Abstract
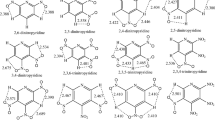
The molecular and high-energy density properties of isolated (3,4-dinitropyrazole, 34DNP) and fused pyrazole ring (1H,4H-3,6-dinitropyrazolo[4,3-c]pyrazole, DNPP) systems were studied by density functional theory (DFT) at the B3LYP/G D3BJ level of theory with def2-TZVP basis set. The theoretically optimized geometrical parameters of both molecules are similar to the corresponding experimental geometrical values. With this, atoms in molecules (AIM), electrostatic, intrinsic bond strength index (IBSI), and natural bond orbital (NBO) properties of the 34DNP and DNPP molecules were studied. These studies show that one of the nitro groups in 34DNP is less stable than other nitro groups present in 34DNP and DNPP molecules. The optimized geometry, AIM analysis, and NBO results confirmed that one of the C-NO2 groups in isolated dinitropyrazole 34DNP makes fewer orbital interactions with the pyrazole ring, and deviation in planarity leads to relatively less stability and acetic than the fused dinitropyrazole DNPP molecule. These studies reveal that the effect of ring fusion in dinitropyrazole derivatives may be used to design high-quality high-energy density materials.









Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Zhang W, Zhang J, Deng M, Qi X, Nie F, Zhang Q (2017) A promising high-energy-density material Nat Commun 8:181. https://doi.org/10.1038/s41467-017-00286-0
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112:1–15. https://doi.org/10.1016/j.jhazmat.2004.04.003
Tsyshevsky R, Smirnov AS, Kuklja MM (2019) Comprehensive end-to-end design of novel high energy density materials: III. Fused heterocyclic energetic compounds. J Phys Chem C 123:8688–8698. https://doi.org/10.1021/acs.jpcc.9b00863
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD (2002) A review of energetic materials synthesis. Thermochim Acta 384:187–204. https://doi.org/10.1016/S0040-6031(01)00805-X
Zhou J, Zhang J, Wang B, Qiu L, Xu R, Sheremetev AB (2022) Recent synthetic efforts towards high energy density materials: how to design high-performance energetic structures? FirePhysChem 2:83–139. https://doi.org/10.1016/j.fpc.2021.09.005
Song Q, Zhang L, Mo Z (2022) Alleviating the stability–performance contradiction of cage-like high-energy-density materials by a backbone-collapse and branch-heterolysis competition mechanism. Phys Chem Chem Phys 24:19252–19262. https://doi.org/10.1039/D2CP02061K
Bykov M, Bykova E, Ponomareva AV, Abrikosov IA, Chariton S, Prakapenka VB, Mahmood MF, Dubrovinsky L, Goncharov AF (2021) Stabilization of polynitrogen anions in tantalum–nitrogen compounds at high pressure. Angew Chemie Int Ed 60:9003–9008. https://doi.org/10.1002/anie.202100283
Yao Y, Adeniyi AO (2021) Solid nitrogen and nitrogen-rich compounds as high-energy-density materials. Phys status solidi 258:2000588. https://doi.org/10.1002/pssb.202000588
Chen S, Jin Y, Xia H, Wang K, Liu Y, Zhang Q (2020) Synthesis of fused tetrazolo[1,5-b]pyridazine-based energetic compounds. Energ Mater Front. https://doi.org/10.1016/j.enmf.2020.05.001
Xiong H, Yang H, Cheng G (2019) 3-Trinitromethyl-4-nitro-5-nitramine-1H-pyrazole: a high energy density oxidizer. New J Chem 43:13827–13831. https://doi.org/10.1039/C9NJ02732G
Xu J, Wu J, Li H, Zhang J (2020) Molecular design of a new family of bridged bis(multinitro-triazole) with outstanding oxygen balance as high-density energy compounds. Int J Quantum Chem 120:e26056. https://doi.org/10.1002/qua.26056
Kumar D, Tang Y, He C, Imler GH, Parrish DA, Shreeve JM (2018) Multipurpose energetic materials by shuffling nitro groups on a 3,3′-bipyrazole moiety. Chem – A Eur J 24:17220–17224. https://doi.org/10.1002/chem.201804418
Yin P, Zhang J, Parrish DA, Shreeve JM (2014) Energetic N,N′-ethylene-bridged bis(nitropyrazoles): diversified functionalities and properties. Chem – A Eur J 20:16529–16536. https://doi.org/10.1002/chem.201404991
Jeong K, Sung I, Joo HU, Kwon T, Yuk JM, Kwon Y, Kim H (2020) Molecular design of nitro-oxide-substituted cycloalkane derivatives for high-energy-density materials. J Mol Struct 1212:128128. https://doi.org/10.1016/j.molstruc.2020.128128
Singh HJ, Upadhyay MK (2013) Nitro derivatives of 1,3,5-triazepine as potential high-energy materials. J Energ Mater 31:301–313. https://doi.org/10.1080/07370652.2012.725121
Edgehouse KJ, Ball DW (2018) Nitro derivatives of triazetidine: potential high energy density materials. J Mol Model 24:189. https://doi.org/10.1007/s00894-018-3733-5
Zlotin SG, Dalinger IL, Makhova NN, Tartakovsky VA (2020) Nitro compounds as the core structures of promising energetic materials and versatile reagents for organic synthesis. Russ Chem Rev 89:1–54. https://doi.org/10.1070/rcr4908
Srinivasan P, Asthana SN, Pawar RB, Kumaradhas P (2011) A theoretical charge density study on nitrogen-rich 4,4′,5,5′-tetranitro-2,2′-bi-1H-imidazole (TNBI) energetic molecule. Struct Chem 22:1213–1220. https://doi.org/10.1007/s11224-011-9815-y
Hervé G, Roussel C, Graindorge H (2010) Selective preparation of 3,4,5-trinitro-1H-pyrazole: a stable all-carbon-nitrated arene. Angew Chemie Int Ed 49:3177–3181. https://doi.org/10.1002/anie.201000764
Zhang Y, Li Y, Yu T, Liu Y, Chen S, Ge Z, Sun C, Pang S (2019) Synthesis and properties of energetic hydrazinium 5-nitro-3-dinitromethyl-2H-pyrazole by unexpected isomerization of N-nitropyrazole. ACS Omega 4:19011–19017. https://doi.org/10.1021/acsomega.9b01910
Lei C, Yang H, Cheng G (2020) New pyrazole energetic materials and their energetic salts: combining the dinitromethyl group with nitropyrazole. Dalt Trans 49:1660–1667. https://doi.org/10.1039/C9DT04235K
Li B-T, Li L-L, Liu L-L (2020) Thermal stability and detonation characters of nitro-substituted derivatives of pyrazole. Mol Phys 118:e1708491. https://doi.org/10.1080/00268976.2019.1708491
Zhang W, Xia H, Yu R, Zhang J, Wang K, Zhang Q (2020) Synthesis and properties of 3,6-dinitropyrazolo[4,3-c]-pyrazole (DNPP) derivatives. Propellants, Explos Pyrotech 45:546–553. https://doi.org/10.1002/prep.201900205
Yin P, Zhang J, Mitchell LA, Parrish DA, Shreeve JM (2016) 3,6-Dinitropyrazolo[4,3-c]pyrazole-based multipurpose energetic materials through versatile N-functionalization strategies. Angew Chemie Int Ed 55:12895–12897. https://doi.org/10.1002/anie.201606894
Zhang J, Parrish DA, Shreeve JM (2015) Curious cases of 3,6-dinitropyrazolo[4,3-c]pyrazole-based energetic cocrystals with high nitrogen content: an alternative to salt formation. Chem Commun 51:7337–7340. https://doi.org/10.1039/C5CC01745A
Bennion JC, McBain A, Son SF, Matzger AJ (2015) Design and synthesis of a series of nitrogen-rich energetic cocrystals of 5,5′-dinitro-2H,2H′-3,3′-bi-1,2,4-triazole (DNBT). Cryst Growth Des 15:2545–2549. https://doi.org/10.1021/acs.cgd.5b00336
Shevelev SA, Dalinger IL, Shkineva TK, Ugrak BI, Gulevskaya VI, Kanishchev MI (1993) Nitropyrazoles Russ Chem Bull 42:1063–1068. https://doi.org/10.1007/BF00704200
Janssen JWAM, Koeners HJ, Kruse CG, Habrakern CL (1973) Pyrazoles. XII. Preparation of 3(5)-nitropyrazoles by thermal rearrangement of N-nitropyrazoles. J Org Chem 38:1777–1782. https://doi.org/10.1021/jo00950a001
Neese F (2022) Software update: the ORCA program system—version 5.0. WIREs Comput Mol Sci 12:e1606. https://doi.org/10.1002/wcms.1606
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
AIMAll (Version 19.10.12), Todd A. Keith, TK Gristmill Software, Overland Park KS, USA, 2019 (aim.tkgristmill.com)
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Klein J, Khartabil H, Boisson J-C, Contreras-García J, Piquemal J-P, Hénon E (2020) New way for probing bond strength. J Phys Chem A 124:1850–1860. https://doi.org/10.1021/acs.jpca.9b09845
Lefebvre C, Khartabil H, Hénon E (2023) New insight into atomic-level interpretation of interactions in molecules and reacting systems. Phys Chem Chem Phys 25:11398–11409. https://doi.org/10.1039/D2CP02839E
Lefebvre C, Klein J, Khartabil H, Boisson J-C, Hénon E (2023) IGMPlot: a program to identify, characterize, and quantify molecular interactions. J Comput Chem 44:1750–1766. https://doi.org/10.1002/jcc.27123
Glendening ED, Landis CR, Weinhold F (2019) NBO 7.0: new vistas in localized and delocalized chemical bonding theory. J Comput Chem 40:2234–2241. https://doi.org/10.1002/jcc.25873
Chemcraft - graphical software for visualization of quantum chemistry computations. Version 1.8, build 654. https://www.chemcraftprog.com
Henson BF, Smilowitz L (2022) Applications of chemical kinetics to detonation: wave coalescence and homogeneous initiation in single crystal 1,3-propanediol-2,2-bis[(nitrooxy)methyl]-tetranitrate (PETN). J Appl Phys 131:175903. https://doi.org/10.1063/5.0085292
Rai NK, Lee Perry W, Duque AL (2022) Novel method to control explosive shock sensitivity: a mesoscale study to understand the effect of thermally expandable microsphere (TEM) inclusions in high explosives (HE) microstructure. J Appl Phys 131:175105. https://doi.org/10.1063/5.0084115
Revil-Baudard B, Cazacu O (2022) Dynamic response of polycrystalline high energetic systems: constitutive modeling and application to impact. J Appl Phys 131:145101. https://doi.org/10.1063/5.0080848
Zhang J, Parrish DA, Shreeve JM (2014) Thermally stable 3,6-dinitropyrazolo[4,3-c]pyrazole-based energetic materials. Chem – An Asian J 9:2953–2960. https://doi.org/10.1002/asia.201402538
Lizhen Chen, Song Liang, Cao Duanlin WJ (2016) Crystal structure of 3,4-dinitropyrazole, C3H2N4O4. Zeitschrift für Krist - New Cryst Struct 231:1099–1100. https://doi.org/10.1515/ncrs-2016-0075
Zhang C, Shu Y, Huang Y, Zhao X, Dong H (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109:8978–8982. https://doi.org/10.1021/jp0512309
Lu T, Chen F (2013) Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J Phys Chem A 117:3100–3108. https://doi.org/10.1021/jp4010345
Tian LUQC (2018) Revealing molecular electronic structure via analysis of valence electron density. Acta Physico-Chimica Sin 34:503–513
Politzer P, Murray JS (2021) Molecular electrostatic potentials: significance and applications. In: Chemical Reactivity in Confined Systems. pp 113–134
Prasath M, Govindammal M, Sathya B (2017) Spectroscopic investigations (FT-IR & FT-Raman) and molecular docking analysis of 6-[1-methyl-4-nitro-1H-imidazol-5-yl) sulfonyl]-7H-purine. J Mol Struct 1146:292–300. https://doi.org/10.1016/j.molstruc.2017.05.136
Mashhadi SMA, Bhatti MH, Jabeen E, Yunus U, Ashfaq M, Akhtar M, Tahir MN, Alshehri SM, Ahmed S, Ojha SC (2023) Synthesis and Antioxidant studies of 2,4-dioxothiazolidine-5-acetic acid based organic salts: SC-XRD and DFT approach. ACS Omega 8:30186–30198. https://doi.org/10.1021/acsomega.3c02895
Malik AN, Tahir MN, Ali A, Ashfaq M, Ibrahim M, Kuznetsov AE, Assiri MA, Sameeh MY (2023) Preparation, crystal structure, supramolecular assembly, and DFT studies of two organic salts bearing pyridine and pyrimidine. ACS Omega 8:25034–25047. https://doi.org/10.1021/acsomega.3c01659
Anbu V, Vijayalakshmi KA, Karunathan R, Stephen AD, Nidhin P V (2019) Explosives properties of high energetic trinitrophenyl nitramide molecules: a DFT and AIM analysis. Arab J Chem 12:621–632. https://doi.org/10.1016/j.arabjc.2016.09.023
Tahir MN, Ali A, Khalid M, Ashfaq M, Naveed M, Murtaza S, Shafiq I, Asghar MA, Orfali R, Perveen S (2023) Efficient synthesis of imine-carboxylic acid functionalized compounds: single crystal, Hirshfeld surface and quantum chemical exploration. Molecules 28
Shehnaz, Siddiqui WA, Ashraf A, Ashfaq M, Tahir MN, Niaz S (2023) Sulfonamide derived Schiff base Mn (II), Co (II), and Ni (II) complexes: crystal structures, density functional theory and Hirshfeld surface analysis. Appl Organomet Chem 37:e7077. https://doi.org/10.1002/aoc.7077
Acknowledgements
Thangavel Subramani acknowledges the Bioinformatics Resources and Applications Facility (BRAF), C-DAC, Pune, for providing the computational facility for this work.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Thangavel Subramani and Srinivasan Ponnusamy conceived the idea and designed the theoretical calculation. Thangavel Subramani and Jothibaskar Natarajan, Sathya Lakshmanan carried out computer simulation and data analyses. Thangavel Subramani, Logesh Ganesan and Manivasakan Palanisamy carried out NBO and IBSI analysis and prepared the manuscript. All authors checked the draft.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The molecular geometry, AIM analysis, electrostatic and IBSI, PDA, and NBO properties of the 34DNP and DNPP molecules were analyzed.
• The tilted -NO2 in the isolated pyrazole ring is favorable for increasing the O–O bond character in -NO2 and leads to less favorable bonding with the pyrazole ring.
• The dinitropyrazole ring fusion provides coplanarity to -NO2 with the pyrazole ring and less steric interaction with the molecule.
• The isolated pyrazole ring provides higher acidity to the attached hydrogen than the fused pyrazole ring.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subramani, T., Natarajan, J., Lakshmanan, S. et al. In silico investigations of high-energy density properties and effect of ring fusion on dinitropyrazole derivatives. Struct Chem 35, 871–884 (2024). https://doi.org/10.1007/s11224-023-02240-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02240-x