Abstract
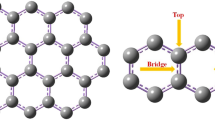
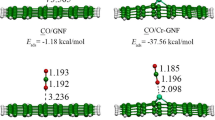
Finding cost-effective and sustainable methods for the removal of hydrogen sulfide (H2S), a highly toxic gas released as a byproduct in many industrial activities, is crucial for environmental health. In this study, the adsorption and electronic sensor properties of Ti, V, Cr and Sc doped graphene nanosheets (GN) for H2S molecule have been investigated using Density Functional Theory (DFT) method. The WB97XD method with 6-31G(d,p)/LanL2DZ basis sets have been utilized in DFT calculations. The charge distribution indicates that the charge transfer occurred between metal doped graphenes and H2S. DFT calculations of H2S molecule adsorption on Ti, V, Cr and Sc doped graphenes demonstrate that the ability to adsorb H2S molecule. The obtained adsorption energy (∆E) values vary in the range of -54.4 to -71.0 kJ/mol. Furthermore, the electrical conductivity of the Cr doped graphene nanosheet (Cr-GN) changed due to the change in the HOMO–LUMO gap (∆Eg = 24.8 kJ/mol). This result indicates that the Cr-GN structure is a potential candidate as an electronic sensor for H2S molecule at room temperature. Through methods like DFT, which are cost-effective and highly compatible with experimental results, predicting suitable adsorbents, understanding their properties, and enhancing them are expected to make substantial contributions to the industrial-scale production of these materials in terms of cost and accuracy in the future.












Similar content being viewed by others
Data availability
There is no data availability.
References
Zeng H, Wang L, Zhang D, Yan P, Nie J, Sharma VK, Wang C (2019) Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem Eng J. https://doi.org/10.1016/j.cej.2018.10.001
Wang S, Nam H, Lee D, Nam H (2022) H2S gas adsorption study using copper impregnated on KOH activated carbon from coffee residue for indoor air purification. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2022.108797
Salih E, Ayesh AI (2020) DFT investigation of H2S adsorption on graphenenanosheets and nanoribbons: Comparative study. Superlattices Microstruct. https://doi.org/10.1016/j.spmi.2020.106650
Ozekmekci M, Salkic G, Fellah MF (2015) Use of zeolites for the removal of H2S: A mini-review. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2015.08.015
Agbroko OW, Piler K, Benson TJ (2017) A comprehensive review of H2S scavenger technologies from oil and gas streams. ChemBioEng Rev. https://doi.org/10.1002/cben.201600026
Nam H, Wang S, Jeong HR (2018) TMA and H2S gas removals using metal loaded on rice husk activated carbon for indoor air purification. Fuel. https://doi.org/10.1016/j.fuel.2017.10.089
Shah MS, Tsapatsis M, Siepmann JI (2017) Hydrogen sulfide capture: From absorption in polar liquids to oxide, zeolite, and metal-organic framework adsorbents and membranes. Chem Rev. https://doi.org/10.1021/acs.chemrev.7b00095
Habeeb O, Ramesh K, Ali GA, Yunus R, Thanusha T, Olalere O (2016) Modeling and optimization for H2S adsorption from wastewater using coconut shell based activated carbon. Aust J Basic Appl Sci 10:136–147
Srivastava R, Suman H, Shrivastava S, Srivastava A (2019) DFT Analysis of Pristine and functionalized Zigzag CNT: A case of H2S sensing. Chem Phys Lett. https://doi.org/10.1016/j.cplett.2019.07.003
El-Sherbiny I, Salih E (2018) In: Kanchi S, Ahmed S (ed) Green synthesis of metallic nanoparticles using biopolymers and plant extracts: Synthesis, characterization and their applications. Wiley, New York. https://doi.org/10.1002/9781119418900.ch10
Dresselhaus M, Terrones M (2013) Carbon-based nanomaterials from a historical perspective. Proc IEEE. https://doi.org/10.1109/JPROC.2013.2261271
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature. https://doi.org/10.1038/363603a0
Baptista F, Belhout S, Giordani S, Quinn S (2015) Recent developments in carbon nanomaterial sensors. Chem Soc Rev. https://doi.org/10.1039/C4CS00379A
Lan G, Tang L, Dong J, Nong J, Luo P, Li X, Li Z, Zhang Y, Dai Y, Wang W, Shi H, Wei W (2023) Enhanced asymmetric light-plasmon coupling in graphene nanoribbons for high-efficiency transmissive infrared modulation. Laser Photonics Rev. https://doi.org/10.1002/lpor.202300469
Tang T, Zhou M, Lv J, Cheng H, Wang H, Qin D, Hu G, Liu X (2022) Sensitive and selective electrochemical determination of uric acid in urine based on ultrasmall iron oxide nanoparticles decorated urchin-like nitrogen-doped carbon. Colloids Surf B. https://doi.org/10.1016/j.colsurfb.2022.112538
Mubeen S, Zhang T, Chartuprayoon N, Rheem Y, Mulchandani A, Myung NV, Deshusses MA (2010) Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal Chem. https://doi.org/10.1021/ac901871d
Tsai JH, Jeng FT, Chiang HL (2001) Removal of H2S from exhaust gas by use of alkaline activated carbon. Adsorption. https://doi.org/10.1023/A:1013142405297
Bandosz TJ (2002) On the adsorption/oxidation of hydrogen sulfide on activated carbons at ambient temperatures. J Colloid Interface Sci. https://doi.org/10.1006/jcis.2001.7952
Yan R, Liang DT, Tsen L, Tay JH (2002) Kinetics and mechanisms of H2S adsorption by alkaline activated carbon. Environ Sci Technol. https://doi.org/10.1021/es0205840
Kaliva AN, Smith JW (1983) Oxidation of low concentrations of hydrogen sulfide by air on a fixed activated carbon bed. Can J Chem Eng. https://doi.org/10.1002/cjce.5450610210
Águila JEC, Cocoletzi HH, Cocoletzi GH (2013) A theoretical analysis of the role of defects in the adsorption of hydrogen sulfide on graphene. AIP Adv. https://doi.org/10.1063/1.4794953
Wang S, Sun H, Ang HM, Tadé MO (2013) Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem Eng J. https://doi.org/10.1016/j.cej.2013.04.070
Ou L, Song B, Liang H, Liu J, Feng X, Deng B, Sun T, Shao L (2016) Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol. https://doi.org/10.1186/s12989-016-0168-y
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater. https://doi.org/10.1038/nmat1849
Dutta S, Manna A, Pati S (2009) Intrinsic half-metallicity in modified graphene nanoribbons. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.102.096601
Ren H, Li Q, Luo Y, Yang J (2009) Graphene nanoribbon as a negative differential resistance device. Appl Phys Lett doi 10(1063/1):3126451
Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, De S, McGovern IT, Holland B, Byrne M, Gun’Ko YK, Boland JJ, Niraj P, Duesberg G, Krishnamurthy S, Goodhue R, Hutchison J, Scardaci V, Ferrari AC, Coleman JN (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. https://doi.org/10.1038/nnano.2008.215
Li X, Zhang G, Bai X, Sun X, Wang X, Wang E, Dai H (2008) Highly conducting graphene sheets and Langmuir-Blodgett films. Nat Nanotechnol. https://doi.org/10.1038/nnano.2008.210
Tang Y, Yang Z, Dai X, Ma D, Fu Z (2013) Formation, stabilities, and electronic and catalytic performance of platinum catalyst supported on non-metal-doped graphene. J Phys Chem C. https://doi.org/10.1021/jp400202e
Ganji M, Sharifi N, Ahangari M (2014) Adsorption of H2S molecules on non-carbonic and decorated carbonic graphenes: A van der Waals density functional study. Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2014.05.035
Zhang YH, Han LF, Xiao YH, Jia DZ, Guo ZH, Li F (2013) Understanding dopant and defect effect on H2S sensing performances of graphene: A first-principles study. Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2012.11.048
Ganji MD, Sharifi N, Ardjmand M, Ahangari MG (2012) Pt-decorated graphene as superior media for H2S adsorption: a first-principles study. App Surf Sci. https://doi.org/10.1016/j.apsusc.2012.08.083
Zhang HP, Luo XG, Song HT, Lin XY, Lu X, Tang Y (2014) DFT study of adsorption and dissociation behavior of H2S on Fe-doped graphene. App Surf Sci. https://doi.org/10.1016/j.apsusc.2014.08.141
Borisova D, Antonov V, Proykova A (2013) Hydrogen sulfide adsorption on a defective graphene. Int J Quantum Chem. https://doi.org/10.1002/qua.24077
Hegde VI, Shirodkar SN, Tit N, Waghmare UV, Yamani ZH (2014) First principles analysis of graphene and its ability to maintain long-ranged interaction with H2S. Surf Sci. https://doi.org/10.1016/j.susc.2013.11.015
Mohammadi-Manesh E, Vaezzadeh M, Saeidi M (2015) Cu- and CuO-decorated graphene as a nanosensor for H2S detection at room temperature. Surf Sci. https://doi.org/10.1016/j.susc.2015.02.002
Gao X, Zhou Q, Wang J, Xu L, Zeng W (2020) Performance of intrinsic and modified graphene for the adsorption of H2S and CH4: a DFT study. Nanomaterials. https://doi.org/10.3390/nano10020299
Salmankhani A, Karami Z, Mashhadzadeh AH, Ganjali MR, Vatanpour V, Esmaeili A, Habibzadeh S, Saeb MR, Fierro V, Celzard A (2020) New insights into H2S adsorption on graphene and graphene-like structures: a comparative DFT study. C. https://doi.org/10.3390/c6040074
Bayatsarmadi B, Zheng Y, Vasileff A, Qiao SZ (2017) Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small. https://doi.org/10.1002/smll.201700191
Qiu HJ, Ito Y, Cong W, Tan Y, Liu P, Hirata A, Fujita T, Tang Z, Chen M (2015) Nanoporous graphene with single-atom nickel dopants: an efficient and stable catalyst for electrochemical hydrogen production. Angew Chem Int Ed Engl. https://doi.org/10.1002/anie.201507381
Sun H, Yang L, Wu H, Zhao L (2023) Effects of element doping on the structure and properties of diamond-like carbon films: a review. Lubricants. https://doi.org/10.3390/lubricants11040186
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2015) Gaussian 09, Revision D. 01; Gaussian, Inc: Wallingford, CT
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev. https://doi.org/10.1103/PhysRev.140.A1133
Chai JD, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys doi 10(1063/1):2834918
Tsuneda T, Hirao K (2014) Long-range correction for density functional theory. Wiley Interdiscip Rev: Comput Mol Sci. https://doi.org/10.1002/wcms.1178
Natan A, Kronik L, Shapira Y (2006) Computing surface dipoles and potentials of self-assembled monolayers from first principles. App Surf Sci. https://doi.org/10.1016/j.apsusc.2006.03.052
Kumar S, Sharma S, Karmaker R, Sinha D (2021) DFT study on the structural, optical and electronic properties of platinum group doped graphene. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2020.101755
Wong MW (1996) Vibrational frequency prediction using density functional theory. Chem Phys Lett. https://doi.org/10.1016/0009-2614(96)00483-6
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. https://doi.org/10.1002/jcc.22885
Kumar N, Sharma M, Sharma JD, Ahluwalia PK (2015) Study of magnetism in nano structures of graphene and functionalized graphene: a first principle study. Indian J Phys. https://doi.org/10.1007/s12648-014-0526-2
Nagarajan V, Nivedhana R, Chandiramouli R (2023) Nucleobases adsorption studies on chair graphane nanosheets – A DFT outlook. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2023.110683
Bokhimi X (2019) Atomic and electronic properties of a 155 H2S cluster under pressure. ACS Omega. https://doi.org/10.1021/acsomega.9b00705
Cook RL, De Lucia FC, Helminger P (1975) Molecular force field and structure of hydrogen sulfide: recent microwave results. J Mol Struct. https://doi.org/10.1016/0022-2860(75)80094-9
Yousefian Z, Ghasemy E, Askarieh M, Rashidi A (2019) Theoretical studies on B, N, P, S, and Si doped fullerenes toward H2S sensing and adsorption. Phys E. https://doi.org/10.1016/j.physe.2019.113626
Ayesh AI (2022) H2S and SO2 adsorption on Cu doped MoSe2: DFT investigation. Phys Lett A. https://doi.org/10.1016/j.physleta.2021.127798
Salih E, Ayesh AI (2021) Computational study of metal doped graphene nanoribbon as a potential platform for detection of H2S. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2020.101823
Bo Z, Guo X, Wei X, Yang H, Yan J, Cen K (2019) Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene. Phys E. https://doi.org/10.1016/j.physe.2019.01.012
Aghaei SM, Monshi MM, Calizo I (2016) A theoretical study of gas adsorption on silicene nanoribbons and its application in a highly sensitive molecule sensor. RSC Adv. https://doi.org/10.1039/C6RA21293J
Faye O, Raj A, Mittal V, Beye AC (2016) H2S adsorption on graphene in the presence of sulfur: A density functional theory study. Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2016.01.034
Wei H, Gui Y, Kang J, Wang W, Tang C (2018) A DFT study on the adsorption of H2S and SO2 on Ni doped MoS2 monolayer. Nanomaterials. https://doi.org/10.3390/nano8090646
Zhang X, Yu L, Wu X, Hu W (2015) Experimental sensing and density functional theory study of H2S and SOF2 adsorption on Au-modified graphene. Adv Sci. https://doi.org/10.1002/advs.201500101
Lin C, Qin W, Dong C (2016) H2S adsorption and decomposition on the gradually reduced α-Fe2O3(001) surface: A DFT study. App Surf Sci. https://doi.org/10.1016/j.apsusc.2016.06.104
Peng X, Liu D, Zhao F, Tang C (2022) Gas sensing properties of Mg-doped graphene for H2S, SO2, SOF2, and SO2F2 based on DFT. Int J Quantum Chem. https://doi.org/10.1002/qua.26989
Gecim G, Ozekmekci M (2021) A density functional theory study of molecular H2S adsorption on (4,0) SWCNT doped with Ge, Ga and B. Surf Sci. https://doi.org/10.1016/j.susc.2021.121876
Khodadadi Z (2018) Evaluation of H2S sensing characteristics of metals–doped graphene and metals-decorated graphene: Insights from DFT study. Phys E. https://doi.org/10.1016/j.physe.2018.02.022
Fellah MF (2016) Adsorption of hydrogen sulfide as initial step of H2S removal: A DFT study on metal exchanged ZSM-12 clusters. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2016.01.003
Fellah MF (2019) Pt doped (8,0) single wall carbon nanotube as hydrogen sensor: A density functional theory study. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.08.169
Ahmadi A, Hadipour NL, Kamfiroozi M, Bagheri Z (2012) Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde. Sens Actuators B. https://doi.org/10.1016/j.snb.2011.12.001
Hadipour NL, Peyghan AA, Soleymanabadi H (2015) Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J Phys Chem C. https://doi.org/10.1021/jp513019z
Peyghan AA, Hadipour NL, Bagheri Z (2013) Effects of Al doping and double-antisite defect on the adsorption of HCN on a BC2N nanotube: Density functional theory studies. J Phys Chem C. https://doi.org/10.1021/jp312503h
Eslami E, Vahabi V, Peyghan AA (2016) Sensing properties of BN nanotube toward carcinogenic 4-chloroaniline: A computational study. Phys E. https://doi.org/10.1016/j.physe.2015.09.043
Khan MS, Khan MS (2012) Comparative theoretical study of iron and magnesium incorporated porphyrin induced carbon nanotubes and their interaction with hydrogen molecule. Phys E. https://doi.org/10.1016/j.physe.2012.05.010
Utsu PM, Gber TE, Nwosa DO, Nwagu AD, Benjamin I, Ikot IJ, Eno EA, Offiong OE, Adeyinka AS, Louis H (2023) Modeling of anthranilhydrazide (HL1) salicylhydrazone and its copper complexes Cu(I) and Cu(II) as a potential antimicrobial and antituberculosis therapeutic candidate. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2023.2186444
Sjoberg P, Politzer P (1990) Use of the electrostatic potential at the molecular surface to interpret and predict nucleophilic processes. J Phys Chem. https://doi.org/10.1021/j100373a017
Baydir E, Altun A, Fellah MF (2022) Molecular adsorption of silane on Ge, Ga and Al-doped CNT structures: a density functional theory study. Prot Met Phys Chem Surf. https://doi.org/10.1134/S2070205122050033
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc. https://doi.org/10.1021/ja100936w
Peng S, Cho K, Qi P, Dai H (2004) Ab initio study of CNT NO2 gas sensor. Chem Phys Lett. https://doi.org/10.1016/j.cplett.2004.02.026
Acknowledgements
The numerical calculations reported in this paper were partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Methodology, Software, Writing—Original Draft were performed by [Ömer Faruk TUNALI] and [Numan YUKSEL]. Validation, Formal analysis, Writing—Review & Editing, Supervision were performed by [Gökhan GECE]. Conceptualization, Validation, Formal analysis, Writing—Review & Editing, Supervision were performed by [Mehmet Ferdi FELLAH]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tunalı, Ö.F., Yuksel, N., Gece, G. et al. A DFT study of H2S adsorption and sensing on Ti, V, Cr and Sc doped graphene surfaces. Struct Chem 35, 759–775 (2024). https://doi.org/10.1007/s11224-023-02265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02265-2