, 2006). The reduced sensitivity of the right dlPFC towards unfamiliarity is consistent with findings of its role in shaping people’s risk attitudes from fMRI (Christopoulos et al., 2009), rTMS (Knoch et al., 2006) and tDCS (Fecteau et al., 2007) studies.
We test if the genetic effect on the behavioral sensitivity is mediated by the brain sensitivity identified in the whole brain search. The extracted values of amygdala and dlPFC are entered into two separate mediation analyses with the genotype as the independent variable and the behavioral sensitivity as the dependent variable. We found that both amygdala sensitivity (Sobel’s z = 2.14, p < 0.04; see SOM for details) and the dlPFC sensitivity (Sobel’s z = 1.85, p < 0.07) mediated the GABRB2 effect on the behavioral sensitivity.
The perceptual epoch is modeled as a box car function with duration of 2 s from the onset of the presentation of the gamble and the decision epoch is modeled as an event with the onset when participants press the button. The expected value (EV) of the lottery and the utility of the chosen option entered as parametric modulation in the perceptual and decision epoch respectively. The right caudate head is found to encode EV (p < 0.001, uncorrected; k = 7) while the bilateral striatum is found to encode the utility of the chosen option (p < 0.001, uncorrected; k ≥ 10; Fig. 6).
Rights and permissions
About this article
Cite this article
Chark, R., Zhong, S., Tsang, S.Y. et al. A gene–brain–behavior basis for familiarity bias in source preference. Theory Decis 92, 531–567 (2022). https://doi.org/10.1007/s11238-022-09871-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11238-022-09871-2