Abstract
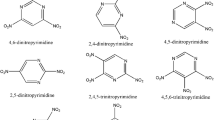
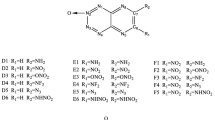
A series of derivatives of pyridine were designed through substituting hydrogen atoms by nitro groups systematically. By using the density functional theory at B3PW91/6-311++G(d,p)//MP2/311++G(d,p) level, heats of formation, bond orders, and bond dissociation energies were calculated to explore the thermodynamic stabilities of title molecules. Furthermore, the regularity of stability was explained based on the electronic population. Our results indicated that title molecules had enough stability to exist. To evaluate the potential usage as a high-energy-density molecule, the detonation pressure and detonation velocity were explored by using the semi-empirical Kamlet–Jacobs equation and excellent detonation character was confirmed. Overall consideration of the thermal stability and energetic character, four molecules (2,3,4,5-tetranitropyridine, 2,3,5,6-tetranitropyridine, 2,4,5,6-tetranitrop-pyridine, 2,3,4,5,6-pentanitropyridine) were confirmed to be better than RDX and filtered as potential energetic molecules.

Similar content being viewed by others
References
Sieranski T (2017) Discovering the stacking landscape of a pyridine–pyridine system. J. Mol. Model. 23(12):338. https://doi.org/10.1007/s00894-017-3496-4
Politzer P, Lane P, Edward Grice M, Concha MC, Redfern PC (1995) Comparative computational analysis of some nitramine and difluoramine structures, dissociation energies and heats of formation. J. Mol. Struct. THEOCHEM 338(1):249–256. https://doi.org/10.1016/0166-1280(94)04064-Y
Archibald TG, Gilardi R, Baum K, George C (1990) Synthesis and X-ray crystal structure of 1,3,3-trinitroazetidine. J Org Chem 55(9):2920–2924. https://doi.org/10.1021/jo00296a066
Rahm M, Dvinskikh SV, Furo I, Brinck T (2011) Experimental detection of trinitramide, N(NO2)3. Angew Chem Int Ed Engl 50(5):1145–1148. https://doi.org/10.1002/anie.201007047
Knobel YK, Miroshnichenko EA, Lebedev YA (1971) Heats of combustion of nitromethane and dinitromethane: enthalpies of formation of nitromethyl radicals and energies of dissociation of bonds in nitro derivatives of methane. Bull Acad Sci USSR Div Chem Sci 20:425–428
Zhang W, Zhang J, Deng M, Qi X, Nie F, Zhang Q (2017) A promising high-energy-density material. Nat. Commun. 8(1):181. https://doi.org/10.1038/s41467-017-00286-0
Zhang Y, Sun X, Yu S, Bao L, Sun C, Pang S (2018) Energetic di- and trinitromethylpyridines: synthesis and characterization. Molecules 23(1):2–14. https://doi.org/10.3390/molecules23010002
Lian P, Lai WP, Wang BZ, Wang XJ, Luo YF (2014) Theoretical study on structures and properties of high-energy-density derivatives of pyridine. Asian J. Chem. 26(8):2357. https://doi.org/10.14233/ajchem.2014.15951
Zhao G, Lu M (2013) Theoretical investigations of pyridine derivatives as potential high energy density materials. J. Phys. Org. Chem. 26(3):211–217. https://doi.org/10.1002/poc.3068
Ritter H, Licht HH (1993) Synthesis and characterization of methylnitramino-substituted pyridines and triazines. Propellants Explos Pyrotechnics 18(2):81–88. https://doi.org/10.1002/prep.19930180207
Licht H, Ritter H (1993) Neue explosivstoffe: Dinitropyridine. 24th Int. In: Annual Conference of ICT, Karlsruhe, Germany, p 6
Becke AD (1992) Density-functional thermochemistry. II. The effect of the Perdew--Wang generalized-gradient correlation correction. J. Chem. Phys. 97(12):9173–9177
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1
Kamlet MJ, Ablard JE (1968) Chemistry of detonations. II. Buffered equilibria. J. Chem. Phys. 48(1):36–42
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J. Chem. Phys. 48(1):23–35. https://doi.org/10.1063/1.1667908
Jeffrey GA, Jeffrey GA (1997) An introduction to hydrogen bonding, vol 12. Oxford University Press, New York
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, B., Zhou, M., Peng, J. et al. Theoretical calculations about nitro-substituted pyridine as high-energy-density compounds (HEDCs). J Mol Model 25, 23 (2019). https://doi.org/10.1007/s00894-018-3904-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3904-4