Abstract
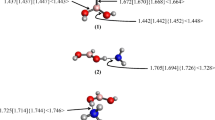
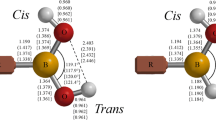
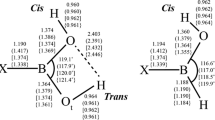
Boronic acids and their conjugate esters have been employed in a variety of biomedical applications. This work reports structural, bonding, and thermochemical calculations for the boronic acids, RB(OH)2 (R = H, methyl, phenyl, and ortho-methyl-phenyl) and the corresponding ethylene glycol diesters, RB(−OCH2CH2O−), in the presence of explicit NH3 and/or H2O molecules. Calculations were performed in vacuo and in polarizable continuum model (PCM) aqueous media using density functional theory (DFT) and second-order Moller-Plesset perturbation theory (MP2) with the Dunning-Woon aug-cc-pVTZ basis set. These results quantify the relative stability of N→B dative-bonded, water-inserted Owater→B (Ow→B) dative-bonded, Zwitterionic, and hydrogen-bonded conformers on the model RB(OH)2⋅NH3⋅H2O and RB(−OCH2CH2O−)⋅NH3⋅H2O potential energy surfaces (PESs). In the PCM aqueous media model, conformers containing an H3N→B dative bond and a water molecule bridging the NH3 and acid/ester moieties were found consistently to be lower in energy than arrangements with either a H3N⋯H(H)Ow→B linkage or Zwitterionic forms, [H4N]+[HOw−BR(OH)2]− or [H4N]+[HOw−BR(−OCH2CH2O−)]−. For the acids, however, conformers with a H3N⋯HOwH moiety hydrogen bonded to the boronate hydroxyl group(s) proved to be lowest in energy.







Similar content being viewed by others
Data availability
Cartesian coordinates for all structures, NBO analysis output, and additional figures are available in the Supplementary Materials section.
Code availability
Not applicable.
References
Kuivila HG, Keough AH, Soboczenski EJ (1954) Areneboronates from diols and polyols. J Organ Chem 19(5):780–783
Silva MP, Saraiva L, Pinto M, Sousa ME (2020) boronic acids and their derivatives in medicinal chemistry: Synthesis and biological applications. Molecules 25(18):4323–4363
Maslah H, Pethe S, Labruere R (2021) Boronic acid/boronate prodrugs for cancer treatment: current status and perspectives. Future Med Chem. 13(10):859–861
Graham BJ, Windsor IW, Gold B, Rains RT (2021) Boronic acid with high oxidative stability and utility in biological contexts. Proc Natl Acad Sci USA 118(10):e2013691118. PMID:33653951; PMCID: PMC7958229. https://doi.org/10.1073/pnas.2013691118
Williams GT, Kedge JL, Fossey JF (2021) Molecular Boronic acid-based saccharide sensors. ACS Sens 6:1508–1528
Banach L, Williams GT, Fossey JS (2021) Insulin delivery using dynamic covalent boronic acid/ester controlled release. Rev Adv Ther. 4:2100118. Wiley-VCH GmbH. https://doi.org/10.1002/adtp.20210018
Smithmyer ME, Deng CC, Cassel SE, LeValley PJ, Sumerlin BS, Kloxin AM (2018) Self-healing boronic acid-based hydrogels for 3D co-cultures. ACS Macro Lett 7(9):1105–1110
Xu S, Sedgwick AC, Elfry SA, Chen W, Jones AS, Williams GT, Toby A, Jenkins A, Bull SD, Fossey JS, James TD (2020) A boronic acid-based fluorescent hydrogel for monosaccharide detection. Front Chem Sci Eng 14:112–116
Irving AM, Vogels CM, Nikolcheva LG, Edwards JP, He X-F, Hamilton MG, Baerlocher MO, Baerlocher FJ, Decken A, Wescott SA (2003) Synthesis and antifungal and antibacterial bioactivity of cyclic diamines containing boronate esters. New J Chem 27:1419–1424
Ketuly KA, Aziz K, Hamid A, Hadi A (2010) Boronate derivatives of functionally diverse catechols: stability studies. Molecules 15(4):2347–2356
Santucci M, Spyrakis F, Cross S, Quotadamo A, Farina D, Tondi D, DeLuca F, Docquier J-D, Prieto I, Ibacache C, Blazquez J, Venturelli A, Cruciani G, Costi MP (2017) Computational and biological profile of boronic acids for the detection of bacterial serine- and metallo-β-lactamases. Sci Rep 7:17716
Zheng H, Hajizadeh S, Gong H, Lin H, Ye L (2021) Preparation of boronic acid-functionalized cryogels using modular and clickable building blocks for bacterial separation. J Agric Food Chem 69:1135–1145
Jung SJ, Lee JY, Kim TH, Lee DE, Jeon J, Yang SD, Hur MG, Min JJ, Park YD (2016) Discovery of boronic acid-based flurorescent probes targeting amyloid-beta plaques in Alzheimer’s disease. Bioorgan Med Chem Letters 26(7):1784–1788
Maiti P, Manna J, Burch ZN, Flaherty DB, Larkin JD, Dunbar GL (2020) Ameliorative properties of boronic compounds in vitro and in vivo models of Alzheimer’s disease. Int J Mol Sci 21(18):6664
Fang G, Wang H, Bian Z, Sun J, Liu A, Fang H, Liu B, Yao Q, Wu Z (2018) Recent development of boronic acid-based fluorescent sensors. RCS ADV 8:29400–29427
Gao X, Zhang Y, Wang B (2003) New Boronic acid fluorescent reporter compounds. 2. A Napthalene-based on-off sensor functional at physiological pH. Organ Lett 5:4615–4618
Pushina M, Penavic A, Farshbaf S, Anzenbacher P (2021) Fluorescent Sensor array for quantitative determination of saccharides. ACS Sensors 6(11):4001–4008
Anslyn EV (2019) As We Like It - Boronic acid Sensors and Loose Bolts - The Final Act in a Shakespearian Drama. https://www.chemistrycommunity.nature.com/posts/52576
Sun X, Chapin BM, Metola P, Collins B, Wang B, James TD, Anslyn EV (2019) The mechanisms of boronate ester formation and fluorescent turn-on in ortho-aminomethylphenylboronic acids. Nat Chem 11:768–778
Sun X, James TD, Anslyn EV (2018) Arresting “loose bolt” internal conversion from −b(oh)2 groups is the mechanism for emission turn-on in ortho-aminomethylphenylboronic acid-based saccharide sensors. J Am Chem Soc 140:2348–2354
Zhu L, Shabbir SH, Gray M, Lynch VM, Sorey S, Anslyn EV (2006) A Structural investigation of the N-B interaction in an o-(N, N-dialkylaminomethyl)arylboronate system. J Am Chem Soc 128:1222–1232
Markham GD, Larkin JD, Bock CW (2021) Models for Boronic acid receptors: a computational structural, bonding, and thermochemical investigation of the HB(OH)2∙H2O∙NH3 and HB(-O-CH-CH2-O-)∙NH3∙H2O potential energy surfaces. Struct Chem 32:607–621
Ernzerhof M, Perdew JP (1998) Generalized gradient approximation to the angle- and system-averaged exchange hole. J Chem Phys 108:3313–3320
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Errata: Generalized gradient approximation made simple. Phys Rev Lett 1396
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Woon DE, Dunning TH Jr (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian Inc, Wallingford CT (2013) Revision D.01
Tomasi J, Mennucci B, Cammi T (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3094
Grimme S, Antony J, Erlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) Effect of damping function in dispersion corrected density functional theory. J Comput Chem 32(7):1456–1465
Frisch MJ, Head-Gordon M, Pople JA (1990) A direct MP2 gradient method. Chem Phys Lett 166:275–280
Head-Gordon M, Pople JA, Frisch MJ (1988) MP2 energy evaluation by direct methods. Phys Lett 153:503–506
Moller C, Plesset MS (1934) Note on the approximation treatment for many-electron systems. Phys Rev 46:0618–0622
Cyranski MK, Klimentowska P, Rydzewska A, Serwatowski J, Sporzynski A, Stepien DK (2012) Towards a monomeric structure of phenylboronic acid: the influence of ortho-alkoxy substituents on the crystal structure. Cryst Eng Comm 14(19):6282
Cyranski MK, Jezierska A, Klimentowska P, Panek JJ, Zukowska GZ, Sporzynski A (2008) Structural and spectroscopic properties of an aliphatic boronic acid studied by combination of experimental and theoretical methods. J Chem Phys 128:124512
Cyranski MK, Jezierska A, Klimentowska P, Panek JJ, Sporzynski A (2008) Impact of intermolecular hydrogen bond on structural properties of phenylboronic acid: quantum chemical and X-ray study. J Phys Organ Chem 21(6):472–482
Rao NZ, Larkin JD, Bock CW (2016) A comparison of the structure and bonding in the aliphatic boronic R-B(OH)2 and borinic R-BH(OH) acids (R=H, NH2, OH, and F): a computational investigation. Struct Chem 27:1081–1091
Rettig SJ, Trotter J (1977) Crystal and molecular structure of phenylboronic acid, C6H5B(OH)2. Can J Chem 55:3071–3075
Larkin JD, Bhat KL, Markham GD, Brooks BR, Schaefer HF III, Bock CW (2006) Structure of the boronic acid dimer and the relative stabilities of its conformers. J Phys Chem A 110(36):10633–10642
Larkin JD, Milkevitch M, Bhat KL, Markham GD, Brooks BR, Bock CW (2008) Dimers of boroglycine and methylamine boronic acid: a computational comparison of the relative importance of dative versus hydrogen bonding. J Phys Chem A 112(1):125–133
Kuttler A, Durganala S, Fratini A, Morgan A, Benin V (2013) Structure, theoretical studies and coupling reactions of some new cyclic boronic esters. Heteroatom Chem 24(5):361–371
Chase Jr. MC (1998) NIST-JANAF Thermochemical Tables, 4th ed., J Phys Chem Ref Data 9
Gurvich LV, Veyts IV, Alcock CB (1994) Thermodynamic Properties of Individual Substances 3, CRC Press
Bock CW, Larkin JD (2012) Heats of formation for the boronic acids R-B(OH)2 and Boroxines R3B3O3 (R = H, Li, HBe, H3C, H2N, HO, and Cl) calculated at the G2, G3, and G4 levels of theory. Comput Theor Chem 986:35–42
Curtiss LA, Redfern PC, Raghavachari K (2005) Assessment of Gaussian-3 and density functional theories on the G3/05 test set of experimental energies. J Chem Phys 123:124107–124119
Kim H, Park JY, Choi S (2019) Highly accurate G4(MP2) benchmark on QM9 database: Energy refinement and analysis of structures. https://doi.org/10.6084/m9.figshare.c.4351631.v1
Ruscic B (2014) Uncertainty Quantification in thermochemistry, benchmarking electronic structure computations, and active thermochemical tables. Int J Quantum Chem 114:1097–1101
NIST Chemistry WebBook, NIST Standard Reference Database Number 69
Potter RG, Camaioni DM, Vasiliu M, Dixon DA (2010) Thermochemistry of Lewis adducts of BH3 and Nucleophilic substitution of triethylamine on NH3BH3 in tetrahydrofuran. Inorg Chem 49:10512–10521
Zhao Y, Truhlar DG (2005) Benchmark databases for nonbonded interactions and their use to test density functional theory. J Chem Theory Comput 1(3):415–432
Rao NZ, Larkin JD, Bock CW (2017) Monosubstituted phenylboronic acids, R-B(OH) 2 (R = C6H5, C6H4CH3, C6H4NH2, C6H4OH, and C6H4F): a computational investigation. Struct Chem 28(4):945–955
Rodriquez-Cuamatzi P, Arillo-Flores O, Bernal-Uruchurtu M, Hopfl H (2005) Theoretical and experimental evaluation of homo- and hetero-dimeric hydrogen-bonded motifs containing boronic acids, carboxylic acids, and carboxylate anions: application for the generation of highly stable hydrogen-bonded supramolecular systems. Cryst Growth Des 5:167–175
Bhat KL, Hayik S, Corvo JN, Marycz DM, Bock CW (2004) A computational study of the formation of 1,3,2-dioxaborolane fron the reaction of dihydroxy borane with 1,2-ethanediol. J Mol Struct (Theochem) 673:145–154
Raghavachari K, Frisch MJ, Pople JA (1980) Contribution of triple substitutions to the electron correlation in fourth-order perturbation theory. Chem Phys Lett 72:4244–4245
Raghavachari K, Pople JA (1978) Approximate 4th order perturbation-theory of electron correlation energy. Int J Quantum Chem 14:91–100
Bhat KL, Hayik S, Bock CW (2003) A computational study of the formation of a boron–oxygen–carbon linkage. The reaction of monohydroxy borane with methanol. J Mol Struct (Theochem) 638:107–117
Boeser R, Niederpruem N, Blaser, D (1992) in In Molecules in Natural Science and Medicine, Maksie ZB, Eckert-Masie M eds. E Horwood Chichester, England
Demaison J, Lievin J, Csaszar AG, Cutle C (2008) Equilibrium structure and torsional barrier of BH3NH3. J Phys Chem A 112:447–4482
Kloster WT, Koetzle TF, Siegbahn PEM, Richardson TB, Crabtre RH (1999) Study of the N-H⋯H-B dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. JACS 121:6337–6343
Thorne LR, Suenrum RD, Lovas FJ (1983) Microwave spectrum, torsional barrier, and structure of BH3NH3. J Chem Phys 78:167–171
Larkin JD, Bock CW (2018) A Comparison of the structure and bonding in the donor-acceptor complexes H3N→BR(OH)2 and H3N→BRH(OH) (R = H; NH2, OH, and F): a computational investigation. Struct Chem 30:361–368
Bhat KL, Howard NJ, Rostami H, Lai JH, Bock CW (2005) Intramolecular dative bonds involving boron with oxygen and nitrogen in boronic acids and esters: a computational study. J Mol Struct (Theochem) 723:147–157
Chapin BM, Metola P, Vankayala SL, Woodcock HL, Mooibroek TJ, Lynch VM, Larkin JD, Anslyn EV (2017) Disaggregation is a mechanism for emission turn-on of ortho-aminomethylphenylboronic acid-based saccharide sensors. J Am Chem Soc 139:5568
Franzen S, Ni W, Wang B (2003) Study of the mechanism of electron-transfer quenching by boron−nitrogen adducts in fluorescent sensors. J Phys Chem B 107:12942–12948
James TD, Sandanayake KRAS, Shinkai S (1995) Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature 374:345–347
James TD, Sandayake KRAS, Iguchi R, Shinkai SJ (1995) Novel Saccaride-photoinduced electron transfer sensors based on the interaction of boronic acid and amines. JACS 117:8982–8987
Ni W, Kaur G, Springsteen G, Wang B, Franzen S (2004) Regulating the fluorescence intensity of an anthrtracene boronic acid system: a B-N bond or a hydrolysis mechanism? Bioorg Chem 32:571–581
Sun X, Zhai W, Fossey JS, James TD (2016) Boronic acids for fluorescence imaging of carbohydrates. Chem Commun. 52:3456–3469
Collins BE, Sorey S, Hargrove AE, Shabbir SH, Lynch VM, Anslyn EV (2009) Probing intramolecular B-N Interactions in Ortho-aminomethyl arylboronic acids. J Org Chem 11:4055–4060
Author information
Authors and Affiliations
Contributions
C.W.B., G.D.M., H.R., and J.D.L. prepared the manuscript along with the accompanying figures. All authors have reviewed and approved the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Markham, G.D., Rostami, H., Larkin, J.D. et al. Models for boronic acid receptors II: a computational structural, bonding, and thermochemical investigation of the RB(OH)2∙H2O∙NH3 and RB(−OCH2CH2O−)∙NH3∙H2O potential energy surfaces (R = H, methyl, phenyl, and ortho-methyl-phenyl). Struct Chem 34, 1951–1972 (2023). https://doi.org/10.1007/s11224-023-02131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02131-1